Abstract
Background: Atypical antipsychotics increase the risk of atrial arrhythmias and sudden cardiac death. This study investigated whether ziprasidone, a second-generation antipsychotic, affected intracellular Ca2+ and Na+ regulation and oxidative stress, providing proarrhythmogenic substrates in atriums. Methods: Electromechanical analyses of rabbit atrial tissues were conducted. Intracellular Ca2+ monitoring using Fluo-3, the patch-clamp method for ionic current recordings, and a fluorescence study for the detection of reactive oxygen species and intracellular Na+ levels were conducted in enzymatically dissociated atrial myocytes. Results: Ziprasidone-treated atriums showed sustained triggered activities after rapid pacing, which were inhibited by KN-93 and ranolazine. A reduced peak L-type Ca2+ channel current and enhanced late Na+ current were observed in ziprasidone-treated atrial myocytes, together with an increased cytosolic Na+ level. KN-93 suppressed the enhanced late Na+ current in ziprasidone-treated atrial myocytes. Atrial myocytes treated with ziprasidone showed reduced Ca2+ transient amplitudes and sarcoplasmic reticulum (SR) Ca2+ stores, and increased SR Ca2+ leakage. Cytosolic and mitochondrial reactive oxygen species production was increased in atrial myocytes treated with ziprasidone. TNF-α and NLRP3 were upregulated in ziprasidone-treated myocytes, and the level of phosphorylated calcium/calmodulin-dependent protein kinase II protein was increased. Conclusions: Our results suggest that ziprasidone increases the occurrence of atrial triggered activity and causes intracellular Ca2+ and Na+ dysregulation, which may result from enhanced oxidative stress and activation of the TNF-α/NLRP3 inflammasome pathway in ziprasidone-treated myocytes.
1. Introduction
Ziprasidone is a second-generation antipsychotic (SGA) used to treat schizophrenia and bipolar disorder [1]. In clinical practice, patients taking antipsychotics have an increased risk of cardiomyopathy, myocarditis, arrhythmias, and even life-threatening cardiac events, with a 1.53-fold increased risk of sudden cardiac death (SCD) [2,3,4]. A multicenter drug-surveillance program for cardiovascular adverse reactions (CARs) during antipsychotic treatment revealed that CARs were the highest during treatment with ziprasidone [5]. Ziprasidone has also been shown to induce a high risk for cardiac arrhythmias [6]. Patients with administered ziprasidone had increased heart rates according to electrocardiogram analysis in an observational study [7]. Among antipsychotic-associated arrhythmias, sinus tachycardia is the most common type, followed by atrial fibrillation (AF) and ventricular extrasystoles [5].
While antipsychotics’ involvement in atrial arrhythmias has not been well investigated, a national database analysis demonstrated that exposure to antipsychotics, especially SGAs, increased the occurrence of AF [8]. While antipsychotic-treatment-associated QT prolongation, which produces an increased rate of SCD, is one of the major concerns in psychiatric patients, AF has been shown to facilitate the induction of ventricular arrhythmias, which, in turn, increases the risk of SCD [9,10]. However, the mechanism for ziprasidone-induced atrial arrhythmias, especially in terms of cardiomyocyte ionic regulation and Ca2+ cycling, remains poorly understood, and further investigation is needed [5].
The objective of the present study was to evaluate the atrial electrophysiological characteristics, cardiomyocyte Ca2+ and Na+ homeostasis, and levels of oxidative stress/inflammasomes under ziprasidone treatment. We hypothesized that ziprasidone caused intracellular Ca2+ and Na+ dysregulation and oxidative stress, leading to atrial arrhythmogenesis. Our results suggest that ziprasidone impaired homeostasis of intracellular Ca2+ and Na+ homeostasis, enhanced reactive oxygen species (ROS) production, and activated the tumor necrosis factor alpha/NOD-, LRR-, and pyrin domain-containing protein 3 (TNF-α/NLRP3) inflammasome signaling pathway in atrial myocytes, which provides proarrhythmogenic substrates.
2. Methods
2.1. Preparation of Atrial Tissues for Electromechanical and Pharmacological Tests
All the animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the National Defense Medical Center, Taipei, Taiwan (IACUC-22-061), and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals guidelines of the National Institute of Health.
Male New Zealand white rabbits (weight, 2–3 kg; age, 6–8 months) were used in the present study. The rabbits were kept in stainless steel cages in a controlled environment (20–22 °C; 50–70% humidity) under a 12:12 h light–dark cycle with ad libitum access to standard food and deionized drinking water.
The rabbits were anaesthetized using an intramuscular injection of a mixture of zoletil 50 (10 mg/kg) and xylazine (5 mg/kg), with an overdose of inhaled isoflurane (5% oxygen) in a vaporizer, after which they were killed. The anesthetic dose was confirmed as adequate after the rabbits did not exhibit corneal reflexes and motor responses to pain stimuli induced with a scalpel tip. After heparin (1000 units/kg) was administered intravenously, the hearts were harvested through midline thoracotomy, as described previously [11]. For the LA preparation, the LA was opened by an incision along the mitral valve annulus and extending from the coronary sinus to the septum in normal Tyrode’s solution composed (in mM) of 137 NaCl, 4 KCl, 15 NaHCO3, 0.5 NaH2PO4, 0.5 MgCl2, 2.7 CaCl2, and 11 dextrose gases, with a mixture of 95% O2/5% CO2. The dissected LA tissue was pinned with needles onto the bottom of a tissue bath. The other end of the LA tissue was connected to a Grass FT03C force transducer (Grass Instrument Co., Quincy, MA, USA) with silk thread. The epicardial side of the LA preparations faced upward. The preparations were superfused with normal Tyrode’s solution at a constant rate (3 mL/min) and saturated with a 95% O2/5% CO2 gas mixture. The bath temperature was maintained at 37 °C. Before the electrophysiological assessments, the preparations were allowed to equilibrate for 1 h in the bath.
The transmembrane action potentials were recorded using glass microelectrodes (filled with 3M KCL) connected to a WPI Duo 773 electrometer (World Precision Instruments, Sarasota, FL, USA), as described previously [12]. The signals were digitally recorded using a data-acquisition system (cut-off frequency of 10 kHz), through a low-pass filter (16-bit accuracy), at a rate of 125 kHz. A pulse stimulation of 1 ms duration was produced using a Grass S48 stimulator (Grass Instruments, Norfolk, MA, USA) through a Grass SIU5B stimulus unit (Grass Instruments). The action-potential durations (APDs) in the left atrial preparations were recorded under 2 Hz-pulse stimulation. The action-potential amplitude (APA) was calculated through the difference between the peak potential of depolarization and the resting membrane potential (RMP). Repolarization extents of 20%, 50%, and 90% of the APA were termed APD20, APD50, and APD90, respectively. Atrial preparations were acutely perfused with ziprasidone (100 μg/mL) at a constant rate to assess the treatment responses. The rapid atrial pacing (RAP) protocol (a 20 Hz pacing rate for 1 s) was performed with or without ziprasidone to evaluate the triggered electric activity evoked by ziprasidone treatment. Triggered activity was defined as the occurrence of spontaneous APs in the absence of electrical stimuli. Burst firing was defined as the occurrence of accelerated spontaneous APs. The occurrence rate, sustained frequency, and duration of the triggered activity after RAP were recorded.
2.2. Cardiomyocyte Isolation
Atrial myocytes were enzymatically dissociated, as previously described, with some modifications [13]. Briefly, the rabbits were anesthetized by using an intramuscular injection of a mixture of zoletil 50 (10 mg/kg) and xylazine (5 mg/kg), with an overdose of inhaled isoflurane (5% oxygen). The hearts were procured and cannulated to a Langendorff perfusion apparatus through the aorta at 37 °C. The hearts were initially perfused using NT solution (137 mmol/L NaCl, 1.8 mmol/L CaCl2, 0.5 mmol/L MgCl2, 5.4 mmol/L KCl, 10 mmol/L glucose, and 10 mmol/L 4-(2 Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), pH adjusted to 7.4 with NaOH) for 10 min, and were then digested using a Ca2+-free solution (120 mM NaCl, 5.4 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 6 mM HEPES, 10 mM glucose, and 10 mM taurine (pH adjusted to 7.4 using NaOH)) containing 300 units/mL of collagenase (Type I; Sigma-Aldrich, St. Louis, MO, USA) and 0.25 units/mL of proteinase (type XIV; Sigma-Aldrich) for 8 to 12 min. After perfusion, the hearts were removed from the cannulas, and the left atriums were excised. The left atriums were cut into small pieces, gently triturated using a plastic transfer pipette in 50 mL of Ca2+-free solution and filtered through a nylon mesh until single cardiomyocytes were obtained. The solution for the dissociated cells was then gradually changed to NT solution. The isolated cells were stored in NT solution at 20–22 °C and were allowed to stabilize in the solution for at least 30 min before the experiments. Rod-shaped myocytes with clear striation and without granulation were used within 6–8 h. To test the effects of ziprasidone, the cells were incubated with ziprasidone (100 μg/mL) for 30 min before intracellular Ca2+ monitoring, electrophysiological measurements, assessing intracellular Na+ levels, and detecting reactive oxygen species (ROS).
2.3. Electrophysiological Measurement
ICa,L, and INa,L
The ionic currents of the atrial myocytes were measured using whole-cell configuration patch-clamp techniques with an Axopatch 1D amplifier (Axon Instruments, Foster City, CA, USA), as described previously [14]. A small hyperpolarizing voltage step from a holding potential of –50 mV to a potential of –55 mV for 80 ms was given at the beginning of each experiment. The cell capacitance was calculated as the area under the capacitive current divided by the applied voltage step. The series resistance was electronically compensated at approximately 60–80%. The filling solution of microelectrodes for ICa,L was composed (in mM) of 130 CsCl, 1 MgCl2, 5 MgATP, 10 HEPES, 0.1 NaGTP, and 5 Na2-phosphocreatine (pH adjusted to 7.2 with CsOH). The filling solution of microelectrodes for INa,L was composed (in mM) of 130 CsCl, 4 Na2ATP, 1 MgCl2, 10 EGTA, and 5 HEPES (pH adjusted to 7.3 with NaOH). The ICa,L was determined as inward currents during voltage-clamp steps from a holding potential of –50 mV to potentials from –40 to +60 mV in steps of 10 mV for 300 ms at a frequency of 0.1 Hz using a perforated patch clamp with amphotericin B as described previously [15]. The ICa,L was assessed between 5 and 15 min after formation of perforated whole-cell patch in each cardiomyocyte, to avoid ‘run-down’ effects [15,16]. To measure the INa,L, a step/ramp protocol (starting with a potential of –100 mV, then stepping to +20 mV for 100 ms, and afterwards ramping back to –100 mV for 100 ms) was used. The INa,L was determined as the tetrodotoxin (30 µM TTX)-sensitive current, obtained when the voltage was ramped back to –100 mV as described previously [15].
2.4. Intracellular Ca2+ Monitoring
Atrial cardiomyocytes were incubated with Ca2+ indicator (10 μM Fluo-3 AM) at room temperature for 30 min, and fluorescent imaging was performed as previously described [17,18]. Briefly, fluorescence microscopy was conducted using an inverted laser-scanning confocal microscope (Zeiss LSM 510; Carl Zeiss, Jena, Germany). The fluorescent signals were corrected for variations in dye concentrations by normalizing the fluorescent signal (represented by F) against the baseline fluorescence (F0) to obtain reliable data about transient intracellular Ca2+ changes, denoted as (F − F0)/F0, and to exclude variations in the fluorescence intensity caused by different volumes of injected dye. Ca2+ transients were elicited using field stimulation at 2 Hz. SR Ca2+ stores were measured by fast application of 20 mM caffeine following a pulse stimulation train at 2 Hz for 30 s. The SR Ca2+ stores were determined from the peak amplitudes of the caffeine-elicited Ca2+ transient. The SR Ca2+ leak was measured as the tetracaine (1 mM)-induced reduction in intracellular Ca2+, as previously described [19]. Briefly, after steady-state Ca2+ transients with repeated pulses (2 Hz for 15 s), the superfusate was rapidly changed to a 0 Na+/0 Ca2+ solution, composed of (in mM) 140 LiCl, 0.5 MgCl2, 5.4 KCl, 10 glucose, 10 EGTA, and 10 HEPES (pH adjusted to 7.4, with LiOH)) containing 1 mM tetracaine, which was applied for a minimum of 20 s to produce reduction in intracellular Ca2+
2.5. Measurement of ROS Production and Cytosolic Na+ Levels
Atrial myocytes were incubated with 10 μM CellROX green, 2 μM MitoSOX Red (Life Technologies, Carlsbad city, CA, USA), and 5 µM Asante NaTRIUM Green-2 AM (Teflabs, Austin, TX, USA) in NT solution to assess the cytosolic and mitochondrial ROS production and the cytosolic Na+ level, respectively. The measurements were carried out using an inverted laser-scanning confocal microscope (Zeiss LSM 510, Carl Zeiss, Oberkochen, Germany) as previously described [20]. Light with a wavelength of 488 nm for excitation was used, and the emission fluorescence was detected at wavelengths over 505 nm in the XY mode of the confocal microscope setup. During the experiment, atrial myocytes were paced at 2 Hz. The fluorescent signals were analyzed using ImageJ, as described previously [21].
2.6. Western Blot Analysis
Western blotting was carried out to assess the levels of cytokines, inflammasome markers, and Ca2+ regulatory proteins, including NF-κB (p65), TNF-α, NLRP3, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), and calcium/calmodulin-dependent protein kinase II (CaMKII). Whole-cell lysates were prepared from left atrial cardiomyocytes. The cell lysis buffer was composed of 100 mM Tris-HCl (pH 8.0), 0.1% sodium dodecyl sulfate, 1% Triton X-100, 150 mM NaCl, and a protease inhibitor cocktail (Roche, Basel, Switzerland). The myocytes protein extracts were separated by conventional gel electrophoresis, and were then transferred to polyvinylidene difluoride membranes (Merck Millipore, Burlington, MA, USA). After blocking with 5% non-fat milk using TBST (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween 20) at room temperature for 1 h, the membranes were incubated with the following primary antibodies: NF-κB (sc-372; Santa Cruz Biotechnology, Dallas, TX, USA), TNF-α (17590-1-AP; Proteintech, Manchester, UK), NLRP3 (ab-214185; Abcam, Cambridge, UK), SERCA2a (sc-376235; Santa Cruz Biotechnology, Dallas, TX, USA), p-SERCA2a (A010-25AP; Badrilla Ltd., Leeds, UK), p-CaMKII (ab32678; Abcam, Cambridge, UK), and GAPDH (60004-1-ig; Proteintech, Manchester, UK). Subsequently, the membranes were incubated with anti-mouse (AP160P; Millipore, MI, USA) or anti-rabbit (AP132P; Millipore, MI, USA) secondary IgG antibodies. Immunoreactive proteins were detected using enhanced chemiluminescence (GE Healthcare, Chicago, IL, USA) and analyzed using the ImageJ software 1.5 (NIH, Bethesda, MD, USA).
2.7. Data Analysis
Student’s t-tests or Pearson’s chi-square tests using SigmaPlot version 12 (Systat Software, San Jose, CA, USA) were used to exam the differences between treatments. The ‘n’ represents the numbers of total cells from the total hearts (n = cells/hearts), and the ‘N’ is the animal number. Statistical significance is denoted as *, **, and *** for p < 0.05, p < 0.01, and p < 0.005, respectively.
3. Results
3.1. Atrial-Tissue Electrical Activity
To investigate whether ziprasidone treatment induced atrial arrhythmia, we first assessed the effect of ziprasidone on the configuration of the action potentials in atriums. Ziprasidone did not affect the RMP, APA, APD20, APD50, or APD90 in the atrial tissues (Figure 1A,B). KN-93 or ranolazine did not show an effect on RMP, APA, APD20, APD50, or APD90 in the ziprasidone-treated atrial tissues (Figure 1A,B). However, ziprasidone-treated atrial tissues showed post-RAP triggered activity, with a 100% occurrence rate, compared to 0% in pre-ziprasidone treated atrial tissues (Figure 2A,B). KN-93, a CaMKII inhibitor, and ranolazine, a late sodium-current inhibitor, inhibited the occurrence of post-RAP triggered activity in ziprasidone-treated atrial tissues (0% occurrence rate) (Figure 2A,B).
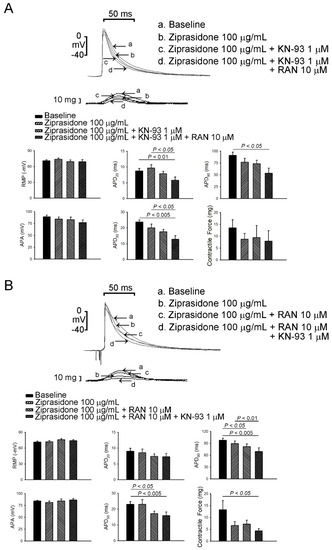
Figure 1.
Effects of ziprasidone on action potentials of rabbit atriums. (A): (Upper) Representative action potentials and contractile forces from atrial tissues treated with ziprasidone, combined with KN-93 or KN-93 plus ranolazine, and (below) the mean data (N = 7) during electrical stimulation at a rate of 2 Hz. (B): (Upper) Representative action potentials and contractile forces from atrial tissues treated with ziprasidone, combined with ranolazine or ranolazine plus KN-93, and (below) the mean data (N = 7) during electrical stimulation at a rate of 2 Hz.
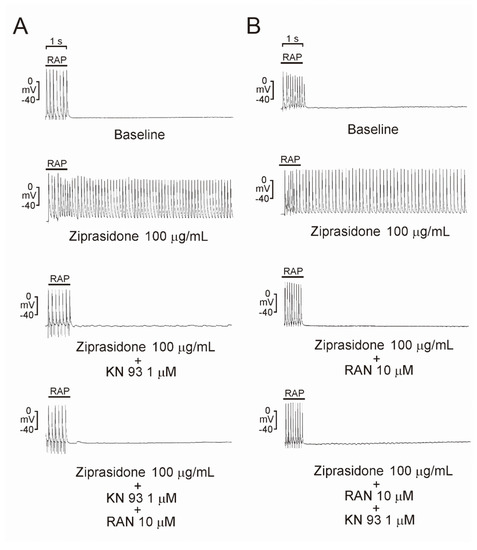
Figure 2.
Triggered activity in atrial tissues with ziprasidone. Representative triggered activities in atrial tissues treated with ziprasidone. (A): Atrial tissues showed triggered activity with ziprasidone (100% occurrence rate, 7.5 ± 0.4 Hz sustained frequency and 19.8 ± 7.9 s sustained duration, n = 7, compared with 0% in atrial tissues without ziprasidone, n = 7). KN-93 and KN-93 with ranolazine inhibited the occurrence of triggered activity (0%, n = 7). (B): Atrial tissues showed triggered activity with ziprasidone (100% occurrence rate, 7.1 ± 0.5 Hz sustained frequency and 22.4 ± 10.4 s sustained duration, n = 7 compared with 0% in atrial tissues without ziprasidone, n = 7). Ranolazine and ranolazine with KN-93 inhibited the occurrence of triggered activity (0%, n = 7).
3.2. ICa,L, INa,L, and Cytosolic Na+ Levels
We then tested if ziprasidone affected the myocyte Ca2+ influx and late Na+ current. The current density of ICa,L in the atrial myocytes was smaller after ziprasidone treatment (Figure 3A). The INa,L (tetrodotoxin-sensitive current) in the atrial myocytes was enhanced after ziprasidone treatment (0.68 ± 0.11 pA/pF to 0.79 ± 0.13 pA/pF; ** p < 0.01; the integral of INa,L: 5.97 ± 1.37 A*ms/F to 9.29 ± 1.45 A*ms/F; *** p < 0.005; Figure 3B). KN-93 reduced the ehnanced INa,L in ziprasidone-treated myocytes (*** p < 0.005; Figure 3C). In addition, the intracellular Na+ concentration ([Na+]i) in the atrial cardiomyocytes was higher after ziprasidone treatment (153 ± 6 ΔF/F0 and 172 ± 4 ΔF/F0, respectively; * p < 0.05; Figure 5A).
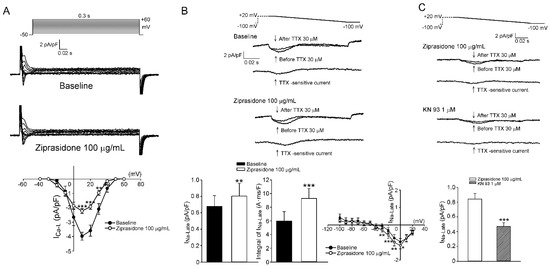
Figure 3.
ICa,L and INa,L. (A): (top and middle section) Representative traces of ICa,L in atrial myocytes with or without ziprasidone and (bottom section) the I–V relationship (n = 9/3; ** p < 0.01; *** p < 0.005). (B): (top and middle section) Representative traces of INa,L (TTX-sensitive current) in atrial myocytes with or without ziprasidone, and (bottom section) the mean data of INa,L and the integral of INa,L and the IV relationship (n = 9/3; * p < 0.05; ** p < 0.01). (C): (top and middle section) Representative traces of INa,L in ziprasidone-treated atrial myocytes with or without KN-93, and (bottom section) the mean data (n = 13/3; *** p < 0.005).
3.3. Ca2+ Transient Amplitudes, SR Ca2+ Stores, and SR Ca2+ Leak
Next, we examined if the intracellular Ca2+ regulation machinery was attenuated by ziprasidone. The steady-state Ca2+ transient amplitudes in atrial myocytes were smaller after treatment with ziprasidone (* p < 0.05, Figure 4A). Ziprasidone-treated myocytes showed decreased SR Ca2+ stores, which were assessed based on the Ca2+ transient elicited by rapid caffeine application (* p < 0.05, Figure 4B) and increased SR Ca2+ leakage (* p < 0.05, Figure 4C).
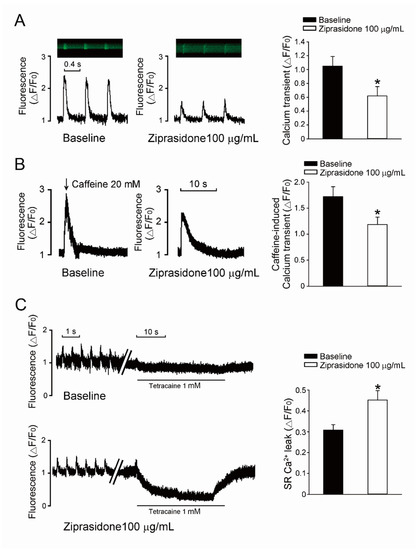
Figure 4.
SR Ca2+ leak. (A): Typical traces of steady-state Ca2+ transient in atrial myocytes treated with or without ziprasidone, and mean data (baseline group n = 22/3, ziprasidone group n = 30/3; * p < 0.05). (B): A: Typical traces of caffeine-provoked Ca2+ transient in atrial myocytes treated with or without ziprasidone, and mean data (baseline group n = 22/3, ziprasidone group n = 16/3; * p < 0.05). (C): Typical recording of SR Ca2+ leakage determined by fast tetracaine application in atrial myocytes treated with or without ziprasidone, and mean data (baseline group n = 13/3, ziprasidone group n = 12/3; * p < 0.05).
3.4. Oxidative Stress
The cytosolic ROS levels in atrial cardiomyocytes were increased by 25% after ziprasidone treatment (108 ± 8 ΔF/F0 and 135 ± 6 ΔF/F0, respectively; * p < 0.05) (Figure 5B). The mitochondrial ROS levels in atrial cardiomyocytes were enhanced by 87% after ziprasidone treatment (80 ± 6 ΔF/F0 and 150 ± 6 Δ F/F0, respectively; * p < 0.05) (Figure 5C).
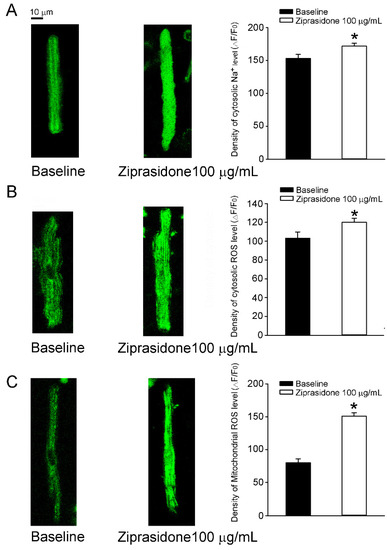
Figure 5.
Cytosolic Na+ level and oxidative stress in atrial myocytes treated with or without ziprasidone. (A): Fluorescent images of cytosolic Na+ levels in atrial myocytes treated with or without ziprasidone and mean data (baseline group n = 26/3, ziprasidone group n = 37/3; * p < 0.05). (B): Fluorescent images of cytosolic levels of reactive oxygen species (ROS) in atrial myocytes treated with or without ziprasidone and mean data (baseline group n = 11/3, ziprasidone group n = 29/3; * p < 0.05). (C). Fluorescent images of mitochondrial ROS levels in atrial myocytes treated with or without ziprasidone and mean data (baseline group n = 26/3, ziprasidone group n = 32/3; * p < 0.05).
3.5. Levels of Proinflammatory Cytokines, Inflammasome Markers, and Ca2+ Regulatory Proteins
While the protein levels of NF-κB (p65) did not change in ziprasidone-treated myocytes, the expression of TNF-α and NLRP3 was upregulated (Figure 6A). Ziprasidone-treated myocytes showed no changes in the protein level of SERCA2a, but an increase in the protein level of phosphorylated CaMKII was observed (Figure 6B).
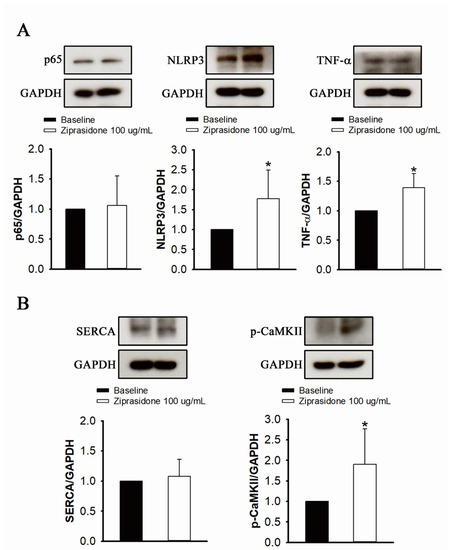
Figure 6.
Proinflammatory cytokine and inflammasome markers, and Ca2+ regulatory proteins, in control and ziprasidone-treated atrial myocytes. (A): Representative immunoblot (upper section) and normalized densitometric p65, NLRP3 and TNF-α protein levels in control and ziprasidone-treated atrial myocytes. (B): Representative immunoblot (upper section) and normalized densitometric SERCA2a and phosphorylated CaMKII protein levels in control and ziprasi-done-treated atrial myocytes. GAPDH was used as an internal control (control group n = 6 and ziprasidone group n = 6; * p < 0.05).
4. Discussion
In this study, we used rabbit atrial tissues and isolated cardiomyocytes to assess whether ziprasidone promoted atrial arrhythmias and altered myocyte Ca2+ and Na+ homeostasis. Our data demonstrate that ziprasidone provoked post-RAP atrial triggered activities, which were suppressed by KN-93 and ranolazine. Ziprasidone reduced the ICa,L and enhanced the INa,L, as well as increased the intracellular Na+ level. Ziprasidone decreased the Ca2+ transient and SR Ca2+ stores, increased SR Ca2+ leakage, and enhanced both cytosolic and mitochondrial ROS production in atrial cardiomyocytes. TNF-α/NLRP3 inflammasome signaling was upregulated in ziprasidone-treated myocytes. These results suggest that ziprasidone impairs the regulation of Ca2+, enhances oxidative stress, and activates inflammatory signaling, which leads to the occurrence of arrhythmic events in atriums. These findings help to elucidate the mechanisms underlying atrial arrhythmias associated with ziprasidone treatment (Figure 7).
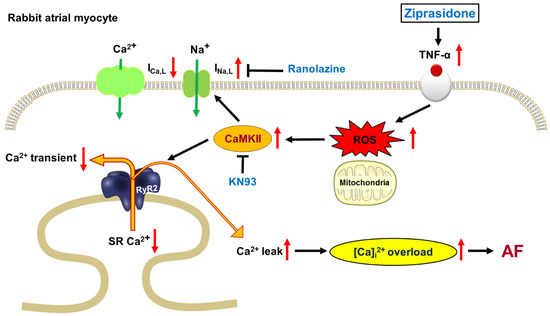
Figure 7.
Proposed mechanism for ziprasidone-induced AF. TNF-α were upregulated in ziprasidone-treated myocytes, which induced cytosolic and mitochondrial ROS production. The increased ROS production activated CaMKII, leading to enhanced INa,L and increased SR Ca2+ leak. As a result, intracellular Ca2+ was overloaded, promoting AF.
Ziprasidone-treated atrial myocytes had smaller Ca2+ transient amplitudes and lower SR Ca2+ stores. We suggest that the smaller SR Ca2+ stores may be partly attributed to the lower Ca2+-loading effect resulting from the smaller ICa,L, leading to a smaller Ca2+ transient and lower SR Ca2+ stores [22]. Depleted SR Ca2+ stores may also result from an increased probability of ryanodine receptor 2 (RyR2) opening, leading to increased SR Ca2+ leakage [23,24]. The CaMKII-mediated hyperphosphorylation of RyR2 enhances diastolic SR Ca2+ leakage, which depletes SR Ca2+ and increases the cytosolic Ca2+ concentration [25,26], which helps to explain the enhanced phosphorylated CaMKII and increased SR Ca2+ leakage in the ziprasidone-treated myocytes. Furthermore, CaMKII-mediated SR Ca2+ leakage has been shown to promote AF in a mouse model [27]. The overload of cytosolic Ca2+ in cardiomyocytes may trigger ectopic electrical activity, such as EADs/DADs, and, consequently, life-threatening arrhythmias [28,29,30]. This creates arrhythmogenic substrates that led to a higher occurrence of triggered activities in the ziprasidone-treated atriums. CaMKII is capable of attenuating intracellular Na+ and Ca2+ regulation by phosphorylating the related proteins and channels [23,26,31,32,33,34]. The triggered arrhythmia was ameliorated by treatment with the CaMKII inhibitor KN-93 or INa,L inhibitor ranolazine, which further confirmed the arrhythmogenic role of the upregulated CaMKII and INa,L induced by ziprasidone.
Enhanced INa,L contributes substantially to cytosolic Na+ levels [35], which may drive NCX to operate in reverse mode to exchange more Ca2+ into the cytosol and, as a result, depolarize the membrane potential and increase the probability of ectopic electric activities [36,37]. Increased INa,L may result from the phosphorylation of the channel Nav1.5 by upregulated CaMKII [34,38,39,40]. Triggered activity in ziprasidone-treated atriums was inhibited by an INa,L inhibitor, ranolazine, which has been shown to reduce intracellular Na+-dependent Ca2+ overload and ameliorate arrhythmic events [41,42]. Furthermore, increased oxidative stress may also enhance late INa,L, thereby stimulating arrhythmogenesis [43], which may help to explain our findings of enhanced ROS production and increased INa,L in ziprasidone-treated atrial myocytes.
Oxidative stress has been shown to increase the risk of AF [44,45]. Our data showed that both cytosolic and mitochondrial ROS production increased in ziprasidone-treated myocytes, which may facilitate the occurrence of AF [46]. The SR Ca2+ leakage-related overaccumulation of cytosolic Ca2+ may result in a mitochondrial overload of Ca2+, which, in turn, would exacerbate mitochondrial ROS production. Increased ROS production has been shown to activate CaMKII, which enhances INa,L and impairs intracellular Ca2+ regulation, leading to arrhythmias [47,48]. While there have been reports of ziprasidone exerting an antioxidative-stress effect in neuroblast cells, protecting against neurotoxic-agent-induced apotosis [49,50], treatment with ziprasidone has been reported to induce pathophysiological alterations in the rat heart, which were suggested to be associated with impaired antioxidant capacity due to the ziprasidone [51]. This implies differential pharmacologic effects of ziprasidone on the neurologic and cardiovascular systems.
Our data show that ziprasidone-treated atrial myocytes presented higher levels of TNF-α and NLRP3. Upregulated NLRP3-inflammasome signaling has been shown to promote AF [52,53]. Ziprasidone has also been shown to increase proinflammatory cytokine levels [54]. Ziprasidone exposure induced higher levels of ROS and proinflammatory cytokines, including IL-1, IL-6, TNF-α, and INF-γ, in a macrophage cell line model [54]. Therefore, we suggest that ziprasidone may promote AF via its immunoendocrine effect, which is suggested to be mediated through the TNF-α/NLRP3 pathway.
Finally, some methodological weaknesses in the present study need to be acknowledged. While ranolazine has been well used as an inhibitor of late Na+ current, it also inhibits the delayed rectifier potassium current (Ikr) and adrenergic receptors in animal models [55]. However, it should be addressed that the concentration of ranolazine (10 μM) we used in the present study is below the half maximal inhibitory concentration (IC50) of ranolazine for Ikr (11.5 μM) [56]. The widely recognized CaMKII inhibitor KN-93 was shown to reversibly inhibits L-type Ca2+ channel in a CaMKII-independent manner [57]. Our data suggest KN-93 inhibits CaMKII, leading to reduction in INa,L and SR Ca2+ leak, which, as a result, producing antiarrhythmic effect in the ziprasidone-treated atriums. ICa,L were decreased in the ziprasidone-treated myocytes. Therefore, the inhibition effect of KN-93 on L-type Ca2+ channel may not be responsible for the primary anti-arrhythmic mechanism in the ziprasidone-treated atriums.
In conclusion, ziprasidone promoted post-rapid-pacing atrial triggered activities. Ziprasidone exposure affected Ca2+ and Na+ homeostasis in atrial myocytes, leading to a higher occurrence of atrial tachyarrhythmias. Oxidative stress and NLRP3 inflammasomes were enhanced in the ziprasidone-treated myocytes. KN-93 and ranolazine inhibited the atrial arrhythmic events. These findings suggest that ziprasidone causes intracellular Ca2+ and Na+ dysregulation, which may result from enhanced oxidative stress and an activated TNF-α/NLRP3 inflammasome pathway in ziprasidone-treated myocytes, providing proarrhythmic substrates in atriums.
Author Contributions
Conceptualization, B.-Y.T. and H.-Y.Y.; methodology, B.-Y.T. and M.-K.L.; software, C.-S.T.; validation, H.-Y.Y.; formal analysis, B.-Y.T. and M.-K.L.; investigation, B.-Y.T.; resources, C.-S.T.; data curation, M.-K.L. and H.-Y.Y.; writing—original draft preparation, H.-Y.Y.; writing—review and editing, H.-Y.Y.; visualization, B.-Y.T. and M.-K.L.; supervision, H.-Y.Y.; project administration, C.-Y.L.; funding acquisition, C.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Ministry of Science and Technology of Taiwan (MOST110-2314-B-016-050-MY3); Tri-Service General Hospital, Taiwan (TSGH-C05-111043 and TSGH-C05-111042); and Ministry of National Defense Medical Affairs Bureau (MND-MAB-D11129 and MND-MAB-110-018).
Institutional Review Board Statement
All the animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the National Defense Medical Center, Taipei, Taiwan (IACUC-22-061), and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals guidelines of the National Institutes of Health.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of the present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Daniel, D.G.; Zimbroff, D.L.; Potkin, S.G.; Reeves, K.R.; Harrigan, E.P.; Lakshminarayanan, M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: A 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1999, 20, 491–505. [Google Scholar] [CrossRef]
- Ray, W.A.; Meredith, S.; Thapa, P.B.; Meador, K.G.; Hall, K.; Murray, K.T. Antipsychotics and the risk of sudden cardiac death. Arch. Gen. Psychiatry 2001, 58, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, W.A.; Chung, C.P.; Murray, K.T.; Hall, K.; Stein, C.M. Atypical antipsychotic drugs and the risk of sudden cardiac death. N. Engl. J. Med. 2009, 360, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.S.; Tsai, Y.T.; Tsai, H.J. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: A nation-wide case-crossover study. J. Am. Heart Assoc. 2015, 4, e001568. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.E.; Winkler, D.; Konstantinidis, A.; Huf, W.; Engel, R.; Toto, S.; Grohmann, R.; Kasper, S. Cardiovascular Adverse Reactions During Antipsychotic Treatment: Results of AMSP, A Drug Surveillance Program between 1993 and 2013. Int. J. Neuropsychopharmacol. 2020, 23, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Beach, S.R.; Celano, C.M.; Sugrue, A.M.; Adams, C.; Ackerman, M.J.; Noseworthy, P.A.; Huffman, J.C. QT Prolongation, Torsades de Pointes, and Psychotropic Medications: A 5-Year Update. Psychosomatics 2018, 59, 105–122. [Google Scholar] [CrossRef]
- Loebel, A.; Miceli, J.; Chappell, P.; Siu, C. Electrocardiographic changes with ziprasidone. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.-H.; Lo, L.-W.; Liou, Y.-J.; Shu, J.-H.; Hsu, H.-C.; Liang, Y.; Huang, C.-C.; Huang, P.-H.; Lin, S.-J.; Chen, J.-W.; et al. Antipsychotic treatment is associated with risk of atrial fibrillation: A nationwide nested case-control study. Int. J. Cardiol. 2017, 227, 134–140. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O.D.; Abildstrøm, S.Z.; Ottesen, M.M.; Rask-Madsen, C.; Bagger, H.; Køber, L. Increased risk of sudden and non-sudden cardiovascular death in patients with atrial fibrillation/flutter following acute myocardial infarction. Eur. Heart J. 2005, 27, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.-S.; Lin, Y.-S.; Lin, Y.-K.; Chen, Y.-C.; Kao, Y.-H.; Hsu, C.-C.; Chen, S.-A.; Chen, Y.-J. Atrial arrhythmogenesis in a rabbit model of chronic obstructive pulmonary disease. Transl. Res. J. Lab. Clin. Med. 2020, 223, 25–39. [Google Scholar] [CrossRef]
- Lee, T.-I.; Chen, Y.-C.; Lin, Y.-K.; Chung, C.-C.; Lu, Y.-Y.; Kao, Y.-H.; Chen, Y.-J. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.K.; Chen, Y.C.; Huang, J.H.; Lin, Y.-J.; Huang, S.-S.; Chen, S.-A.; Chen, Y.-J. Leptin modulates electrophysiological characteristics and isoproterenol-induced arrhythmogenesis in atrial myocytes. J. Biomed. Sci. 2013, 20, 94. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Chen, S.A.; Chen, Y.C.; Yeh, H.I.; Chan, P.; Chang, M.S.; Lin, C.I. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: Implication in initiation of atrial fibrillation. Circulation 2001, 104, 2849–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-Y.; Chen, Y.-C.; Kao, Y.-H.; Hsieh, M.-H.; Lin, Y.-K.; Chen, S.-A.; Chen, Y.-J. Redox and Activation of Protein Kinase a Dysregulates Calcium Homeostasis in Pulmonary Vein Cardiomyocytes of Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e005701. [Google Scholar] [CrossRef]
- Linley, J.E. Perforated whole-cell patch-clamp recording. Methods Mol. Biol. 2013, 998, 149–157. [Google Scholar] [PubMed]
- Chen, Y.-C.; Kao, Y.-H.; Huang, C.-F.; Cheng, C.-C.; Chen, Y.-J.; Chen, S.-A. Heat stress responses modulate calcium regulations and electrophysiological characteristics in atrial myocytes. J. Mol. Cell. Cardiol. 2010, 48, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Wongcharoen, W.; Chen, Y.-C.; Chen, Y.-J.; Chang, C.-M.; Yeh, H.-I.; Lin, C.-I.; Chen, S.-A. Effects of a Na+/Ca2+ exchanger inhibitor on pulmonary vein electrical activity and ouabain-induced arrhythmogenicity. Cardiovasc. Res. 2006, 70, 497–508. [Google Scholar] [CrossRef]
- Shannon, T.R.; Ginsburg, K.S.; Bers, D.M. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ. Res. 2002, 91, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Viatchenko-Karpinski, S.; Kornyeyev, D.; El-Bizri, N.; Budas, G.; Fan, P.; Jiang, Z.; Yang, J.; Anderson, M.E.; Shryock, J.C.; Chang, C.-P. Intracellular Na+ overload causes oxidation of CaMKII and leads to Ca2+ mishandling in isolated ventricular myocytes. J. Mol. Cell. Cardiol. 2014, 76, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-Y.; Chen, Y.-C.; Kao, Y.-H.; Hsieh, M.-H.; Lin, Y.-K.; Chung, C.-C.; Lee, T.-I.; Tsai, W.-C.; Chen, S.-A.; Chen, Y.-J. Fibroblast growth factor 23 dysregulates late sodium current and calcium homeostasis with enhanced arrhythmogenesis in pulmonary vein cardiomyocytes. Oncotarget 2016, 7, 69231–69242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.H.; Herting, J.; Tirilomis, T.; Renner, A.; Neef, S.; Toischer, K.; Ellenberger, D.; Förster, A.; Schmitto, J.D.; Gummer, J.; et al. Ca/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation 2013, 128, 970–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Chiang, D.Y.; Wang, S.; Wang, Q.; Sun, L.; Voigt, N.; Respress, J.L.; Ather, S.; Skapura, D.G.; Jordan, V.K.; et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 2014, 129, 1276–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, L.S.; Zhang, T.; Chen, L.; DeSantiago, J.; Brown, J.H.; Bers, D.M. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ. Res. 2003, 92, 904–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, X.; Curran, J.W.; Shannon, T.R.; Bers, D.M.; Pogwizd, S.M. Ca/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005, 97, 1314–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelu, M.G.; Sarma, S.; Sood, S.; Wang, S.; van Oort, R.J.; Skapura, D.G.; Li, N.; Santonastasi, M.; Müller, F.U.; Schmitz, W.; et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J. Clin. Investig. 2009, 119, 1940–1951. [Google Scholar] [CrossRef] [Green Version]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Shannon, T.R.; Pogwizd, S.M.; Bers, D.M. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ. Res. 2003, 93, 592–594. [Google Scholar] [CrossRef] [Green Version]
- Bers, D.M.; Eisner, D.A.; Valdivia, H.H. Sarcoplasmic reticulum Ca2+ and heart failure: Roles of diastolic leak and Ca2+ transport. Circ. Res. 2003, 93, 487–490. [Google Scholar] [CrossRef] [Green Version]
- Maier, L.S.; Bers, D.M. Role of Ca/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc. Res. 2007, 73, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sag, C.M.; Wadsack, D.P.; Khabbazzadeh, S.; Abesser, M.; Grefe, C.; Neumann, K.; Opiela, M.-K.; Backs, J.; Olson, E.N.; Brown, J.H. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ. Heart Fail. 2009, 2, 664–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehrens, X.H.; Lehnart, S.E.; Reiken, S.R.; Marks, A.R. Ca/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 2004, 94, e61–e70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer-Short, A.; Musa, H.; Alsina, K.M.; Ni, L.; Word, T.A.; Reynolds, J.O.; Gratz, D.; Lane, C.; El-Refaey, M.; Unudurthi, S. Calmodulin kinase II regulates atrial myocyte late sodium current, calcium handling, and atrial arrhythmia. Heart Rhythm 2020, 17, 503–511. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Sabbah, H.N.; Higgins, R.S.; Silverman, N.; Lesch, M.; Undrovinas, A.I. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 1998, 98, 2545–2552. [Google Scholar] [CrossRef]
- Armoundas, A.A.; Hobai, I.A.; Tomaselli, G.F.; Winslow, R.L.; O’Rourke, B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ. Res. 2003, 93, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Shryock, J.C.; Belardinelli, L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2031–H2039. [Google Scholar] [CrossRef] [Green Version]
- Maltsev, V.A.; Reznikov, V.; Undrovinas, N.A.; Sabbah, H.N.; Undrovinas, A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: Similarities and differences. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1597–H1608. [Google Scholar] [CrossRef] [Green Version]
- Sapia, L.; Palomeque, J.; Mattiazzi, A.; Petroff, M.V. Na/K ATPase inhibition by ouabain induces CaMKII-dependent apoptosis in adult rat cardiac myocytes. J. Mol. Cell. Cardiol. 2010, 49, 459–468. [Google Scholar] [CrossRef]
- Koval, O.M.; Snyder, J.S.; Wolf, R.M.; Pavlovicz, R.E.; Glynn, P.; Curran, J.; Leymaster, N.D.; Dun, W.; Wright, P.J.; Cardona, N.; et al. Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation 2012, 126, 2084–2094. [Google Scholar] [CrossRef] [Green Version]
- Belardinelli, L.; Shryock, J.C.; Fraser, H. Inhibition of the late sodium current as a potential cardioprotective principle: Effects of the late sodium current inhibitor ranolazine. Heart 2006, 92 (Suppl. S4), iv6–iv14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenfuss, G.; Maier, L.S. Mechanism of action of the new anti-ischemia drug ranolazine. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2008, 97, 222–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, K.; Davies, S.S.; Nakajima, T.; Ong, B.-H.; Kupershmidt, S.; Fessel, J.; Amarnath, V.; Anderson, M.E.; Boyden, P.A.; Viswanathan, P.C.; et al. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ. Res. 2005, 97, 1262–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korantzopoulos, P.; Letsas, K.; Fragakis, N.; Tse, G.; Liu, T. Oxidative stress and atrial fibrillation: An update. Free Radic. Res. 2018, 52, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Korantzopoulos, P.; Kolettis, T.M.; Galaris, D.; Goudevenos, J.A. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int. J. Cardiol. 2007, 115, 135–143. [Google Scholar] [CrossRef]
- Samman Tahhan, A.; Sandesara, P.B.; Hayek, S.S.; Alkhoder, A.; Chivukula, K.; Hammadah, M.; Mohamed-Kelli, H.; O’Neal, W.T.; Topel, M.; Ghasemzadeh, N.; et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm 2017, 14, 1849–1855. [Google Scholar] [CrossRef]
- Nishio, S.; Teshima, Y.; Takahashi, N.; Thuc, L.C.; Saito, S.; Fukui, A.; Kume, O.; Fukunaga, N.; Hara, M.; Nakagawa, M.; et al. Activation of CaMKII as a key regulator of reactive oxygen species production in diabetic rat heart. J. Mol. Cell. Cardiol. 2012, 52, 1103–1111. [Google Scholar] [CrossRef]
- Wagner, S.; Ruff, H.M.; Weber, S.L.; Bellmann, S.; Sowa, T.; Schulte, T.; Anderson, M.E.; Grandi, E.; Bers, D.M.; Backs, J.; et al. Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ. Res. 2011, 108, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Lee, C.H.; Lee, J.G.; Kim, L.W.; Shin, B.S.; Lee, B.J.; Kim, Y.H. Protective effects of atypical antipsychotic drugs against MPP(+)-induced oxidative stress in PC12 cells. Neurosci. Res. 2011, 69, 283–290. [Google Scholar] [CrossRef]
- Terada, K.; Murata, A.; Toki, E.; Goto, S.; Yamakawa, H.; Setoguchi, S.; Watase, D.; Koga, M.; Takata, J.; Matsunaga, K.; et al. Atypical Antipsychotic Drug Ziprasidone Protects against Rotenone-Induced Neurotoxicity: An In Vitro Study. Molecules 2020, 25, 4206. [Google Scholar] [CrossRef]
- Nikolić-Kokić, A.; Tatalović, N.; Nestorov, J.; Mijović, M.; Mijusković, A.; Miler, M.; Oreščanin-Dušić, Z.; Nikolić, M.; Milošević, V.; Blagojević, D.; et al. Clozapine, ziprasidone, and sertindole-induced morphological changes in the rat heart and their relationship to antioxidant enzymes function. J. Toxicol. Environ. Health Part A 2018, 81, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Brundel, B. Inflammasomes and Proteostasis Novel Molecular Mechanisms Associated with Atrial Fibrillation. Circ. Res. 2020, 127, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Veleva, T.; Scott, L., Jr.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Duarte, T.; Barbisan, F.; do Prado-Lima, P.A.S.; Azzolin, V.F.; Jung, I.E.d.C.; Duarte, M.M.M.F.; Teixeira, C.F.; Mastella, M.H.; da Cruz, I.B.M. Ziprasidone, a second-generation antipsychotic drug, triggers a macrophage inflammatory response in vitro. Cytokine 2018, 106, 101–107. [Google Scholar] [CrossRef]
- Rayner-Hartley, E.; Sedlak, T. Ranolazine: A Contemporary Review. J. Am. Heart Assoc. 2016, 5, e003196. [Google Scholar] [CrossRef] [Green Version]
- Antzelevitch, C.; Belardinelli, L.; Zygmunt, A.C.; Burashnikov, A.; di Diego, J.M.; Fish, J.M.; Cordeiro, J.M.; Thomas, G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 2004, 110, 904–910. [Google Scholar] [CrossRef]
- Gao, L.; Blair, L.A.; Marshall, J. CaMKII-independent effects of KN93 and its inactive analog KN92: Reversible inhibition of L-type calcium channels. Biochem. Biophys. Res. Commun. 2006, 345, 1606–1610. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).