Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI)
Abstract
1. Introduction
2. Materials and Methods
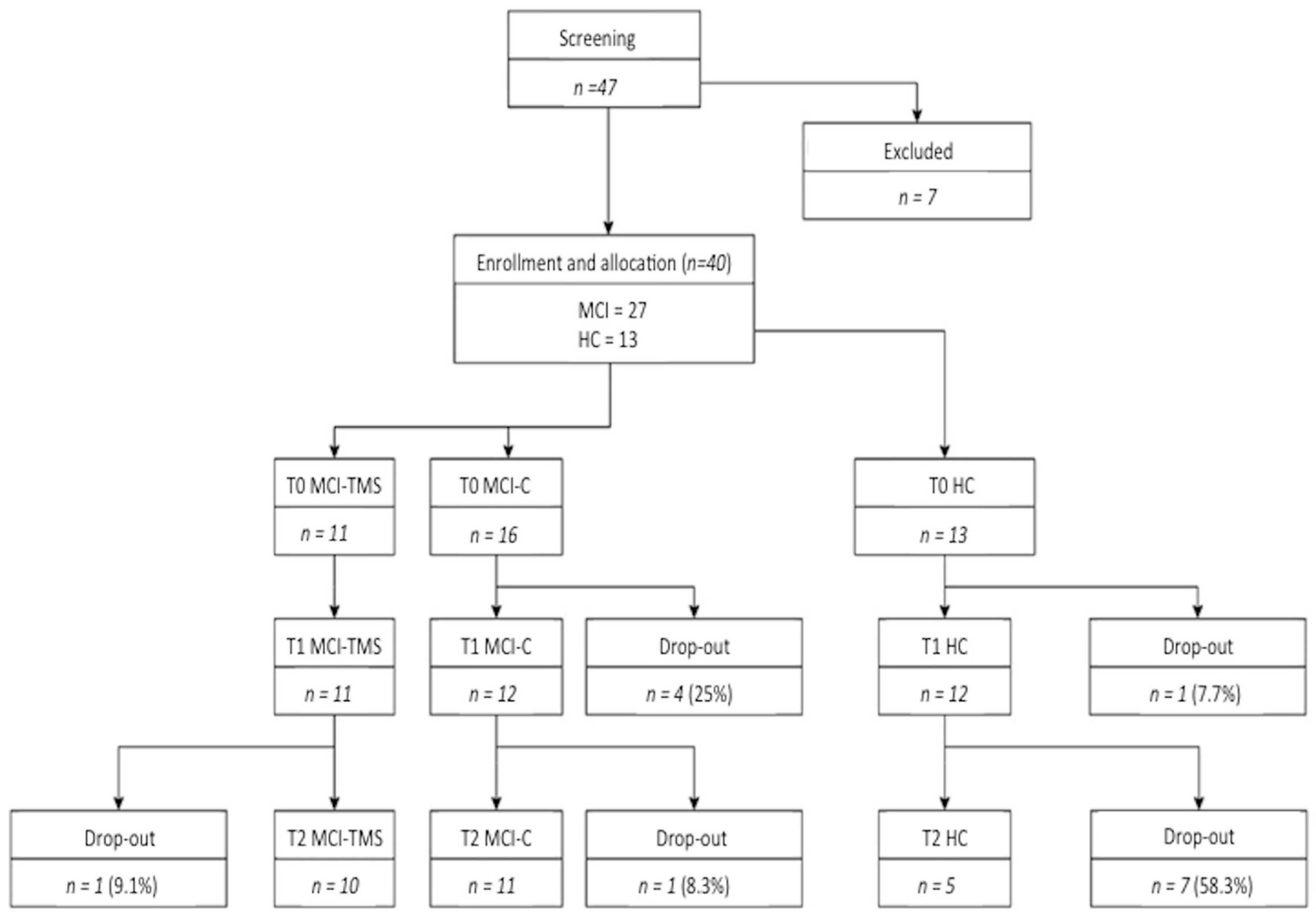
2.1. Case Selection and Study Procedures
Neuropsychological Assessment
2.2. Study Design
2.3. Statistical Analysis: Between-Groups Comparisons of Clinical and Neuropsychological Data
2.4. rTMS Protocol
2.5. MRI Analysis
2.5.1. Magnetic Resonance Imaging
2.5.2. RS-fMRI Data Preparation and Preprocessing
2.5.3. Resting State Network (RSN) Functional Connectivity Analysis
2.5.4. Regional Atrophy Measurements: Voxel-Based Morphometry (VBM)
3. Results
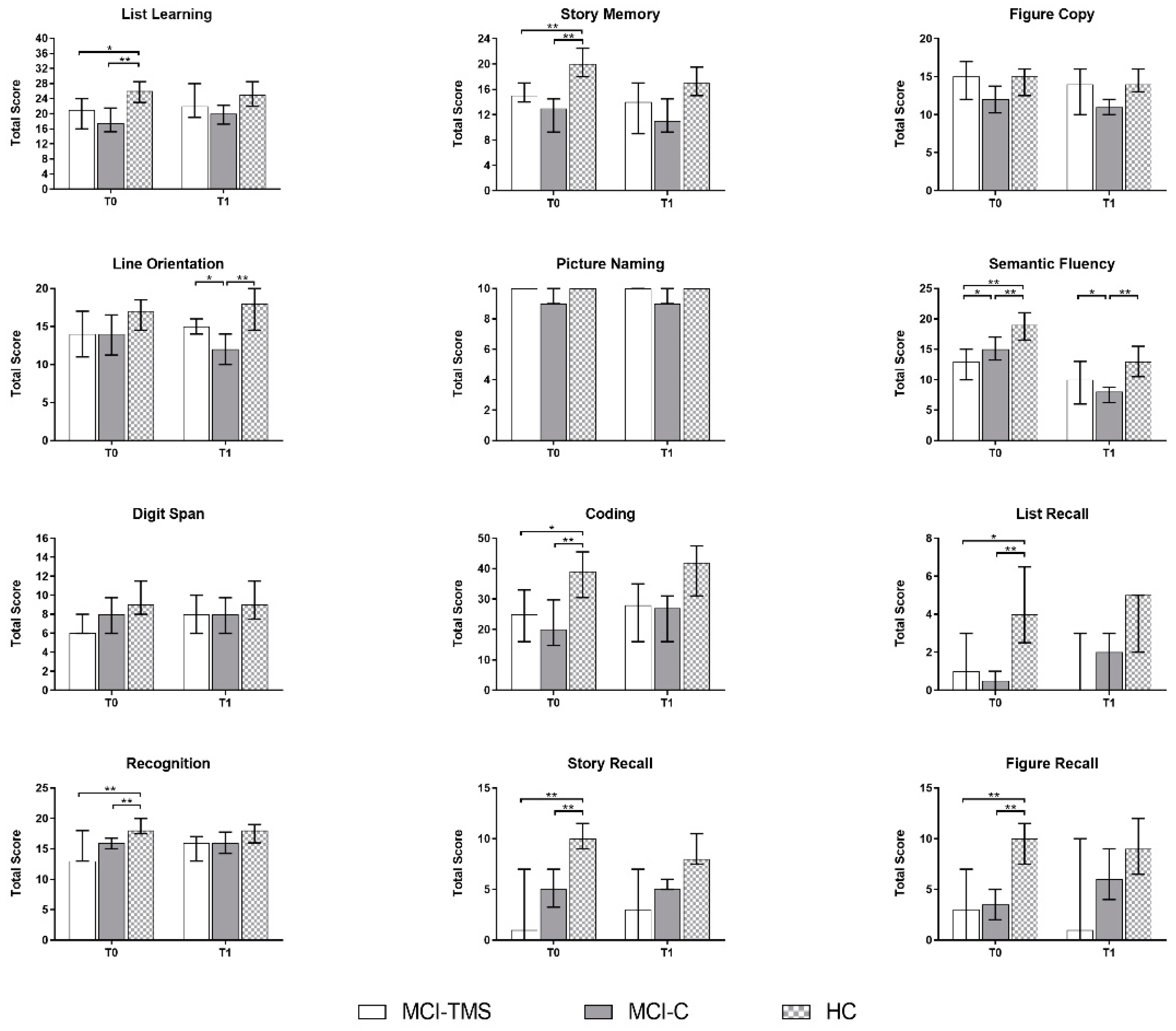
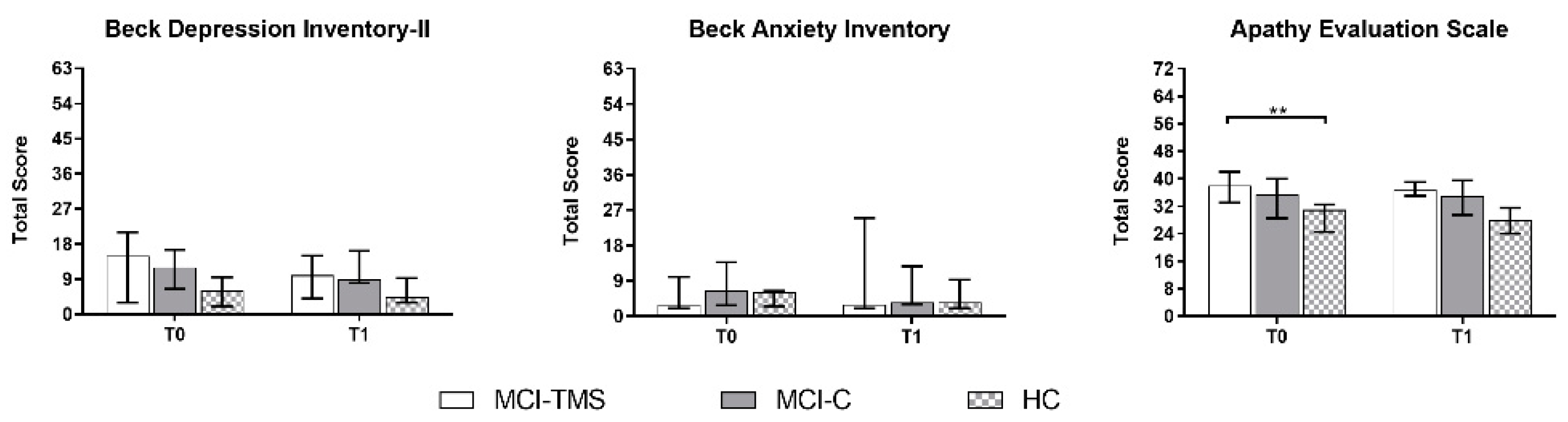
3.1. Clinical and Neuropsychological Assessment
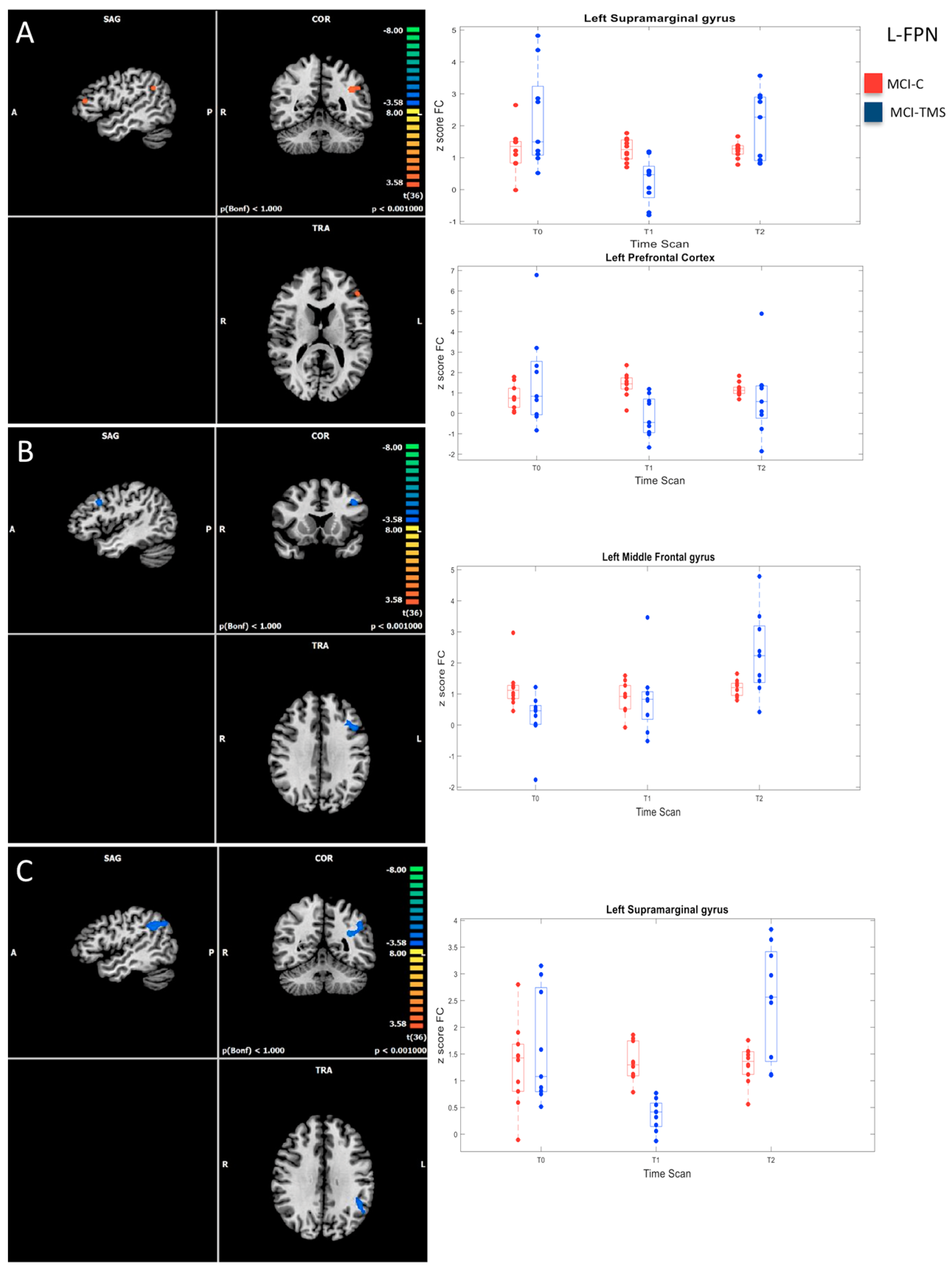
3.2. Baseline RSN Functional Connectivity Analysis
3.3. Five-Week and Six-Month RSN Functional Connectivity Analysis
3.4. VBM Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Mariani, E.; Monastero, R.; Mecocci, P. Mild cognitive impairment: A systematic review. J. Alzheimer’s Dis. 2007, 12, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, E.; Almkvist, O. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease. Acta Neurol. Scand. Suppl. 2003, 107, 34–41. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.A. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2012, 34, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Mangialasche, F.; Camarda, C.; Ercolani, S.; Camarda, R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J. Alzheimer’s Dis. 2009, 18, 11–30. [Google Scholar] [CrossRef]
- Teng, E.; Tassniyom, K.; Lu, P.H. Reduced quality of life ratings in mild cognitive impairment: Analyses of subject and informant responses. Am. J. Geriatr. Psychiatry 2012, 20, 1016–1025. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Wolk, D.A. Alzheimer’s Disease Neuroimaging Initiative. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J. Neurol. Neurosurg. Psychiatry 2011, 82, 45–51. [Google Scholar] [CrossRef]
- Johns, E.K.; Phillips, N.A.; Belleville, S.; Goupil, D.; Babins, L.; Kelner, N.; Ska, B.; Gilbert, B.; Massoud, F.; de Boysson, C.; et al. The profile of executive functioning in amnestic mild cognitive impairment: Disproportionate deficits in inhibitory control. J. Int. Neuropsychol. Soc. 2012, 18, 541–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, B.; Verhaeghen, P.; Nilsson, L.G. Executive functioning in older adults with mild cognitive impairment: MCI has effects on planning, but not on inhibition. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2007, 14, 557–570. [Google Scholar] [CrossRef]
- Zheng, D.; Dong, X.; Sun, H.; Xu, Y.; Ma, Y.; Wang, X. The overall impairment of core executive function components in patients with amnestic mild cognitive impairment: A cross-sectional study. BMC Neurol. 2012, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Chang, C.C.; Lin, K.N.; Huang, C.W.; Chang, W.N.; Chang, Y.T.; Chen, C.; Yeh, Y.C.; Wang, P.N. Patterns of executive dysfunction in amnestic mild cognitive impairment. Int. Psychogeriatr. 2013, 25, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, Y.; Yu, K.H.; Lee, B.C. Disproportionate decline of executive functions in early Mild Cognitive Impairment, Late Mild Cognitive Impairment, and Mild Alzheimer’s Disease. Dement. Neurocogn. Disord. 2016, 15, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Traykov, L.; Raoux, N.; Latour, F.; Gallo, L.; Hanon, O.; Baudic, S.; Bayle, C.; Wenisch, E.; Remy, P.; Rigaud, A.S. Executive functions deficit in mild cognitive impairment. Cogn. Behav. Neurol. 2007, 20, 219–224. [Google Scholar] [CrossRef]
- Summers, M.J.; Saunders, N.L. Neuropsychological measures predict decline to Alzheimer’s dementia from mild cognitive impairment. Neuropsychology 2012, 26, 498–508. [Google Scholar] [CrossRef]
- Rozzini, L.; Chilovi, B.V.; Conti, M.; Bertoletti, E.; Delrio, I.; Trabucchi, M.; Padovani, A. Conversion of amnestic Mild Cognitive Impairment to dementia of Alzheimer type is independent to memory deterioration. Int. J. Geriatr. Psychiatry 2007, 22, 1217–1222. [Google Scholar] [CrossRef]
- Lissek, V.; Suchan, B. Preventing dementia? Interventional approaches in mild cognitive impairment. Neurosci. Biobehav. Rev. 2021, 122, 143–164. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Korai, S.A.; Ranieri, F.; Di Lazzaro, V.; Papa, M.; Cirillo, G. Neurobiological After-Effects of Low Intensity Transcranial Electric Stimulation of the Human Nervous System: From Basic Mechanisms to Metaplasticity. Front. Neurol. 2021, 12, 587771. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Vico, I.A.; Emadi Andani, M.; Morgante, F.; Sepe, G.; Tessitore, A.; Bologna, M.; Tinazzi, M. Changes in corticospinal circuits during premovement facilitation in physiological conditions. Front. Hum. Neurosci. 2021, 15, 684013. [Google Scholar] [CrossRef]
- Demirtas-Tatlidede, A.; Vahabzadeh-Hagh, A.M.; Pascual-Leone, A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology 2013, 64, 566–578. [Google Scholar] [CrossRef]
- Hoogendam, J.M.; Ramakers, G.M.; Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Lisanby, S.H.; Luber, B.; Perera, T.; Sackeim, H.A. Transcranial magnetic stimulation: Applications in basic neuroscience and neuropsychopharmacology. Int. J. Neuropsychopharmacol. 2000, 3, 259–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Chen, R.S.; Rothwell, J.C.; Wen, H.I. The after-effect oh human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol. 2007, 118, 1028–1032. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Wu, X.; Ji, G.J.; Geng, Z.; Zhou, S.; Yan, Y.; Wei, L.; Qiu, B.; Tian, Y.; Wang, K. Strengthened theta-burst transcranial magnetic stimulation as an adjunctive treatment for Alzheimer’s disease: An open-label pilot study. Brain Stimul. 2020, 13, 484–486. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.S.; Chen, R.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin. Neurophysiol. 2021, 132, 2568–2608. [Google Scholar] [CrossRef]
- Cho, Y.H.; Ton That, V.; Sundman, M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2020, 86, 1–10. [Google Scholar] [CrossRef]
- Lara, A.H.; Wallis, J.D. The role of prefrontal cortex in working memory: A mini review. Front. Syst. Neurosci. 2015, 9, 173. [Google Scholar] [CrossRef]
- Cotelli, M.; Calabria, M.; Manenti, R.; Rosini, S.; Zanetti, O.; Cappa, S.F.; Miniussi, C. Improved language performance in Alzheimer disease following brain stimulation. J. Neurol. Neurosurg. Psychiatry 2011, 82, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Drumond Marra, H.L.; Myczkowski, M.L.; Maia Memória, C.; Arnaut, D.; Leite Ribeiro, P.; Sardinha Mansur, C.G.; Lancelote Alberto, R.; Boura Bellini, B.; Alves Fernandes da Silva, A.; Tortella, G.; et al. Transcranial magnetic stimulation to address mild cognitive impairment in the elderly: A randomized controlled study. Behav. Neurol. 2015, 2015, 287843. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, W.; Liu, X.; Xu, Q.; Tang, L.; Wu, S. Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease: A randomized, double-blind, sham-controlled study. Shanghai Arch. Psychiatry 2015, 27, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Padala, P.R.; Padala, K.P.; Lensing, S.Y.; Jackson, A.N.; Hunter, C.R.; Parkes, C.M.; Dennis, R.A.; Bopp, M.M.; Caceda, R.; Mennemeier, M.S.; et al. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 2018, 261, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ren, R.; Lin, G.; Zou, Y.; Jiang, L.; Wei, Z.; Li, C.; Wang, G. Repetitive transcranial magnetic stimulation induced hypoconnectivity within the default mode network yields cognitive improvements in amnestic mild cognitive impairment: A randomized controlled study. J. Alzheimer’s Dis. 2019, 69, 1137–1151. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Darwish, E.S.; Khedr, E.M.; El Serogy, Y.M.; Ali, A.M. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s Dementia. J. Neurol. 2012, 259, 83–92. [Google Scholar] [CrossRef]
- Rutherford, G.; Lithgow, B.; Moussavi, Z. Short andl-term effects of rTMS treatment on Alzheimer’s disease at different stages: A pilot study. J. Exp. Neurosci. 2015, 9, 43–51. [Google Scholar] [CrossRef]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef]
- Nilakantan, A.S.; Bridge, D.J.; Gagnon, E.P.; VanHaerents, S.A.; Voss, J.L. Stimulation of the Posterior Cortical-Hippocampal Network Enhances Precision of Memory Recollection. Curr Biol 2017, 27, 465–470. [Google Scholar] [CrossRef]
- Nilakantan, A.S.; Mesulam, M.M.; Weintraub, S.; Karp, E.L.; VanHaerents, S.; Voss, J.L. Network-targeted stimulation engages neurobehavioral hallmarks of age-related memory decline. Neurology 2019, 92, e2349–e2354. [Google Scholar] [CrossRef]
- Bagattini, C.; Mutanen, T.P.; Fracassi, C.; Manenti, R.; Cotelli, M.; Ilmoniemi, R.J.; Miniussi, C.; Bortoletto, M. Predicting Alzheimer’s disease severity by means of TMS-EEG coregistration. Neurobiol. Aging 2019, 80, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Myers, N.E.; Göttler, J.; Pasquini, L.; Grimmer, T.; Förster, S.; Manoliu, A.; Neitzel, J.; Kurz, A.; Förstl, H.; et al. Disrupted intrinsic networks link amyloid-beta pathology and impaired cognition in prodromal Alzheimer’s disease. Cereb. Cortex 2015, 25, 4678–4688. [Google Scholar] [CrossRef]
- Peña-Gomez, C.; Solé-Padullés, C.; Clemente, I.C.; Junqué, C.; Bargalló, N.; Bosch, B.; Molinuevo, J.L.; Valls-Solé, J.; Pascual-Leone, A.; Bartrés-Faz, D. APOE status modulates the changes in network connectivity induced by brain stimulation in non-demented elders. PLoS ONE 2012, 7, e51833. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Zhao, Z.; Min, B.; Lu, J.; Li, K.; He, Y.; Jia, J. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fMRI study. Neuroimage 2011, 55, 287–295. [Google Scholar] [CrossRef]
- Spetsieris, P.G.; Ko, J.H.; Tang, C.C.; Nazem, A.; Sako, W.; Peng, S.; Ma, Y.; Dhawan, V.; Eidelberg, D. Metabolic resting-state brain networks in health and disease. Proc. Natl. Acad. Sci. USA 2015, 112, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Pievani, M.; Filippini, N.; van den Heuvel, M.P.; Cappa, S.F.; Frisoni, G.B. Brain connectivity in neurodegenerative diseases--from phenotype to proteinopathy. Nat. Rev. Neurol. 2014, 10, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Pievani, M.; Mega, A.; Quattrini, G.; Guidali, G.; Ferrari, C.; Cattaneo, A.; D’Aprile, I.; Mascaro, L.; Gasparotti, R.; Corbo, D.; et al. Targeting Default Mode Network Dysfunction in Persons at Risk of Alzheimer’s Disease with Transcranial Magnetic Stimulation (NEST4AD): Rationale and Study Design. J. Alzheimer’s Dis. 2021, 83, 1877–1889. [Google Scholar] [CrossRef]
- Brier, M.R.; Thomas, J.B.; Snyder, A.Z.; Benzinger, T.L.; Zhang, D.; Raichle, M.E.; Holtzman, D.M.; Morris, J.C.; Ances, B.M. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J. Neurosci. 2012, 32, 8890–8899. [Google Scholar] [CrossRef]
- Bai, F.; Shi, Y.; Yuan, Y.; Wang, Y.; Yue, C.; Teng, Y.; Wu, D.; Zhang, Z.; Jia, J.; Zhang, Z. Altered self-referential network in resting-state amnestic type mild cognitive impairment. Cortex 2012, 48, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Pievani, M.; Geroldi, C.; Copetti, M.; Frisoni, G.B.; Filippi, M. Resting state fMRI in Alzheimer’s disease: Beyond the default mode network. Neurobiol. Aging 2012, 33, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Guse, B.; Falkai, P.; Wobrock, T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: A systematic review. J. Neural. Transm 2010, 117, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cui, H.; Zhang, C.; Cao, X.; Gu, N.; Zhu, Y.; Wang, J.; Yang, Z.; Li, C. Repetitive Transcranial Magnetic Stimulation for Improving Cognitive Function in Patients With Mild Cognitive Impairment: A Systematic Review. Front. Aging Neurosci. 2021, 12, 593000. [Google Scholar] [CrossRef]
- He, W.; Wang, J.C.; Tsai, P.Y. Theta Burst Magnetic Stimulation Improves Parkinson’s-Related Cognitive Impairment: A Randomised Controlled Study. Neurorehabil. Neural. Repair 2021, 1, 1545968321104131. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G. The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996, 36, 378–384. [Google Scholar] [CrossRef]
- Ponteri, M.; Pioli, R.; Padovani, A.; Tunesi, S.; De Girolamo, G. RBANS Repeatable Battery for the Assessment of Neuropsychological Status; Organizzazioni Speciali: Firenze, Italy, 2007. [Google Scholar]
- Sica, C.; Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; Lange, M.A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Isella, V.; Appollonio, I.; Meregalli, L.; Melzi, P.; Iurlaro, S.; Frattola, L. Dati normativi per le versioni italiane delle scale di apatia e di anedonia. Arch. Psicol. Neurol. Psichiatr. 1998, 59, 356–375. [Google Scholar]
- Furneri, G.; Platania, S.; Privitera, A.; Martelli, F.; Smeriglio, R.; Razza, G.; Maci, T.; Castellano, S.; Drago, F.; Santagati, M.; et al. The Apathy Evaluation Scale (AES-C): Psychometric Properties and Invariance of Italian Version in Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 9597. [Google Scholar] [CrossRef]
- Hays, R.D.; Anderson, R.; Revicki, D. Psychometric considerations in evaluating health-related quality of life measures. Qual. Life Res. 1993, 2, 441–449. [Google Scholar] [CrossRef]
- Quade, D. Rank analysis of covariance. J. Am. Stat. Assoc. 1967, 62, 1187–1200. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Trojsi, F.; Di Nardo, F.; Caiazzo, G.; Siciliano, M.; D’Alvano, G.; Passaniti, C.; Russo, A.; Bonavita, S.; Cirillo, M.; Esposito, F.; et al. Between-sex variability of resting state functional brain networks in amyotrophic lateral sclerosis (ALS). J. Neural. Transm. 2021, 128, 1881–1897. [Google Scholar] [CrossRef] [PubMed]
- Cavedo, E.; Redolfi, A.; Angeloni, F.; Babiloni, C.; Lizio, R.; Chiapparini, L.; Bruzzone, M.G.; Aquino, D.; Sabatini, U.; Alesiani, M.; et al. The Italian Alzheimer’s Disease Neuroimaging Initiative (I-ADNI): Validation of structural MR imaging. J. Alzheimer’s Dis. 2014, 40, 941–952. [Google Scholar] [CrossRef]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.; Turner, R. Movement-related effects in fMRI time-series. Magn. Reason. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef]
- Lemieux, L.; Salek-Haddadi, A.; Lund, T.E.; Laufs, H.; Carmichael, D. Modelling large motion events in fMRI studies of patients with epilepsy. Magn. Reason. Imaging 2007, 25, 894–901. [Google Scholar] [CrossRef]
- Satterthwaite, T.D.; Elliott, M.A.; Gerraty, R.T.; Ruparel, K.; Loughead, J.; Calkins, M.E.; Eickhoff, S.B.; Hakonarson, H.; Gur, R.C.; Gur, R.E.; et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 2013, 64, 240–256. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef]
- Kim, J.; Criaud, M.; Cho, S.S.; Díez-Cirarda, M.; Mihaescu, A.; Coakeley, S.; Ghadery, C.; Valli, M.; Jacobs, M.F.; Houle, S.; et al. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain 2017, 140, 2955–2967. [Google Scholar] [CrossRef]
- Goebel, R.; Esposito, F.; Formisano, E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006, 27, 392–401. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Karhunen, J.; Oja, E. Independent Component Analysis; John Wiley and Sons: New York, NY, USA, 2001. [Google Scholar]
- Esposito, F.; Scarabino, T.; Hyvarinen, A.; Himberg, J.; Formisano, E.; Comani, S.; Tedeschi, G.; Goebel, R.; Seifritz, E.; Di Salle, F. Independent component analysis of fMRI group studies by self-organizing clustering. Neuroimage 2005, 25, 193–205. [Google Scholar] [CrossRef]
- Himberg, J.; Hyvärinen, A.; Esposito, F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 2004, 22, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef]
- Eklund, A.; Nichols, T.E.; Knutsson, H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA 2016, 113, 7900–7905. [Google Scholar] [CrossRef] [PubMed]
- Forman, S.D.; Cohen, J.D.; Fitzgerald, M.; Eddy, W.F.; Mintun, M.A.; Noll, D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn. Reason. Med. 1995, 33, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Bisecco, A.; Altieri, M.; Santangelo, G.; Di Nardo, F.; Docimo, R.; Caiazzo, G.; Capuano, R.; Pappacena, S.; d’Ambrosio, A.; Bonavita, S.; et al. Resting-state functional correlates of social cognition in multiple sclerosis: An explorative study. Front. Behav. Neurosci. 2019, 13, 276. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef]
- Mantini, D.; Perrucci, M.G.; Del Gratta, C.; Romani, G.L.; Corbetta, M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA 2007, 104, 13170–13175. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Agosta, F.; Canu, E.; Valsasina, P.; Riva, N.; Prelle, A.; Comi, G.; Filippi, M. Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol. Aging 2013, 34, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.K.; Welton, T.; Lyon, M.; McCorkindale, A.N.; Sutherland, G.T.; Burnham, S.; Fripp, J.; Martins, R.; Grieve, S.M. Structural core of the executive control network: A high angular resolution diffusion MRI study. Hum. Brain Mapp. 2020, 41, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Farb, N.A.; Grady, C.L.; Strother, S.; Tang-Wai, D.F.; Masellis, M.; Black, S.; Freedman, M.; Pollock, B.G.; Campbell, K.L.; Hasher, L.; et al. Abnormal network connectivity in frontotemporal dementia: Evidence for prefrontal isolation. Cortex 2013, 49, 1856–1873. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Agosta, F.; Scola, E.; Canu, E.; Magnani, G.; Marcone, A.; Valsasina, P.; Caso, F.; Copetti, M.; Comi, G.; et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex 2013, 49, 2389–2401. [Google Scholar] [CrossRef]
- Koch, G.; Martorana, A.; Caltagirone, C.; Koch, G. Transcranial magnetic stimulation: Emerging biomarkers and novel therapeutics in Alzheimer’s disease. Neurosci. Lett. 2020, 719, 13435. [Google Scholar] [CrossRef]
- Moser, D.J.; Jorge, R.E.; Manes, M.D.; Paradiso, S.; Benjamin, B.S.; Robinson, R.G. Improved executive functioning following repetitive transcranial magnetic stimulation. Neurology 2002, 58, 1288–1290. [Google Scholar] [CrossRef]
- O’Connor, M.; Brenninkmeyer, C.; Morgan, A.; Bloomingdale, K.; Thall, M.; Vasile, R.; Leone, A.P. Magnetic stimulation and electroconvulsive therapy on mood and memory: A neurocognitive risk-benefit-analysis. Cogn. Behav. Neurol. 2003, 6, 118–127. [Google Scholar] [CrossRef]
- Little, J.T.; Kimbrell, T.A.; Wassermann, E.M.; Grafman, J.; Figueras, S.; Dunn, R.T.; Danielson, A.; Repella, J.; Huggins, T.; George, M.S.; et al. Cognitive effects of 1- and 20-Hertz repetitive transcranial magnetic stimulation in depression: Preliminary report. Neuropsychiatry Neuropsychol. Behav. Neurol. 2000, 13, 119–124. [Google Scholar]
- Martis, B.; Alam, D.; Dowd, S.M.; Hill, S.K.; Sharma, R.P.; Rosen, C.; Pliskin, N.; Martin, E.; Carson, V.; Janicak, P.G. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin. Neurophysiol. 2003, 114, 1125–1132. [Google Scholar] [CrossRef]
- Vanderhasselt, M.A.; De Raedt, R.; Baeken, C.; Leyman, L.; D’haenen, H. The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Exp. Brain Res. 2006, 169, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Yu, L.; Wang, L.; Li, L.; Liu, K.; Xu, S.; Wang, X. The case-control study of the effect of repetitive transcranial magnetic stimulation on elderly mild cognitive impairment patients. J. Clin. Psychiatry 2013, 23, 156–159. [Google Scholar]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hajak, G.; Marienhagen, J.; Langguth, B.; Werner, S.; Binder, H.; Eichhammer, P. High frequency transcranial magnetic stimulation in schizophrenia: A combined treatment and neuroimaging study. Psychol. Med. 2004, 34, 1157–1163. [Google Scholar] [CrossRef]
- Cordes, J.; Mobascher, A.; Arends, M.; Agelink, M.W.; Klimke, A. A new method for the treatment of depression: Transcranial magnetic stimulation. Dtsch. Med. Wochenschr. 2005, 130, 889–892. [Google Scholar] [CrossRef]
- Jin, Y.; Potkin, S.G.; Kemp, A.S.; Huerta, S.T.; Alva, G.; Thai, T.M.; Carreon, D.; Bunney, W.E., Jr. Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (alpha rTMS) on negative symptoms of schizophrenia. Schizophr. Bull. 2006, 32, 556–561. [Google Scholar] [CrossRef]
- Biesbroek, J.M.; Lim, J.S.; Weaver, N.A.; Arikan, G.; Kang, Y.; Kim, B.J.; Kuijf, H.J.; Postma, A.; Lee, B.C.; Lee, K.J.; et al. Anatomy of phonemic and semantic fluency: A lesion and disconnectome study in 1231 stroke patients. Cortex 2021, 143, 148–163. [Google Scholar] [CrossRef]
- Chouiter, L.; Holmberg, J.; Manuel, A.L.; Colombo, F.; Clarke, S.; Annoni, J.M.; Spierer, L. Partly segregated cortico-subcortical pathways support phonologic and semantic verbal fluency: A lesion study. Neuroscience 2016, 329, 275–283. [Google Scholar] [CrossRef]
- Baldo, J.V.; Schwartz, S.; Wilkins, D.; Dronkers, N.F. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J. Int. Neuropsychol. Soc. 2006, 12, 896–900. [Google Scholar] [CrossRef]
- Schmidt, C.S.M.; Nitschke, K.; Bormann, T.; Romer, P.; Kummerer, D.; Martin, M.; Umarova, R.M.; Leonhart, R.; Egger, K.; Dressing, A.; et al. Dissociating frontal and temporal correlates of phonological and semantic fluency in a large sample of left hemisphere stroke patients. Neuroimage Clin. 2019, 23, 101840. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Song, L.; Huang, R.; Ding, J.; Fang, Y.; Xu, Y.; Han, Z. Structural connectivity subserving verbal fluency revealed by lesion-behavior mapping in stroke patients. Neuropsychologia 2017, 101, 85–96. [Google Scholar] [CrossRef]
- Dilkina, K.; McClelland, J.L.; Plaut, D.C. Are there mental lexicons? The role of semantics in lexical decision. Brain Res. 2010, 1365, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Esser, S.K.; Huber, R.; Massimini, M.; Peterson, M.J.; Ferrarelli, F.; Tononi, G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res. Bull. 2006, 69, 86–94. [Google Scholar] [CrossRef]
- May, A.; Hajak, G.; Gänssbauer, S.; Steffens, T.; Langguth, B.; Kleinjung, T.; Eichhammer, P. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cereb. Cortex 2007, 17, 205–210. [Google Scholar] [CrossRef]
- Fu, Y.; Long, Z.; Luo, Q.; Xu, Z.; Xiang, Y.; Du, W.; Cao, Y.; Cheng, X.; Du, L. Functional and Structural Connectivity Between the Left Dorsolateral Prefrontal Cortex and Insula Could Predict the Antidepressant Effects of Repetitive Transcranial Magnetic Stimulation. Front. Neurosci. 2021, 15, 645936. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; May, A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008, 192, 137. [Google Scholar] [CrossRef]

| Variable | MCI-TMS (n = 11) | MCI-C (n = 16) | HC (n = 13) | a H-Test; b U-test; c χ2 Test | p-Value | Adj-p | MCI-TMS vs. MCI-C | MCI-TMS vs. HC | MCI-C vs. HC |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Age, years | 64.00 (60.00, 74.00) | 70.50 (62.50, 77.25) | 68.00 (60.50, 74.50) | a 1.68 | 0.431 | 0.895 | - | - | - |
| Education, years | 13.00 (10.00, 13.00) | 11.00 (8.00, 13.00) | 13.00 (13.00, 18.00) | a 6.06 | 0.048 | 0.544 | - | - | - |
| Sex, male | 6 (46.20%) | 8 (50.00%) | 5 (45.50%) | c 0.06 | 0.967 | 1.000 | - | - | - |
| Neuropsychiatric symptoms | |||||||||
| Neuropsychiatric Inventory dimensions | |||||||||
| Delusions | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | * | b 71.50 | 0.375 | 0.895 | - | - | - |
| Hallucination | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | * | b 77.00 | 1.000 | 1.000 | - | - | - |
| Agitation/aggression | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | * | b 63.00 | 0.103 | 0.544 | - | - | - |
| Dysphoria | 6.00 (4.00, 12.00) | 9.00 (0.00, 9.75) | * | b 75.50 | 0.933 | 1.000 | - | - | - |
| Anxiety | 6.00 (0.00, 12.00) | 9.00 (4.00, 12.00) | * | b 61.00 | 0.370 | 0.895 | - | - | - |
| Euphoria | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | * | b 71.50 | 0.375 | 0.895 | - | - | - |
| Apathy | 4.00 (0.00, 9.00) | 0.00 (0.00, 9.00) | * | b 68.00 | 0.593 | 0.988 | - | - | - |
| Disinhibition | 0.00 (0.00, 0.00) | 0.00 (0.00, 1.50) | * | b 60.50 | 0.109 | 0.544 | - | - | - |
| Irritability | 4.00 (0.00, 9.00) | 4.00 (0.00, 6.75) | * | b 64.50 | 0.478 | 0.895 | - | - | - |
| Aberrant motor activity | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | * | b 77.00 | 1.000 | 1.000 | - | - | - |
| Night-time behavioural disturbances | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | * | b 77.00 | 1.000 | 1.000 | - | - | - |
| Appetite and eating abnormalities | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | * | b 77.00 | 1.000 | 1.000 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, S.; Trojsi, F.; Cirillo, G.; de Stefano, M.; Di Nardo, F.; Siciliano, M.; Caiazzo, G.; Ippolito, D.; Ricciardi, D.; Buonanno, D.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines 2022, 10, 994. https://doi.org/10.3390/biomedicines10050994
Esposito S, Trojsi F, Cirillo G, de Stefano M, Di Nardo F, Siciliano M, Caiazzo G, Ippolito D, Ricciardi D, Buonanno D, et al. Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines. 2022; 10(5):994. https://doi.org/10.3390/biomedicines10050994
Chicago/Turabian StyleEsposito, Sabrina, Francesca Trojsi, Giovanni Cirillo, Manuela de Stefano, Federica Di Nardo, Mattia Siciliano, Giuseppina Caiazzo, Domenico Ippolito, Dario Ricciardi, Daniela Buonanno, and et al. 2022. "Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI)" Biomedicines 10, no. 5: 994. https://doi.org/10.3390/biomedicines10050994
APA StyleEsposito, S., Trojsi, F., Cirillo, G., de Stefano, M., Di Nardo, F., Siciliano, M., Caiazzo, G., Ippolito, D., Ricciardi, D., Buonanno, D., Atripaldi, D., Pepe, R., D’Alvano, G., Mangione, A., Bonavita, S., Santangelo, G., Iavarone, A., Cirillo, M., Esposito, F., ... Tedeschi, G. (2022). Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines, 10(5), 994. https://doi.org/10.3390/biomedicines10050994








