IRW (Isoleucine–Arginine–Tryptophan) Improves Glucose Tolerance in High Fat Diet Fed C57BL/6 Mice via Activation of Insulin Signaling and AMPK Pathways in Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diet, and Body Weight (BW) Measurements
2.2. IRW Dosage
2.3. Oral Glucose Tolerance and Insulin Tolerance Tests
2.4. Tissue Collection
2.5. Protein Extraction and Western Blotting
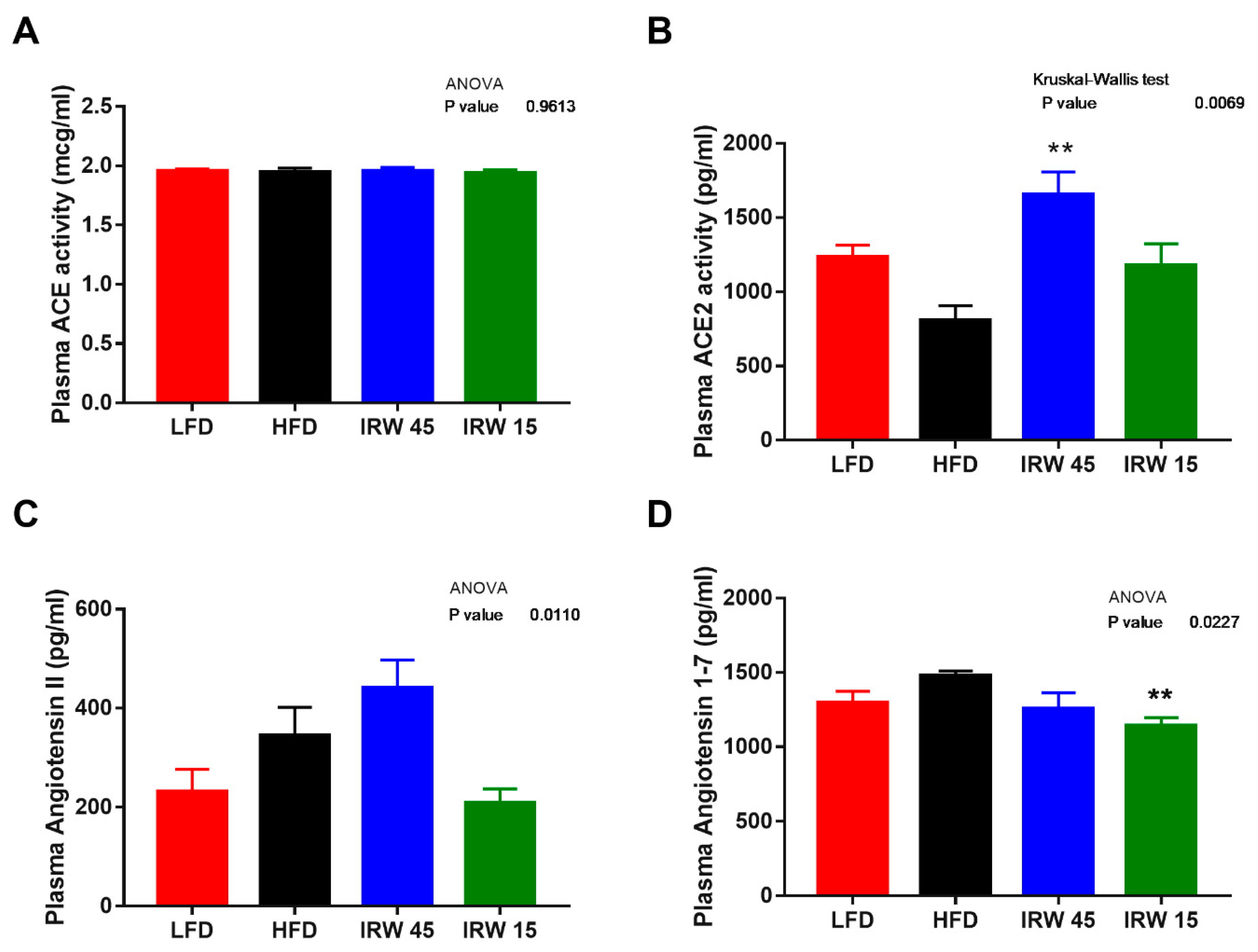
2.6. Plasma RAS Components and Insulin
2.7. RNA Sequencing and Quantitative RT-PCR (qPCR)
2.8. Statistics and Sample Size
3. Results
3.1. Food Intake and Body Composition
3.2. Glucose Homeostasis and Plasma Insulin
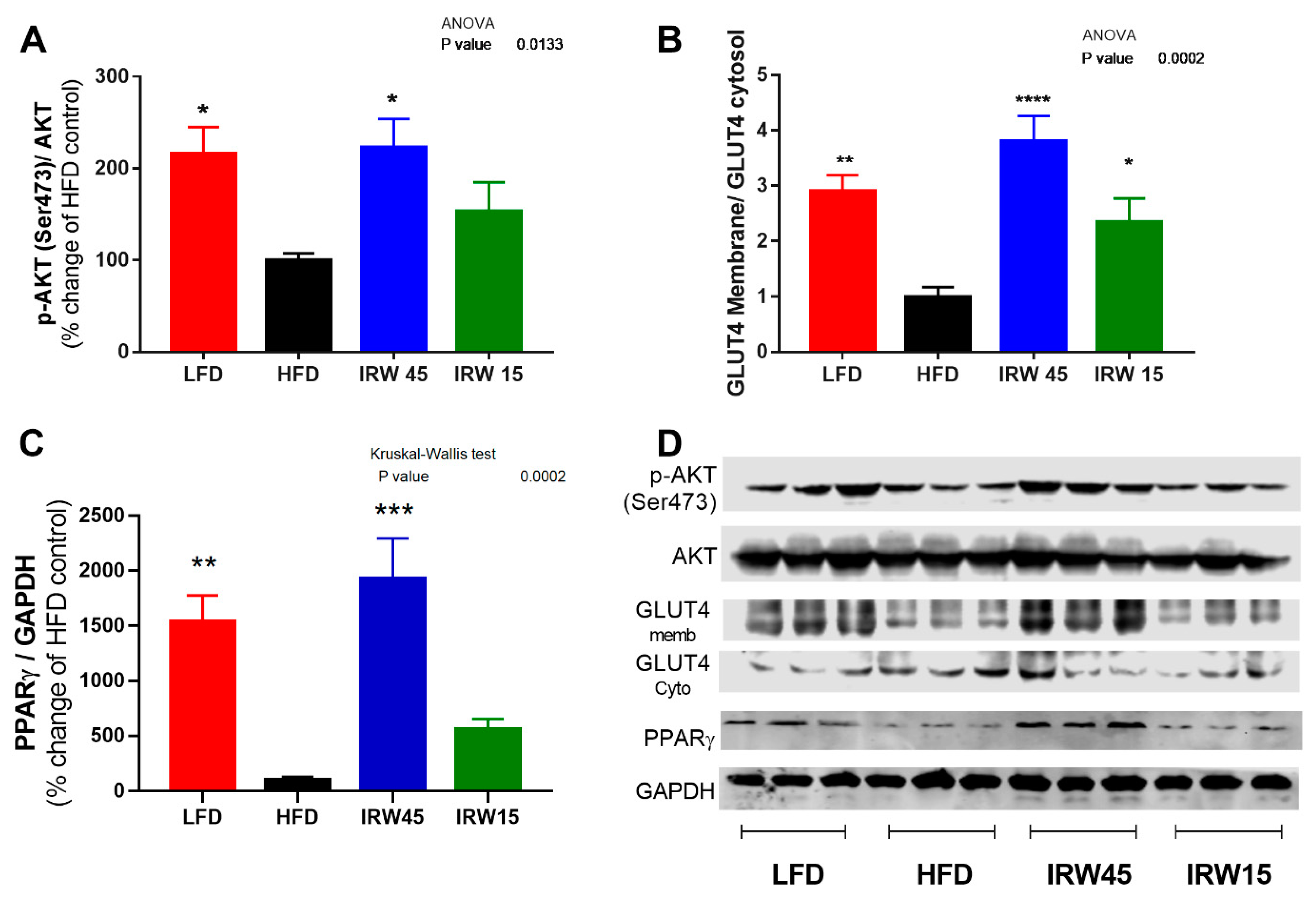
3.3. Insulin Signaling and PPARγ Abundance
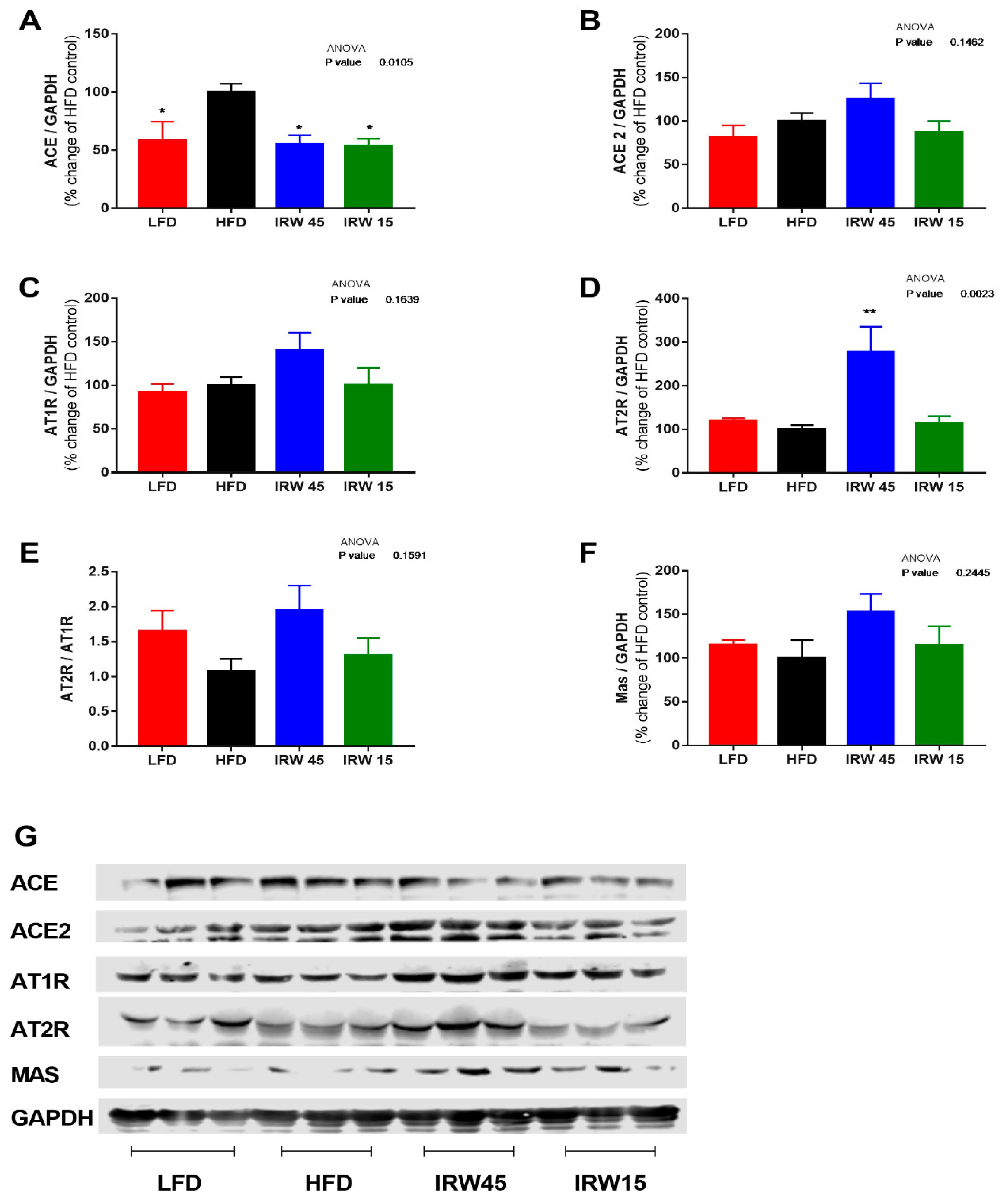
3.4. RAS Components
3.5. Skeletal Muscle Gene Expression
3.6. AMPKα Abundance and mTOR Signaling
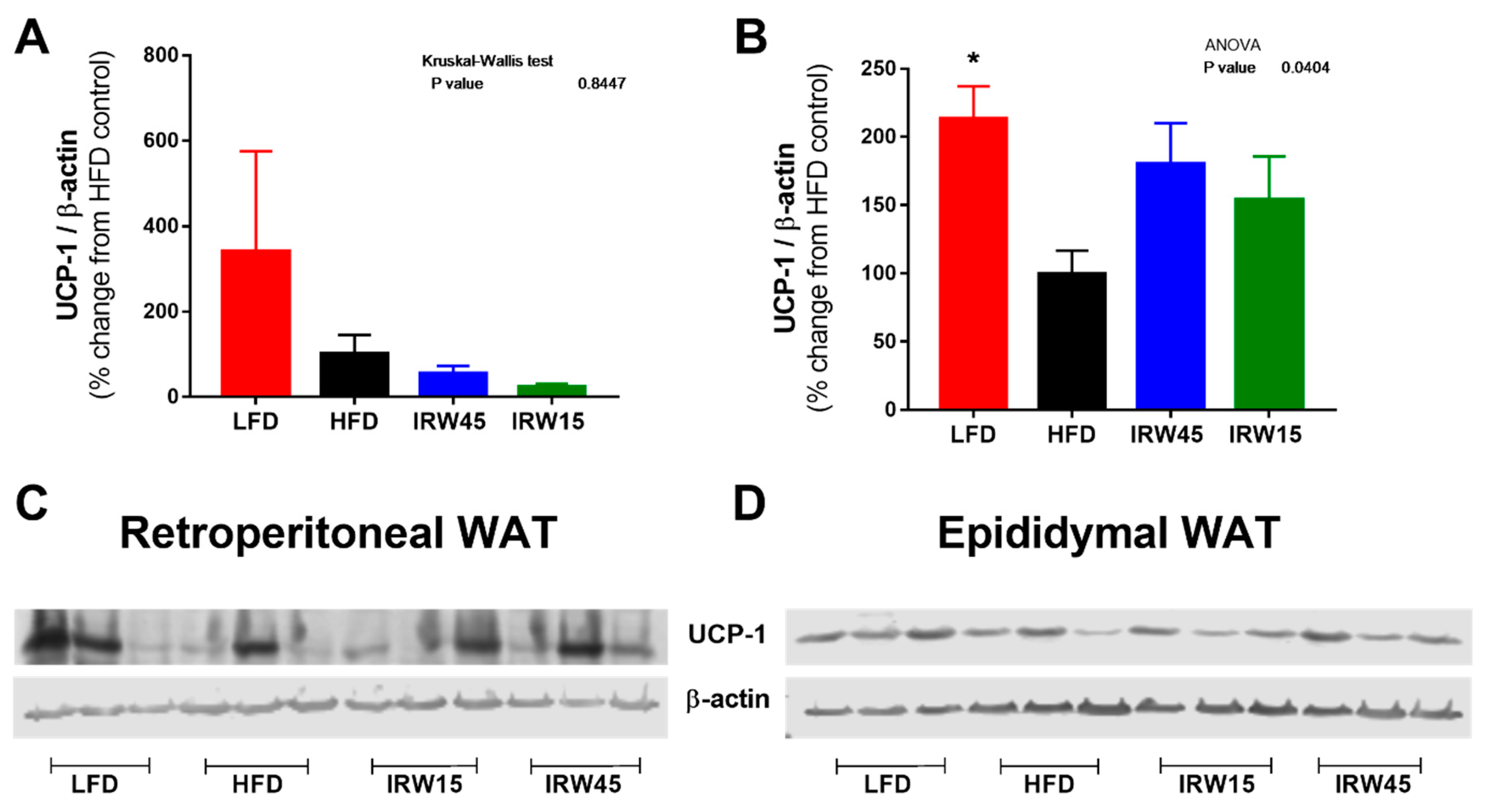
3.7. Adipose Tissue UCP-1 Abundance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Putnam, K.; Shoemaker, R.; Yiannikouris, F.; Cassis, L.A. The renin-angiotensin system: A target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1219–H1230. [Google Scholar] [CrossRef] [PubMed]
- Agoudemos, M.M.; Greene, A.S. Localization of the renin-angiotensin system components to the skeletal muscle microcirculation. Microcirculation 2005, 12, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Wang, W.; Dong, Z.; Cao, W.; Liu, Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes 2011, 60, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Frantz, E.D.; Crespo-Mascarenhas, C.; Barreto-Vianna, A.R.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Renin-angiotensin system blockers protect pancreatic islets against diet-induced obesity and insulin resistance in mice. PLoS ONE 2013, 8, e67192. [Google Scholar] [CrossRef]
- van der Zijl, N.J.; Moors, C.C.; Goossens, G.H.; Hermans, M.M.; Blaak, E.E.; Diamant, M. Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: A randomized controlled trial. Diabetes Care 2011, 34, 845–851. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, R.B.; Yang, Y.; Su, T.Y.; Huang, M.J.; Li, P.; Chen, X.M. Valsartan Alleviates Insulin Resistance in Skeletal Muscle of Chronic Renal Failure Rats. Med. Sci. Monit. 2018, 24, 2413–2419. [Google Scholar] [CrossRef]
- Mitsuishi, M.; Miyashita, K.; Muraki, A.; Itoh, H. Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes 2009, 58, 710–717. [Google Scholar] [CrossRef]
- Diplock, A.T.; Action, E.C. Scientific concepts of functional foods in Europe-consensus document. Br. J. Nutr. 1999, 81, S1–S27. [Google Scholar]
- Jahandideh, F.; Zani, S.C.C.; Son, M.; Proctor, S.D.; Davidge, S.T.; Chan, C.B.; Wu, J. Egg white hydrolysate enhances insulin sensitivity in high-fat diet-induced insulin-resistant rats via Akt activation. Br. J. Nutr. 2019, 122, 14–24. [Google Scholar] [CrossRef]
- Jahandideh, F.; Liu, P.; Wu, J. Purification and identification of adipogenic-differentiating peptides from egg white hydrolysate. Food Chem. 2018, 259, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Bhullar, K.S.; Hubbard, B.P.; Wu, J.P. Tripeptide IRW initiates differentiation in osteoblasts differentiation via the RUNX2 pathway. BBA-Gen. Subj. 2019, 1863, 1138–1146. [Google Scholar] [CrossRef]
- Martin, M.; Deussen, A. Effects of natural peptides from food proteins on angiotensin converting enzyme activity and hypertension. Crit. Rev. Food Sci. Nutr. 2019, 59, 1264–1283. [Google Scholar] [CrossRef] [PubMed]
- de Campos Zani, S.C.; Wu, J.; Chan, C.B. Egg and soy-derived peptides and hydrolysates: A review of their physiological actions against diabetes and obesity. Nutrients 2018, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Wu, J. Purification and characterisation of angiotensin I converting enzyme (ACE) inhibitory peptides derived from enzymatic hydrolysate of ovotransferrin. Food Chem. 2011, 126, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef]
- Huang, W.; Chakrabarti, S.; Majumder, K.; Jiang, Y.; Davidge, S.T.; Wu, J. Egg-derived peptide IRW inhibits TNF-alpha-induced inflammatory response and oxidative stress in endothelial cells. J. Agric. Food Chem. 2010, 58, 10840–10846. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Davidge, S.T.; Wu, J. Egg White-Derived Antihypertensive Peptide IRW (Ile-Arg-Trp) Reduces Blood Pressure in Spontaneously Hypertensive Rats via the ACE2/Ang (1–7)/Mas Receptor Axis. Mol. Nutr. Food Res. 2019, 63, e1900063. [Google Scholar] [CrossRef]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE 2013, 8, e82829. [Google Scholar] [CrossRef]
- Son, M.; Chan, C.B.; Wu, J. Egg white ovotransferrin-derived ACE inhibitory peptide ameliorates angiotensin II-stimulated insulin resistance in skeletal muscle cells. Mol. Nutr. Food Res. 2018, 62, 1700602. [Google Scholar] [CrossRef]
- Son, M.; Wu, J. Egg white hydrolysate and peptide reverse insulin resistance associated with tumor necrosis factor-alpha (TNF-alpha) stimulated mitogen-activated protein kinase (MAPK) pathway in skeletal muscle cells. Eur. J. Nutr. 2019, 58, 1961–1969. [Google Scholar] [CrossRef]
- Williams, L.M.; Campbell, F.M.; Drew, J.E.; Koch, C.; Hoggard, N.; Rees, W.D.; Kamolrat, T.; Thi Ngo, H.; Steffensen, I.L.; Gray, S.R.; et al. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS ONE 2014, 9, e106159. [Google Scholar] [CrossRef]
- Heikkinen, S.; Argmann, C.A.; Champy, M.F.; Auwerx, J. Evaluation of glucose homeostasis. Curr. Protoc. Mol. Biol. 2007, 77, 29B.3.1–29B.3.22. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Non-Prescription Health Products Doctorate (NNHPD). What Are Natural Health Products; Government of Canada: Ottawa, ON, Canada, 2004.
- Li, S.; Liu, L.; He, G.; Wu, J. Molecular targets and mechanisms of bioactive peptides against metabolic syndromes. Food Funct. 2018, 9, 42–52. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Antidiabetic Food-Derived Peptides for Functional Feeding: Production, Functionality and In Vivo Evidences. Foods 2020, 9, 983. [Google Scholar] [CrossRef]
- Grootaert, C.; Jacobs, G.; Matthijs, B.; Pitart, J.; Baggerman, G.; Possemiers, S.; Van der Saag, H.; Smagghe, G.; Van Camp, J.; Voorspoels, S. Quantification of egg ovalbumin hydrolysate-derived anti-hypertensive peptides in an in vitro model combining luminal digestion with intestinal Caco-2 cell transport. Food Res. Int. 2017, 99, 531–541. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Jain, S.K.; Rains, J.; Croad, J.; Larson, B.; Jones, K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid. Redox Signal. 2009, 11, 241–249. [Google Scholar] [CrossRef]
- Kawabeta, K.; Hase-Tamaru, S.; Yuasa, M.; Suruga, K.; Sugano, M.; Koba, K. Dietary beta-Conglycinin Modulates Insulin Sensitivity, Body Fat Mass, and Lipid Metabolism in Obese Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. J. Oleo Sci. 2019, 68, 339–350. [Google Scholar] [CrossRef]
- Matsui, T.; Sato, M.; Tanaka, M.; Yamada, Y.; Watanabe, S.; Fujimoto, Y.; Imaizumi, K.; Matsumoto, K. Vasodilating dipeptide Trp-His can prevent atherosclerosis in apo E-deficient mice. Br. J. Nutr. 2010, 103, 309–313. [Google Scholar] [CrossRef]
- Tanaka, M.; Hong, S.M.; Akiyama, S.; Hu, Q.Q.; Matsui, T. Visualized absorption of anti-atherosclerotic dipeptide, Trp-His, in Sprague-Dawley rats by LC-MS and MALDI-MS imaging analyses. Mol. Nutr. Food Res. 2015, 59, 1541–1549. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Ali, B.H.; Al Suleimani, Y.; Adham, S.A.; Ali, H.; Manoj, P.; Ashique, M.; Nemmar, A. The Effect of Metformin in Diabetic and Non-Diabetic Rats with Experimentally-Induced Chronic Kidney Disease. Biomolecules 2021, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Liu, Z.H.; Nie, Q.; Zhang, X.M.; Yang, L.Q.; Wang, C.; Yang, L.L.; Song, G.Y. Metformin improves high-fat diet-induced insulin resistance in mice by downregulating the expression of long noncoding RNA NONMMUT031874.2. Exp. Ther. Med. 2022, 23, 332. [Google Scholar] [CrossRef]
- Garces-Rimon, M.; Gonzalez, C.; Vera, G.; Uranga, J.A.; Lopez-Fandino, R.; Lopez-Miranda, V.; Miguel, M. Pepsin egg white hydrolysate improves glucose metabolism complications related to metabolic syndrome in Zucker fatty rats. Nutrients 2018, 10, 441. [Google Scholar] [CrossRef]
- Zhou, X.; Shentu, P.; Xu, Y. Spatiotemporal regulators for insulin-stimulated GLUT4 vesicle exocytosis. J. Diabetes Res. 2017, 2017, 1683678. [Google Scholar] [CrossRef]
- Kurth-Kraczek, E.J.; Hirshman, M.F.; Goodyear, L.J.; Winder, W.W. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 1999, 48, 1667–1671. [Google Scholar] [CrossRef]
- Bergeron, R.; Russell, R.R., 3rd; Young, L.H.; Ren, J.M.; Marcucci, M.; Lee, A.; Shulman, G.I. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am. J. Physiol. 1999, 276, E938–E944. [Google Scholar] [CrossRef]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg White-Derived Tripeptide IRW (Ile-Arg-Trp) Is an Activator of Angiotensin Converting Enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Chai, W.; Wang, W.; Liu, J.; Barrett, E.J.; Carey, R.M.; Cao, W.; Liu, Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 2010, 55, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Liu, Q.; Zhang, J.; Zhang, Z.; Wu, T.; Tang, Q.; Huang, C.; Li, R.; Zhou, J.; et al. Telmisartan attenuates obesity-induced insulin resistance via suppression of AMPK mediated ER stress. Biochem. Biophys. Res. Commun. 2020, 523, 787–794. [Google Scholar] [CrossRef]
- Shinshi, Y.; Higashiura, K.; Yoshida, D.; Togashi, N.; Yoshida, H.; Miyazaki, Y.; Ura, N.; Shimamoto, K. Angiotensin II inhibits glucose uptake of skeletal muscle via the adenosine monophosphate-activated protein kinase pathway. J. Am. Soc. Hypertens. 2007, 1, 251–255. [Google Scholar] [CrossRef]
- Ohshima, K.; Mogi, M.; Jing, F.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Ogimoto, A.; Dahlof, B.; Steckelings, U.M.; Unger, T.; et al. Direct angiotensin II type 2 receptor stimulation ameliorates insulin resistance in type 2 diabetes mice with PPARgamma activation. PLoS ONE 2012, 7, e48387. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J. Clin. Investig. 2000, 106, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Dallas-Yang, Q.; Li, Z.; Szalkowski, D.; Liu, F.; Shen, X.; Wu, M.; Zhou, G.; Doebber, T.; Berger, J.; et al. Potentiation of insulin signaling in tissues of Zucker obese rats after acute and long-term treatment with PPARgamma agonists. Diabetes 2002, 51, 2412–2419. [Google Scholar] [CrossRef]
- Hevener, A.L.; He, W.; Barak, Y.; Le, J.; Bandyopadhyay, G.; Olson, P.; Wilkes, J.; Evans, R.M.; Olefsky, J. Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 2003, 9, 1491–1497. [Google Scholar] [CrossRef]
- Li, G.S.; Liu, X.H.; Zhu, H.; Huang, L.; Liu, Y.L.; Ma, C.M. Skeletal muscle insulin resistance in hamsters with diabetes developed from obesity is involved in abnormal skeletal muscle LXR, PPAR and SREBP expression. Exp. Ther. Med. 2016, 11, 2259–2269. [Google Scholar] [CrossRef][Green Version]
- Amankwaah, A.F.; Hudson, J.L.; Kim, J.E.; Campbell, W.W. Reductions in whole-body fat mass but not increases in lean mass predict changes in cardiometabolic health indices with exercise training among weight-stable adults. Nutr. Res. 2019, 63, 63–69. [Google Scholar] [CrossRef]
- Kim, G.H.; Ju, J.Y.; Chung, K.S.; Cheon, S.Y.; Gil, T.Y.; Cominguez, D.C.; Cha, Y.Y.; Lee, J.H.; Roh, S.S.; An, H.J. Rice Hull Extract (RHE) Suppresses Adiposity in High-Fat Diet-Induced Obese Mice and Inhibits Differentiation of 3T3-L1 Preadipocytes. Nutrients 2019, 11, 1162. [Google Scholar] [CrossRef]
- Quiroga, D.T.; Munoz, M.C.; Gil, C.; Pffeifer, M.; Toblli, J.E.; Steckelings, U.M.; Giani, J.F.; Dominici, F.P. Chronic administration of the angiotensin type 2 receptor agonist C21 improves insulin sensitivity in C57BL/6 mice. Physiol. Rep. 2018, 6, e13824. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Patel, S.; Mani, S.; Hussain, T. Role of angiotensin type 2 receptor in improving lipid metabolism and preventing adiposity. Mol. Cell Biochem. 2019, 461, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Fromme, T.; Klingenspor, M. Uncoupling protein 1 expression and high-fat diets. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1–R8. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Perez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef]



| LFD | HFD | IRW15 | IRW45 | |
|---|---|---|---|---|
| Casein (g/kg) | 210.0 | 245.0 | 245.0 | 245.0 |
| L-Cystine (g/kg) | 3.0 | 3.5 | 3.5 | 3.5 |
| Corn Starch (g/kg) | 445.0 | 85.0 | 85.0 | 85.0 |
| Maltodextrin (g/kg) | 50.0 | 115.0 | 115.0 | 115.0 |
| Sucrose (g/kg) | 160.0 | 200.0 | 200.0 | 200.0 |
| Lard (g/kg) | 20.0 | 195.0 | 195.0 | 195.0 |
| Soybean Oil (g/kg) | 20.0 | 30.0 | 30.0 | 30.0 |
| Cellulose (g/kg) | 37.15 | 58.0 | 58.0 | 58.0 |
| Mineral Mix, AIN-93G-MX (94046) (g/kg) | 35.0 | 43.0 | 43.0 | 43.0 |
| Calcium Phosphate, dibasic (g/kg) | 2.0 | 3.4 | 3.4 | 3.4 |
| Vitamin Mix, AIN-93-VX (94047) (g/kg) | 15.0 | 19.0 | 19.0 | 19.0 |
| Choline Bitartrate (g/kg) | 2.75 | 3.0 | 3.0 | 3.0 |
| IRW (mg/kg BW) | n/a | n/a | 15 | 45 |
| LFD | HFD | IRW45 | IRW15 | |
|---|---|---|---|---|
| Body composition | ||||
| BW week 6 (g) | 28.1 ± 0.8 a | 31.3 ± 0.7 a | 30.9 ± 0.9 a | 31.7 ± 1.0 a |
| BW week 14 (g) | 35.0 ± 0.7 a | 41.6 ± 0.8 b | 36.9 ± 0.5 a | 40.4 ± 1.0 b |
| BW gain (g) (week 6–14) | 7.0 ± 0.2 a | 10.2 ± 0.3 b | 6.0 ± 0.6 a | 8.6 ± 0.6 b |
| BW gain (% of week 6) | 25.1 ± 1.2 a | 32.8 ± 1.3 b | 19.9 ± 2.3 a | 27.5 ± 2.2 b |
| Fat mass gain (g) | 3.9 ± 0.5 a | 7.0 ± 0.3 b | 3.6 ± 0.4 a | 6.1 ± 0.4 b |
| Fat mass gain (% BW) | 8.2 ± 1.1 b | 9.5 ± 0.8 b | 4.8 ± 0.9 a | 8.9 ± 0.9 b |
| Lean mass change (g) | 0.8 ± 0.3 a | 2.6 ± 0.1 b | 2.0 ± 0.1 b | 2.3 ± 0.2 b |
| Lean mass change (% BW) | −7.8 ± 1.0 b | −9.2 ± 0.7 b | −5.0 ± 0.9 a | −7.9 ± 0.9 b |
| Metabolic profile | ||||
| Fasting glucose week 6 (mmol/L) | 4.5 ± 0.06 a | 4.7 ± 0.3 a | 5.0 ± 0.3 a | 4.7 ± 0.2 a |
| Fasting glucose week 14 (mmol/L) | 4.8 ± 0.1 a | 6.3 ± 0.4 b | 4.4 ± 0.2 a | 5.7 ± 0.2 b |
| Fasting insulin week 14 (uU/mL) | 192.4 ± 37.3 a | 780.0 ± 56.4 b | 383.1 ± 72.5 a | 623.7 ± 112.7 b |
| HOMA-IR | 5.7 ± 0.8 a | 32.0 ± 3.2 b | 11.0 ± 2.0 a | 23.0 ± 4.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Campos Zani, S.C.; Son, M.; Bhullar, K.S.; Chan, C.B.; Wu, J. IRW (Isoleucine–Arginine–Tryptophan) Improves Glucose Tolerance in High Fat Diet Fed C57BL/6 Mice via Activation of Insulin Signaling and AMPK Pathways in Skeletal Muscle. Biomedicines 2022, 10, 1235. https://doi.org/10.3390/biomedicines10061235
de Campos Zani SC, Son M, Bhullar KS, Chan CB, Wu J. IRW (Isoleucine–Arginine–Tryptophan) Improves Glucose Tolerance in High Fat Diet Fed C57BL/6 Mice via Activation of Insulin Signaling and AMPK Pathways in Skeletal Muscle. Biomedicines. 2022; 10(6):1235. https://doi.org/10.3390/biomedicines10061235
Chicago/Turabian Stylede Campos Zani, Stepheny C., Myoungjin Son, Khushwant S. Bhullar, Catherine B. Chan, and Jianping Wu. 2022. "IRW (Isoleucine–Arginine–Tryptophan) Improves Glucose Tolerance in High Fat Diet Fed C57BL/6 Mice via Activation of Insulin Signaling and AMPK Pathways in Skeletal Muscle" Biomedicines 10, no. 6: 1235. https://doi.org/10.3390/biomedicines10061235
APA Stylede Campos Zani, S. C., Son, M., Bhullar, K. S., Chan, C. B., & Wu, J. (2022). IRW (Isoleucine–Arginine–Tryptophan) Improves Glucose Tolerance in High Fat Diet Fed C57BL/6 Mice via Activation of Insulin Signaling and AMPK Pathways in Skeletal Muscle. Biomedicines, 10(6), 1235. https://doi.org/10.3390/biomedicines10061235








