A New Topical Candidate in Acne Treatment: Characterization of the Meclozine Hydrochloride as an Anti-Inflammatory Compound from In Vitro to a Preliminary Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
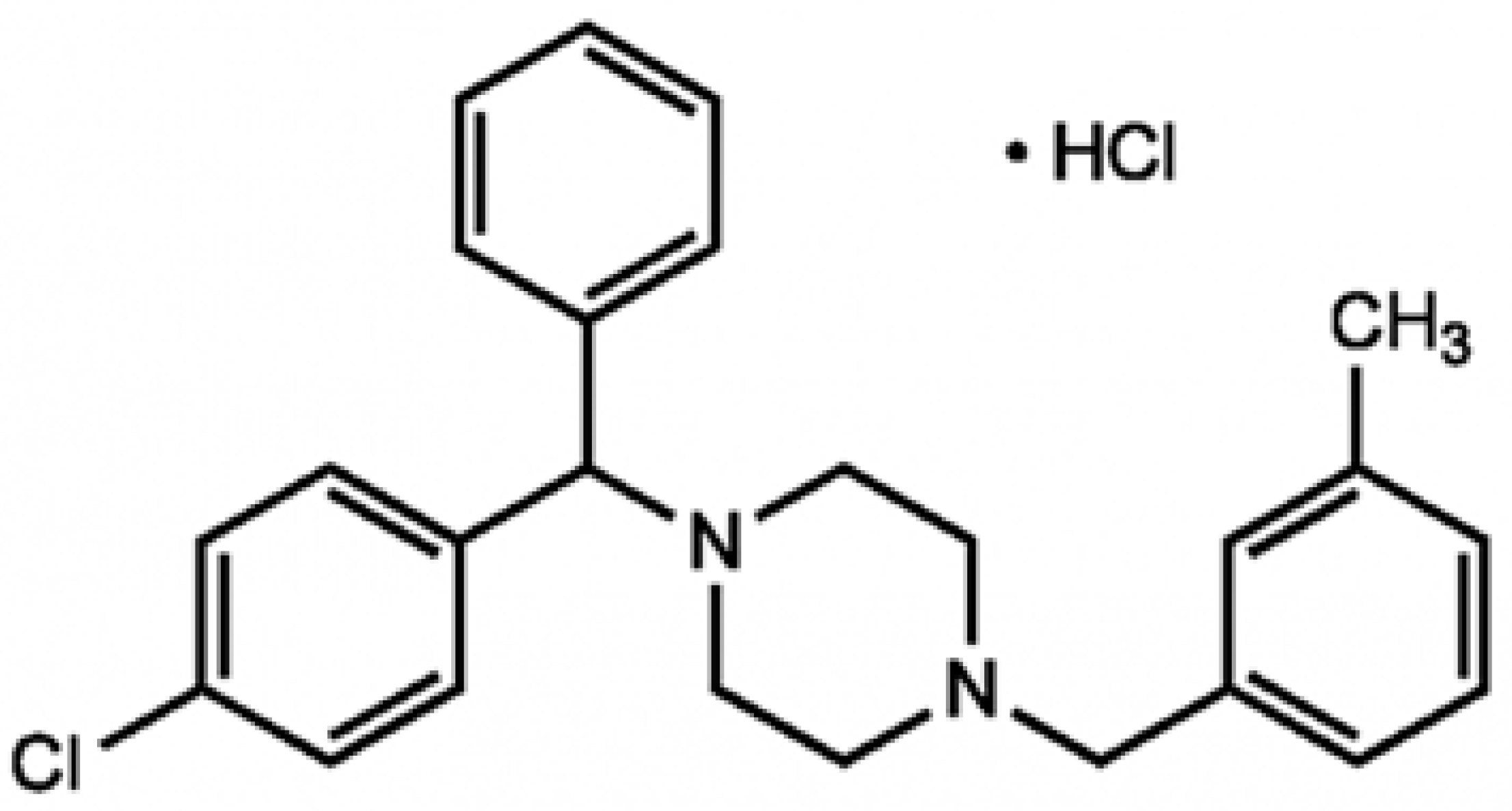
2.1. Chemicals
2.2. Cell Culture, Pre-Treatment and Stimulation
2.3. Bacterial Strain and Growth Conditions
2.4. Screening of 2145 FDA-Approved Compounds
2.5. Cell Viability Assays
2.6. ELISA
2.7. Luminex Assay
2.8. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.9. Immunoblotting Analysis
2.10. C. acnes-Induced Inflammation in the Mouse Ear Model In Vivo
2.11. C. acnes-Induced Inflammation Assay Ex Vivo
2.12. Clinical Trial
2.12.1. Study Design
2.12.2. Efficacy Assessment
2.12.3. Safety Assessments
2.12.4. Study Product
2.12.5. Participants
2.12.6. Clinical Test Procedure and Outline
2.13. Statistical Analysis
3. Results
3.1. Chemical Library Screening
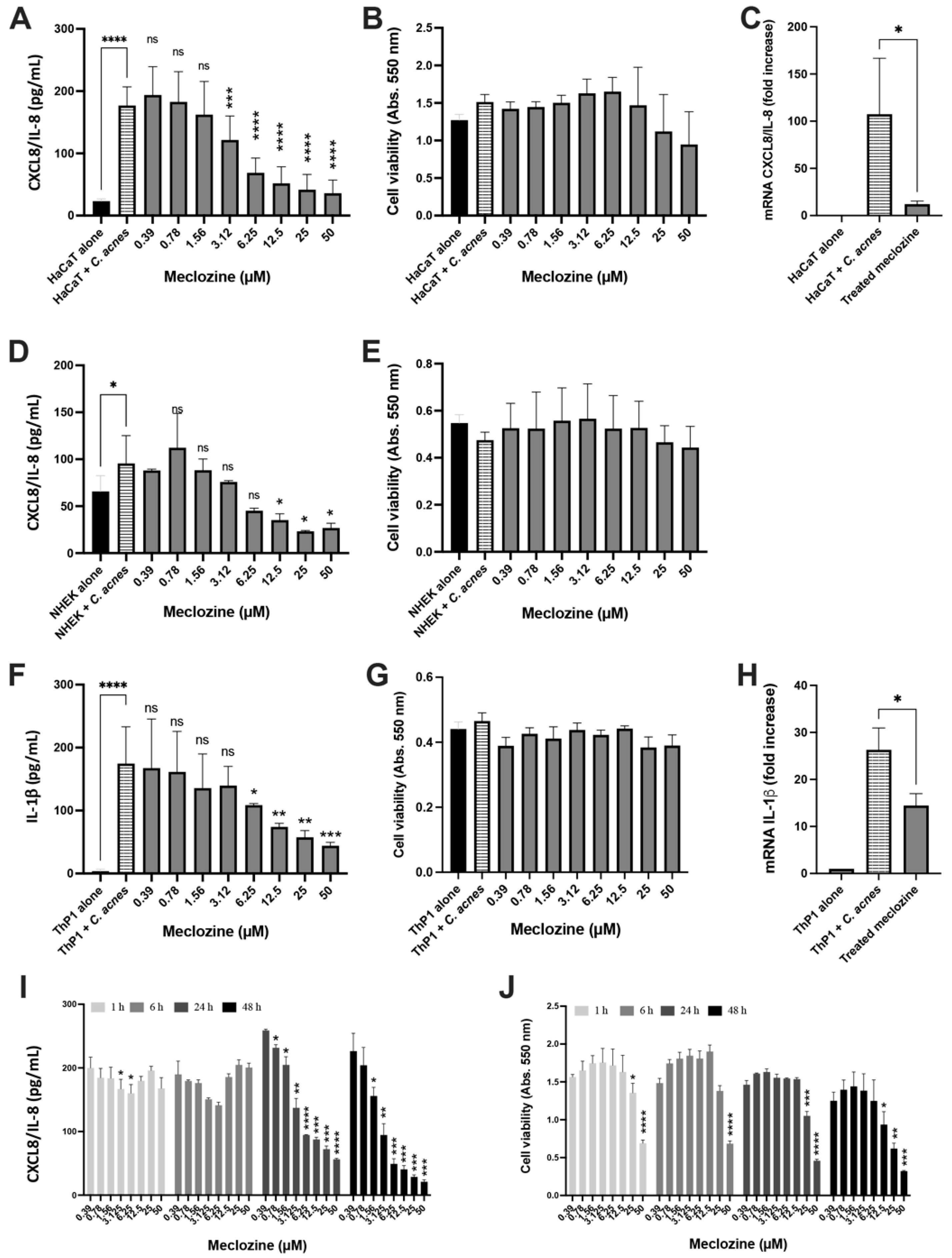
3.2. Meclozine Inhibits the C. acnes-Induced Production of CXCL8/IL-8 and IL-1β in Keratinocytes and Monocytes
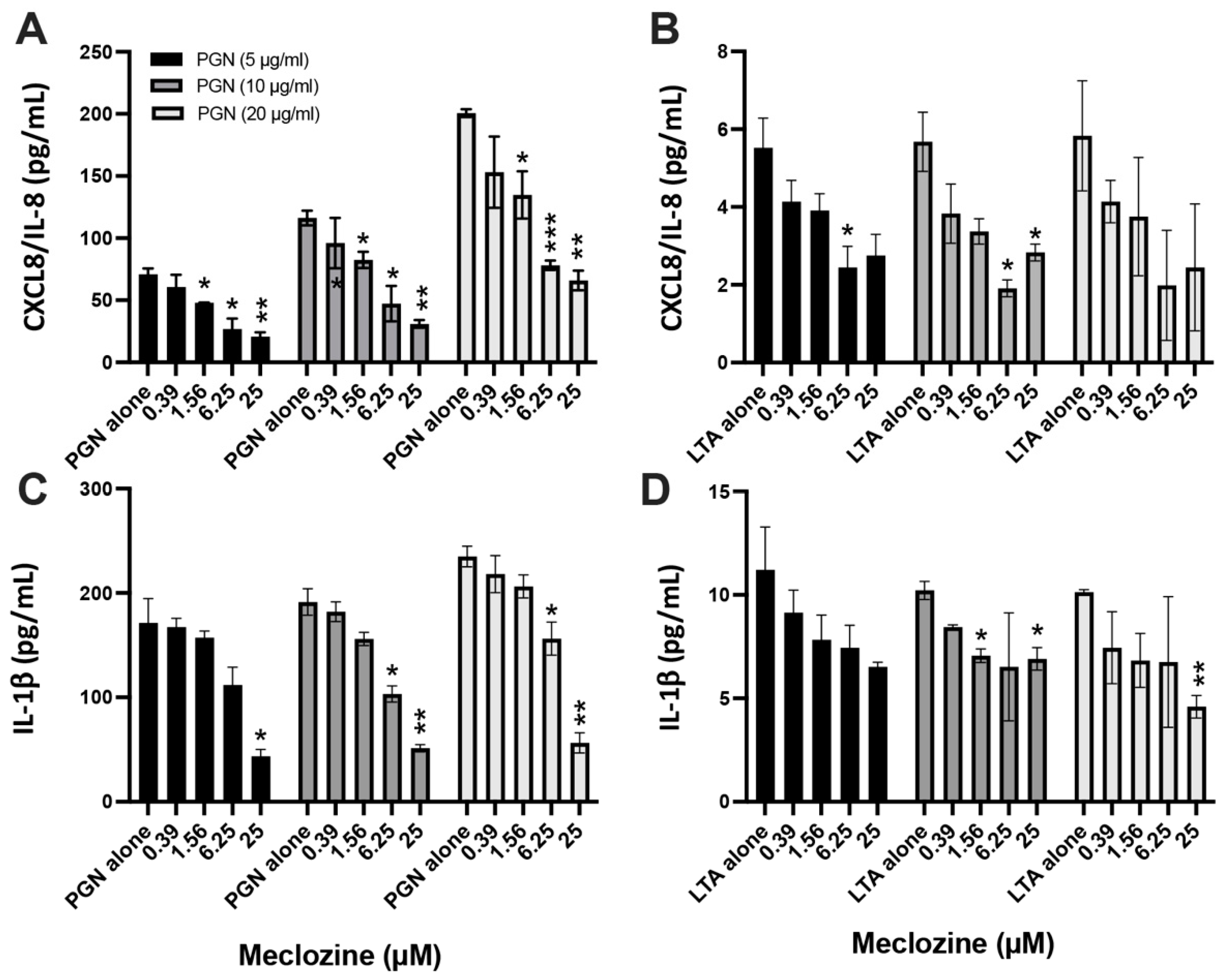
3.3. Meclozine Inhibits the PGN- and LTA-Induced Production of CXCL8/IL-8 and IL-1β
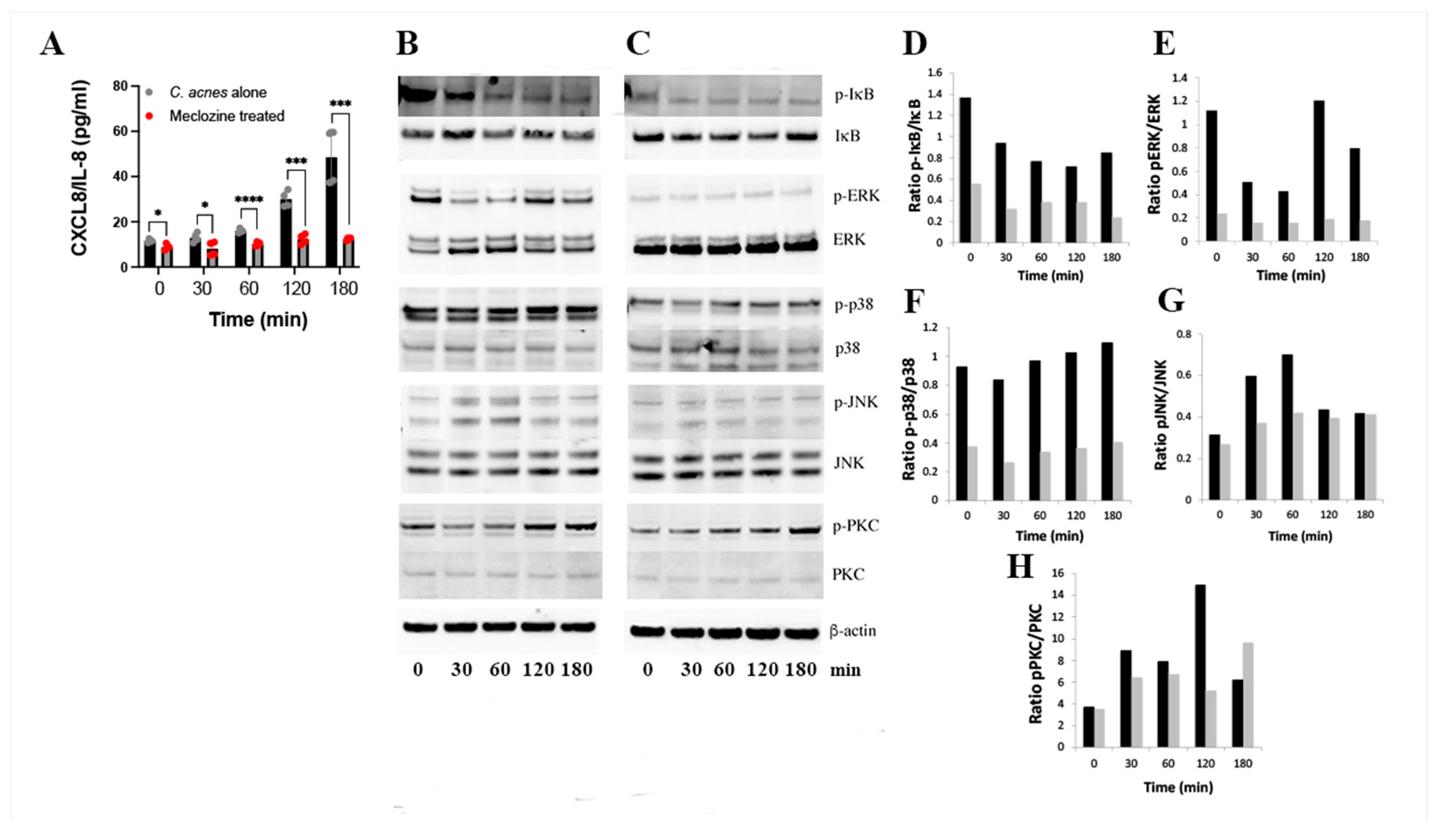
3.4. Molecular Mechanism of Action of Meclozine
3.5. Meclozine Reduces C. acnes-Induced Inflammation In Vivo
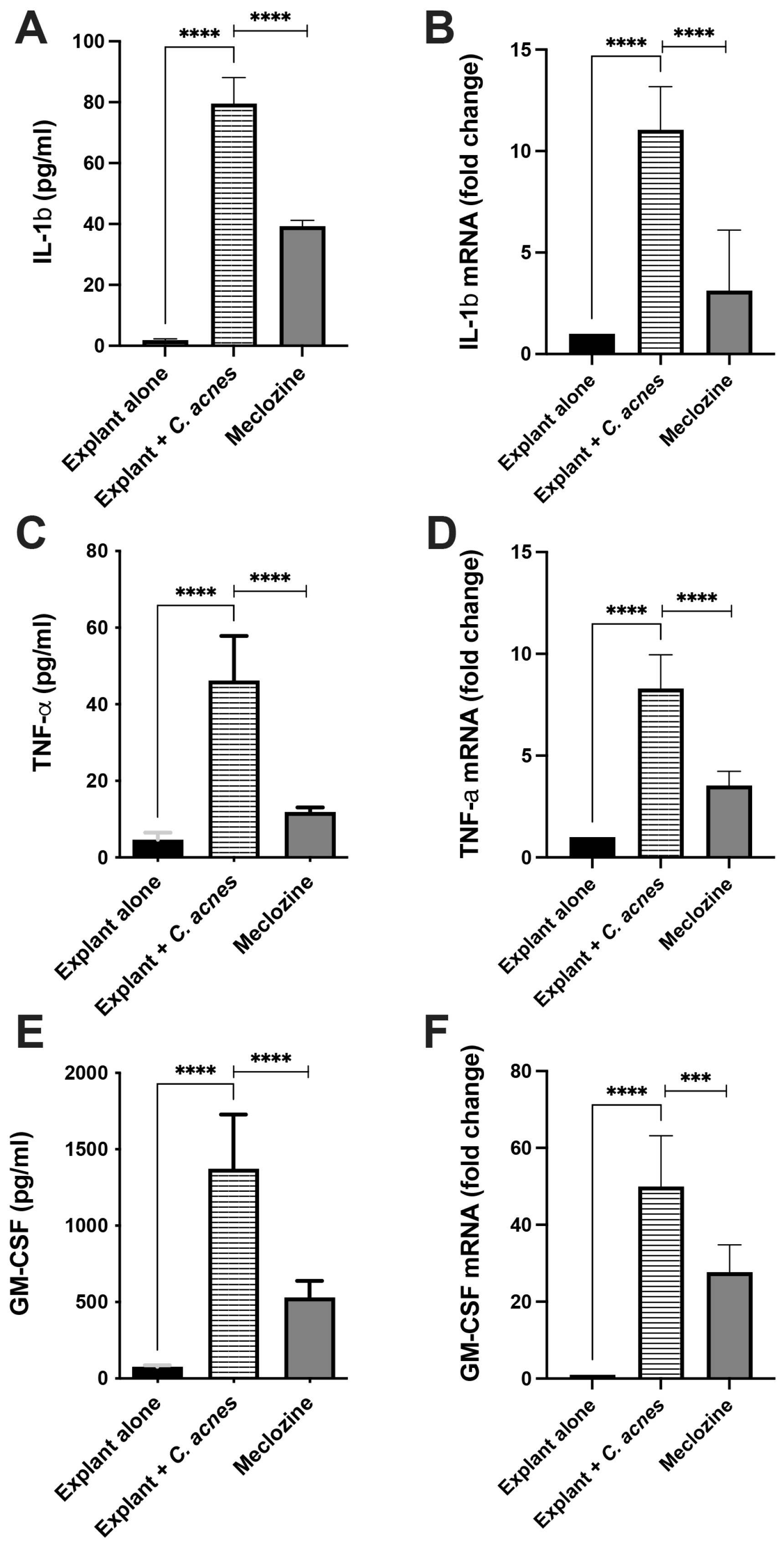
3.6. Meclozine Reduced C. acnes-Induced Inflammation Ex Vivo
3.7. Preclinical Toxicological Evaluation
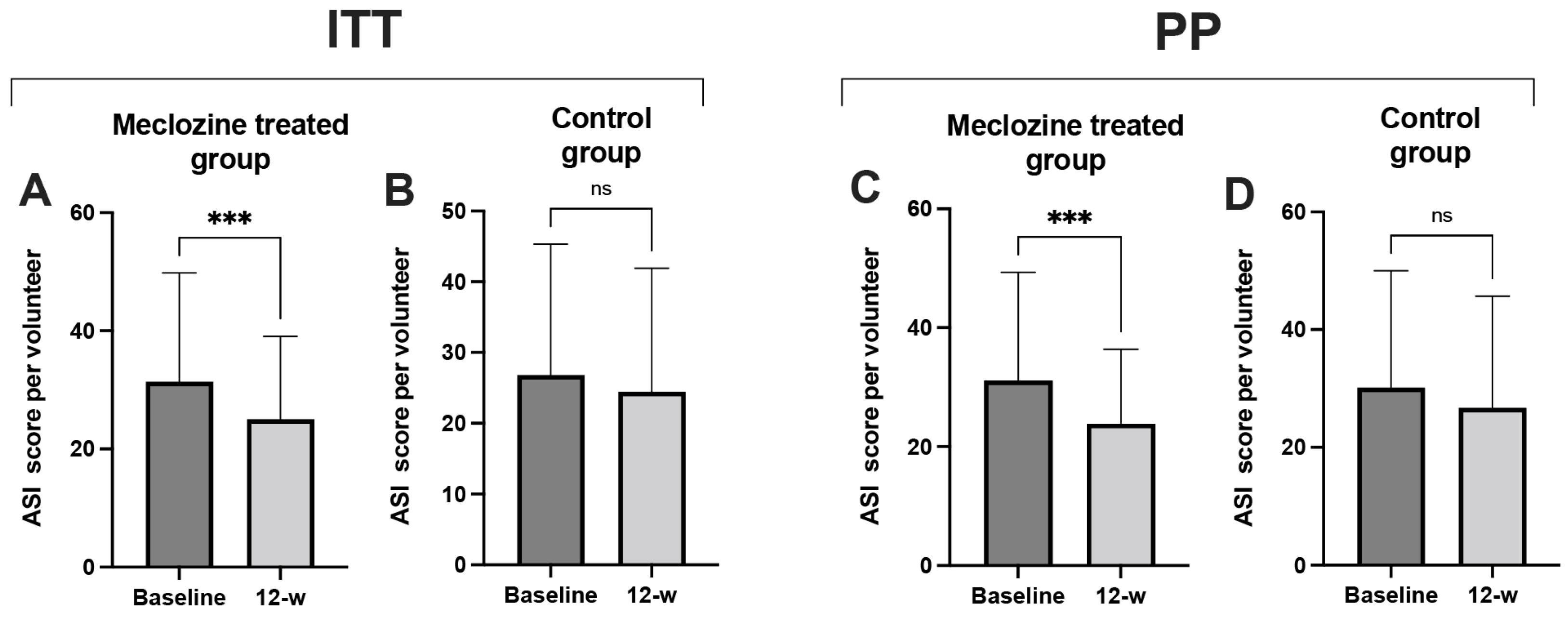
3.8. Clinical Study Proof-of-Concept
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bocquet-Trémoureux, S.; Corvec, S.; Khammari, A.; Dagnelle, M.-A.; Bolsrobert, A.; Dreno, B. Acne fulminans and Cutibacterium acnes phylotypes. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Ofenloch, R.F.; Bruze, M.; Naldi, L.; Cazzaniga, S.; Elsner, P.; Goncalo, M.; Schuttelaar, M.A.; Diepgen, T.L. Prevalence of skin disease in a population-based sample of adults from five European countries. Br. J. Dermatol. 2018, 178, 1111–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deplewski, D.; Rosenfield, R.L. Role of hormones in pilosebaceous unit development. Endocr. Rev. 2000, 21, 363–392. [Google Scholar] [CrossRef] [PubMed]
- Tax, G.; Urbán, E.; Palotás, Z.; Puskás, R.; Kónya, Z.; Bíró, T.; Kemény, L.; Szabó, K. Propionic acid produced by Propionibacterium acnes strains contributes to their pathogenicity. Acta. Dermatol. Venereol. 2016, 96, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomholt, H.B.; Kilian, M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS ONE 2010, 5, e12277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A.; Barnard, E.; Nagy, I.; Gao, A.; Tomida, S.; Li, H.; Eady, A.; Cove, J.; Nord, C.E.; Patrick, S. An expanded multilocus sequence typing scheme for Propionibacterium acnes: Investigation of ‘pathogenic’, ‘commensal’ and antibiotic resistant strains. PLoS ONE 2012, 7, e41480. [Google Scholar] [CrossRef] [Green Version]
- McDowell, A.; Nagy, I.; Magyari, M.; Barnard, E.; Patrick, S. The opportunistic pathogen Propionibacterium acnes: Insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS ONE 2013, 8, e70897. [Google Scholar] [CrossRef] [Green Version]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [Green Version]
- Dreno, B.; Martin, R.; Moyal, D.; Henley, J.B.; Khammari, A.; Seite, S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp. Derm. 2017, 26, 798–803. [Google Scholar] [CrossRef] [Green Version]
- Brüggemann, H.; Salar-Vidal, L.; Gollnick, H.P.; Lood, R. A janus-faced bacterium: Host-beneficial and -detrimental roles of Cutibacterium acnes. Front. Microbiol. 2021, 12, 673845. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.; Sampaio-Barros, P.D. SAPHO syndrome. Rheum. Dis Clin. N. Am. 2013, 39, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Goldstein, E.J.C.; Coenye, T.; Shirtliff, M.E. Propionibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüggemann, H.; Al-Zeer, M. Bacterial signatures and their inflammatory potentials associated with prostate cancer. APMIS 2020, 128, 80–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumgarner, R.E.; Harrison, D.; Hsu, J.E. Cutibacterium acnes isolates from deep tissue specimens retrieved during revision shoulder arthroplasty: Similar colony morphology does not indicate clonality. J. Clin. Microbiol. 2020, 58, e00121-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudek, R.; Brobeil, A.; Brüggemann, H.; Sommer, F.; Gattenlöhner, S.; Gohlke, F. Cutibacterium acnes is an intracellular and intra-articular commensal of the human shoulder joint. J. Shoulder Elbow. Surg. 2020, 30, 16–26. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Koreck, A.; Széll, M.; Urbán, E.; Kemény, L. Distinct strains of Propionibacterium acnes induce selective human β-defensin-2 and interleukin-8 expression in human keratinocytes through Toll-like receptors. J. Investig. Dermatol. 2005, 124, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, N.R.; Gilliland, K.L.; Zhao, W.; Liu, W.; Thiboutot, D.M. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J. Investig. Dermatol. 2006, 126, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Pirouz, A.; Kim, M.-H.; Krutzik, S.R.; Garban, H.J.; Kim, J. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J. Investig. Dermatol. 2014, 134, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Törőcsik, D.; Kovács, D.; Póliska, S.; Szentkereszty-Kovács, Z.; Lovászi, M.; Hegyi, K.; Szegedi, A.; Zouboulis, C.C.; Ståhle, M. Genome wide analysis of TLR1/2- and TLR4-activated SZ95 sebocytes reveals a complex immune-competence and identifies serum amyloid A as a marker for activated sebaceous glands. PLoS ONE 2018, 13, e0198323. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Töröcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Lheure, C.; Grange, P.A.; Ollagnier, G.; Morand, P.; Désiré, N.; Sayon, S.; Corvec, S.; Raingeaud, J.; Marcelin, A.G.; Clavez, V.; et al. TLR-2 recognizes Propionibacterium acnes CAMP Factor 1 from highly inflammatory strains. PLoS ONE 2016, 11, e0167237. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Tschismarov, R.; Pilz, A.; Straubinger, S.; Carotta, S.; McDowell, A.; Decker, T. Cutibacterium acnes infection induces type I interferon synthesis through the cGAS-STING pathway. Front. Immunol. 2020, 11, 571334. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef] [Green Version]
- Zaenglein, A.L. Acne vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef]
- Nakase, K.; Aoki, S.; Sei, S.; Fukumoto, S.; Horiuchi, Y.; Yasuda, T.; Tanioka, M.; Sugai, J.; Huh, W.-K.W.; Kakuta, M.; et al. Characterization of acne patients carrying clindamycin-resistant Cutibacterium acnes: A Japanese multicenter study. J. Dermatol. 2020, 47, 863–869. [Google Scholar] [CrossRef]
- Peterson, G.L. Determination of total protein. Methods Enzymol. 1983, 91, 95–119. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Matsubara, M.; Tamura, T.; Ohmori, K.; Hasegawa, K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/IκB/NF-κB cascades. Biochem. Pharmacol. 2005, 69, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.H.; Elmotasem, H.; Salama, A.A.A. Nanotechnology based blended chitosan-pectin hybrid for safe and efficient consolidative antiemetic and neuro-proctective effect of meclizine hydrochloride in chemotherapy induced emesis. Int. J. Pharm. 2020, 30, 119411. [Google Scholar] [CrossRef]
- Leathem, A.M. Safety and efficacy of antiemetics used to treat nausea and vomiting in pregnancy. Clin. Pharm. 1986, 5, 660–668. [Google Scholar] [PubMed]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Matsushita, M.; Kitoh, H.; Ohkawara, B.; Mishima, K.; Kaneko, H.; Ito, M.; Masuda, A.; Ishiguro, N.; Ohno, K. Meclozine facilitate proliferation and differentiation of chondrocytes by attenuating abnormally activated FGFR3 signaling in achondroplasia. PLoS ONE 2013, 8, e81569. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, W.; Wu, Y.; Jing, X.; Huang, J.; Zhang, J.; Xiang, W.; Ren, R.; Lv, Z.; Xiao, J.; et al. Meclizine prevents ovariectomy-induced bone loss and inhibits osteoclastogenesis partially by upregulating PXR. Front. Pharmacol. 2017, 8, 693. [Google Scholar] [CrossRef] [Green Version]
- Kochevar, I.E.; Moran, M.; Lyon, N.; Flotte, T.; Siebert, E.; Gange, W. Effects of systemic indomethacin, meclizine and BW755C on chronic ultraviolet B-induced effects in hairless mousse skin. J. Investig. Dermatol. 1993, 100, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Kishi, S.; Campanholle, G.; Gohil, V.M.; Perocchi, F.; Brooks, C.R.; Morizane, R.; Sabbisetti, V.; Ichimura, T.; Mootha, V.K.; Bonventre, J.V. Meclizine preconditioning protects the kidney against ischemia-reperfusion injury. EBioMed 2015, 2, 1090–1101. [Google Scholar] [CrossRef] [Green Version]
- Suvanprakorn, P.; Tongyen, T.; Prakhongcheep, O.; Laoratthaphong, P.; Chanvorachote, P. Establishment of an anti-acne vulgaris evaluation method based on TLR2 and TLR4-mediated interleukin-8 production. In Vivo 2019, 33, 1929–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vancurova, I.; Miskolci, V.; Davidson, D. NF-kappa B activation in tumor necrosis factor alpha-stimulated neutrophils is mediated by protein kinase Cdelta. Correlation to nuclear Ikappa Balpha. J. Biol. Chem. 2001, 276, 19746–19752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscella, A.; Greco, S.; Elia, M.G.; Storelli, C.; Marsigliante, S. PKC-zeta is required for angiotensin II-induced activation of ERK and synthesis of C-FOS in MCF-7 cells. J. Cell Physiol. 2003, 197, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Vardarova, K.; Scharf, S.; Lang, F.; Schmeck, B.; Opitz, B.; Eitel, J.; Hocke, A.C.; Slevogt, H.; Flieger, A.; Hippenstiel, S.; et al. PKC(alpha) and PKC(epsilon) differentially regulate Legionella pneumophila-induced GM-CSF. Eur. Respir. 2009, 34, 1171–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iinuma, K.; Sato, T.; Akimoto, N.; Noguchi, N.; Sasatsu, M.; Nishijima, S.; Kurokawa, I.; Ito, A. Involvement of Propionibacterium acnes in the augmentation of lipogenesis in hamster sebaceous glands in vivo and in vitro. J. Investig. Dermatol. 2009, 129, 2113–2119. [Google Scholar] [CrossRef] [Green Version]
- Jani, S.; Da Eira, D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; de Pinho, R.A.; Ceddia, R.B. Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci Rep. 2021, 11, 19160. [Google Scholar] [CrossRef]
- Lin, Z.-C.; Lee, C.-W.; Tsai, M.-H.; Kp, H.-H.; Fang, J.-Y.; Chiang, Y.-C.; Liang, C.-J.; Hsu, L.-F.; Hu, S.C.-S.; Yen, F.-L. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. Int. J. Nanomed. 2016, 11, 3907–3926. [Google Scholar]
- Nakatsuji, T.; Liu, Y.-T.; Huang, C.-P.; Gallo, R.L.; Huang, C.-M. Antibodies elicited by inactivated Propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: Relevance to therapy for acne vulgaris. J. Investig. Dermatol. 2008, 128, 2451–2457. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.A. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002, 23, 403–408. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From growth factor to central mediator of tissue inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.A.; Cook, A.D.; Tak, P.P. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat. Rev. Drug Discov. 2017, 16, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.M.; Farrar, M.D.; Cruse-Sawyer, J.E.; Holland, K.T.; Ingham, E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Bri. J. Dermatol. 2004, 150, 421–428. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, B.; Pearce, D.; Qian, S.; Wang, Y.; Zhang, Q.; Chow, M.S.S. Meclizine metabolism and pharmacokinetics: Formulation on its absorption. J. Clin. Pharmacol. 2012, 52, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Otlewska, A.; Baran, W.; Batycka-Baran, A. Adverse events related to topical drug treatments for acne vulgaris. Expert Opin. Drug Saf. 2015, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, H.B.; Kilian, M. Clonality and anatomic distribution on the skin of antibiotic resistant and sensitive Propionibacterium acnes. Acta Derm Venereol. 2014, 94, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Dessinioti, C.; Katsambas, A. Propionibacterium acnes and antimicrobial resistance in acne. Clin. Dermatol. 2017, 35, 163–167. [Google Scholar] [CrossRef]
- Nast, A.; Dréno, B.; Bettoli, V.; Bukvic Mokos, Z.; Degitz, K.; Dressler, C.; Finlay, A.Y.; Haedersdal, M.; Lambert, J.; Layton, A.; et al. European evidence-based (S3) guideline.for the treatment of acne—Update 2016—Short version. J. Eur. Acad Dermatol. Venereol. 2016, 30, 1261–1268. [Google Scholar] [CrossRef]
- Dreno, B. Bacteriological resistance in acne: A call to action. Eur. J. Dermatol. 2016, 26, 127–132. [Google Scholar] [CrossRef]
- Kawashima, M.; Nagare, T.; Doi, M. Clinical efficacy and safety of benzoyl peroxide for acne vulgaris: Comparison between Japanese and Western patients. J. Dermatol. 2017, 44, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Brammann, C.; Müller-Goymann, C.C. An update on formulation strategies of benzoyl peroxide in efficient acne therapy with special focus on minimizing undesired effects. Int. J. Pharm. 2020, 578, 119074. [Google Scholar] [CrossRef]
- Pelle, E.; McCarthy, J.; Seltmann, H.; Huang, X.; Mammone, T.; Zouboulis, C.C.; Maes, D. Identification of histamine receptors and reduction of squalene levels by an antihistamine in sebocytes. J. Investig. Dermatol. 2008, 128, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.K.; Lee, G.C.; Teng, C.; Frei, C.R. In vitro activity of non-antibiotic drugs against Staphylococcus aureus clinical strains. J. Glob. Antimicrob. Resist 2021, 27, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.; Maund, E.; Wilcox, C.; Sridharan, K.; Sivaramakrishnan, G.; Regas, C.; Newell, D.; Soulsby, I.; Tang, K.F.; Finlay, A.Y.; et al. Topical preparations for the treatment of mild-to-moderate acne vulgaris: Systematic review and network meta-analysis. Br. J. Dermatol. 2021, 185, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, S.; Xia, Y.; Chen, J.; Ye, C.; Zeng, Q.; Lai, W. Efficacy and safety of 2% supramolecular salicylic acid compared with 5% benzoyl peroxide/0.1% adapalene in the acne treatment: A randomized, split-face, open-label, single-center study. Cutan Ocul. Toxicol. 2019, 38, 48–54. [Google Scholar] [CrossRef] [PubMed]

| Treated Group | Control Group | |
|---|---|---|
| Age, n | 30 | 30 |
| Mean (S.D.) | 27 (5.34) | 27 (6.43) |
| Median | 25 | 27 |
| (Min, max) | (19, 38) | (19, 39) |
| Age categories | ||
| >18 | 1 | 5 |
| 20–30 | 20 | 14 |
| 30–40 | 9 | 11 |
| Sex, n (%) | ||
| Man | 14 (46.7) | 6 (20) |
| Woman | 16 (53.3) | 24 (80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grange, P.A.; Ollagnier, G.; Beauvais Remigereau, L.; Nicco, C.; Mayslich, C.; Marcelin, A.-G.; Calvez, V.; Dupin, N. A New Topical Candidate in Acne Treatment: Characterization of the Meclozine Hydrochloride as an Anti-Inflammatory Compound from In Vitro to a Preliminary Clinical Study. Biomedicines 2022, 10, 931. https://doi.org/10.3390/biomedicines10050931
Grange PA, Ollagnier G, Beauvais Remigereau L, Nicco C, Mayslich C, Marcelin A-G, Calvez V, Dupin N. A New Topical Candidate in Acne Treatment: Characterization of the Meclozine Hydrochloride as an Anti-Inflammatory Compound from In Vitro to a Preliminary Clinical Study. Biomedicines. 2022; 10(5):931. https://doi.org/10.3390/biomedicines10050931
Chicago/Turabian StyleGrange, Philippe A., Guillaume Ollagnier, Laurianne Beauvais Remigereau, Carole Nicco, Constance Mayslich, Anne-Geneviève Marcelin, Vincent Calvez, and Nicolas Dupin. 2022. "A New Topical Candidate in Acne Treatment: Characterization of the Meclozine Hydrochloride as an Anti-Inflammatory Compound from In Vitro to a Preliminary Clinical Study" Biomedicines 10, no. 5: 931. https://doi.org/10.3390/biomedicines10050931
APA StyleGrange, P. A., Ollagnier, G., Beauvais Remigereau, L., Nicco, C., Mayslich, C., Marcelin, A.-G., Calvez, V., & Dupin, N. (2022). A New Topical Candidate in Acne Treatment: Characterization of the Meclozine Hydrochloride as an Anti-Inflammatory Compound from In Vitro to a Preliminary Clinical Study. Biomedicines, 10(5), 931. https://doi.org/10.3390/biomedicines10050931









