Application of Bio-Active Elastin-like Polypeptide on Regulation of Human Mesenchymal Stem Cell Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Bone-Marrow-Derived MSCs
2.2. ELPs Expression and Purification
2.3. Cell Adhesion Assay
2.4. Phalloidin Staining
2.5. Cell Viability Assay
2.6. Cell Proliferation Assay
2.7. Population Doubling Assay
2.8. Migration Assay
2.9. Calcium Deposition Analysis
2.10. Lipid Droplet Staining
2.11. Alcian Blue Staining
2.12. Western Blotting of Bone Marrow MSCs
2.13. Analysis of Stemness Properties of MSCs
2.14. Statistical Analysis
3. Results and Discussion
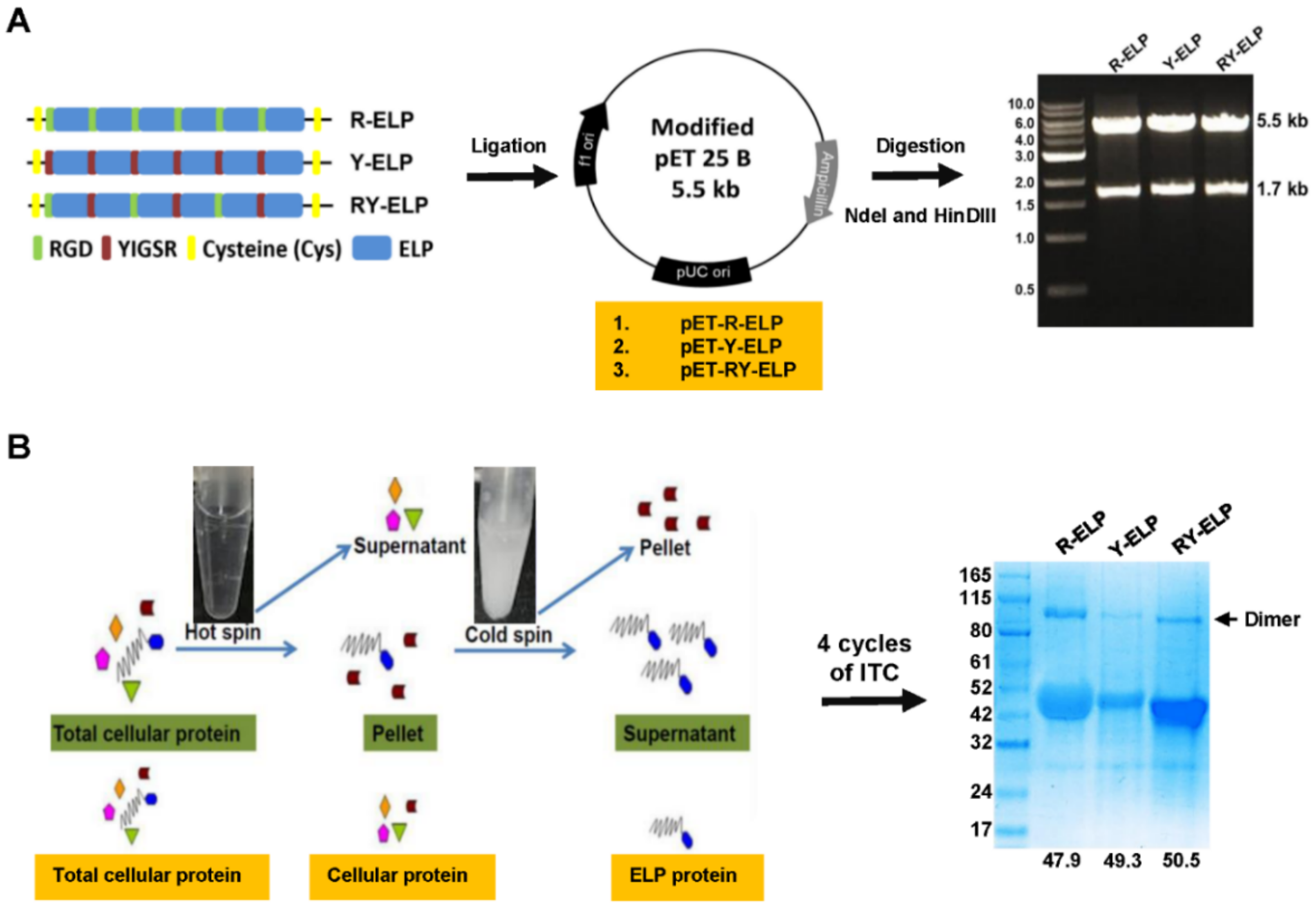
3.1. Genetic-Encoded Synthesis of Fusion ELPs and Protein Expression
3.2. Thermal Characteristics of Fusion-ELPs
3.3. Effect of Fusion ELP on Cell Adhesion and Proliferation
3.4. Induction of Migratory Effect by Fusion ELP on IHF
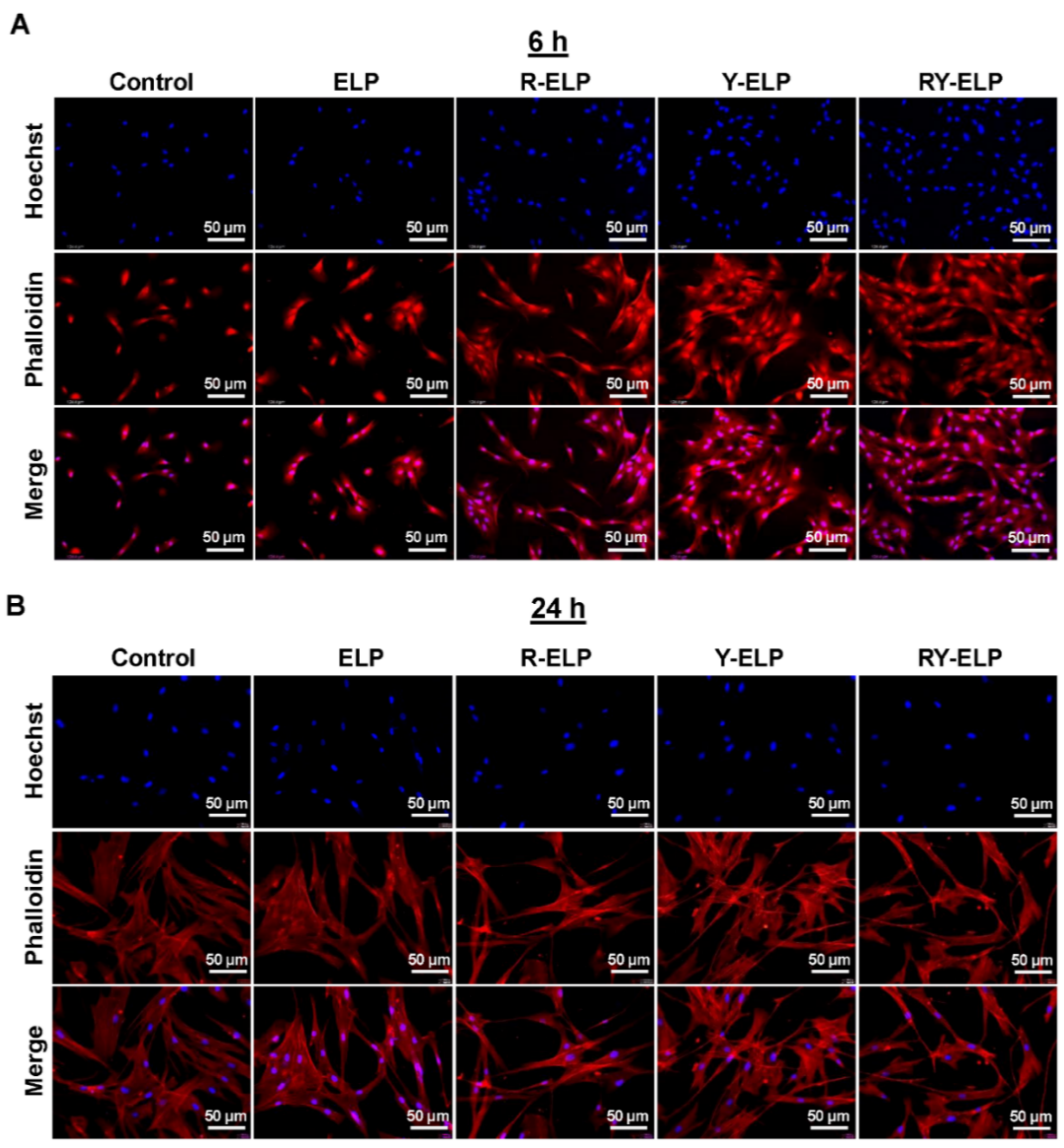
3.5. Interaction of MSCs on ELP Matrix
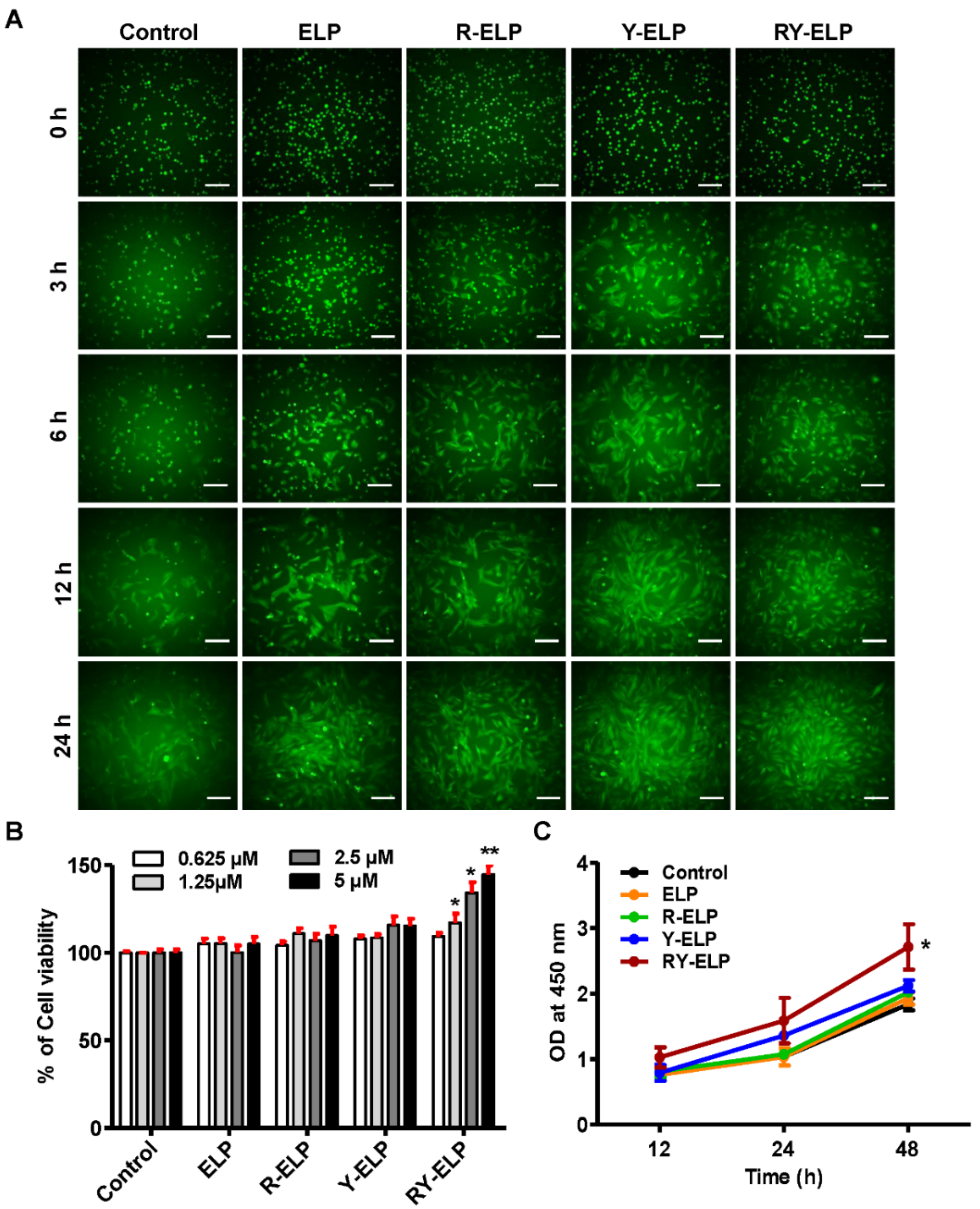
3.6. Effect of Fusion ELP on Viability and Proliferation of MSCs
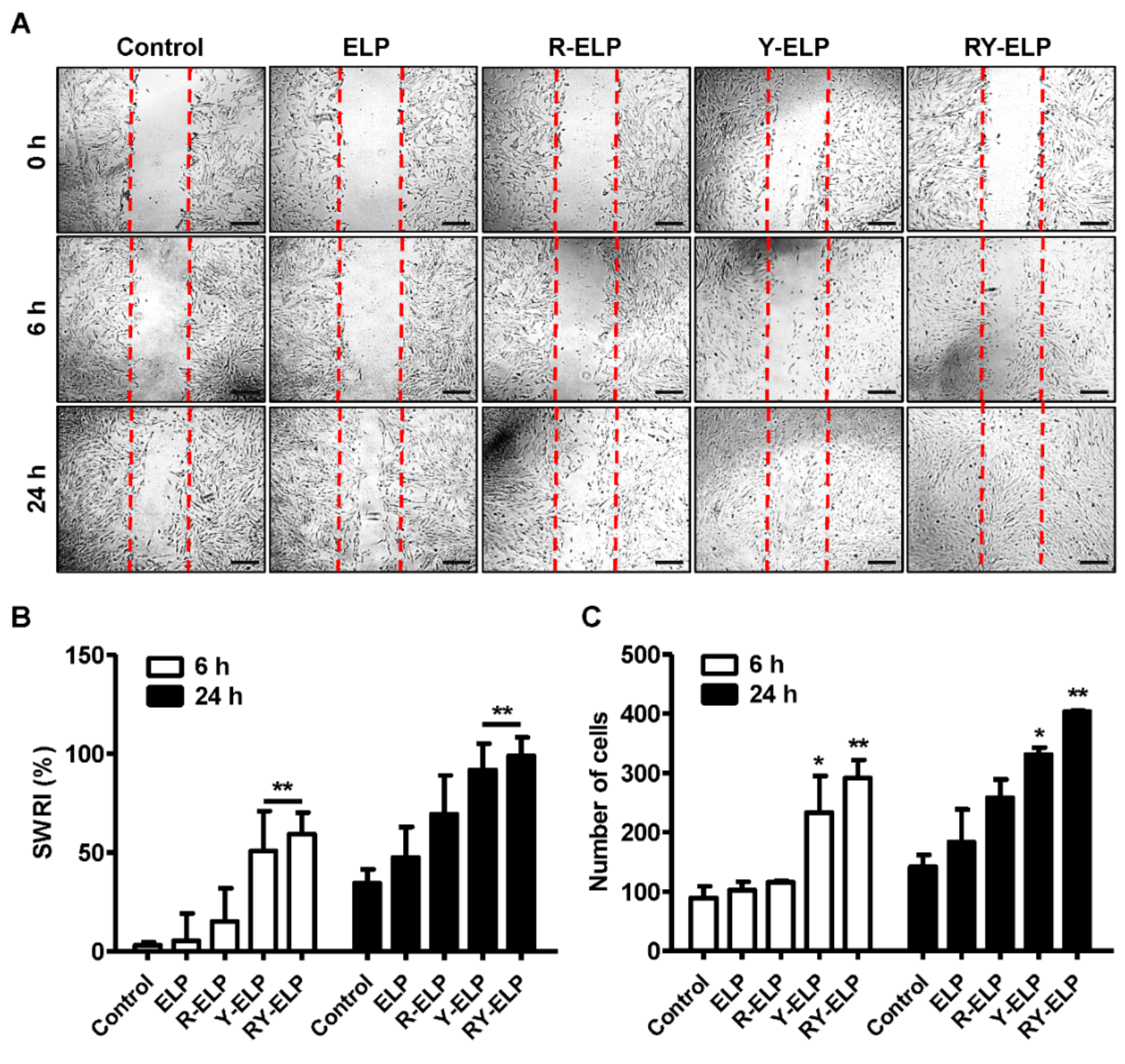
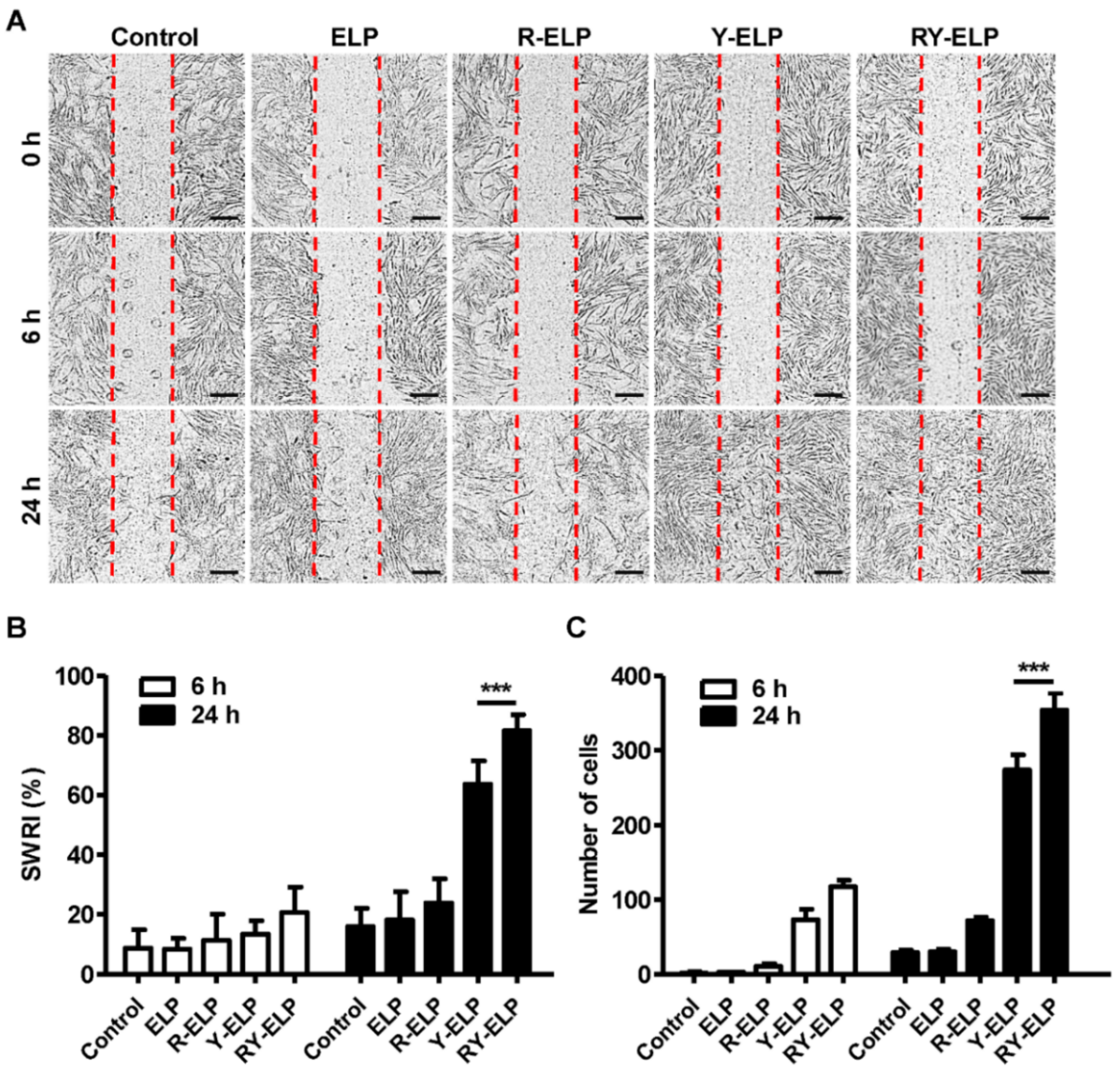
3.7. Effect of Fusion ELP on Migratory Effect of MSCs
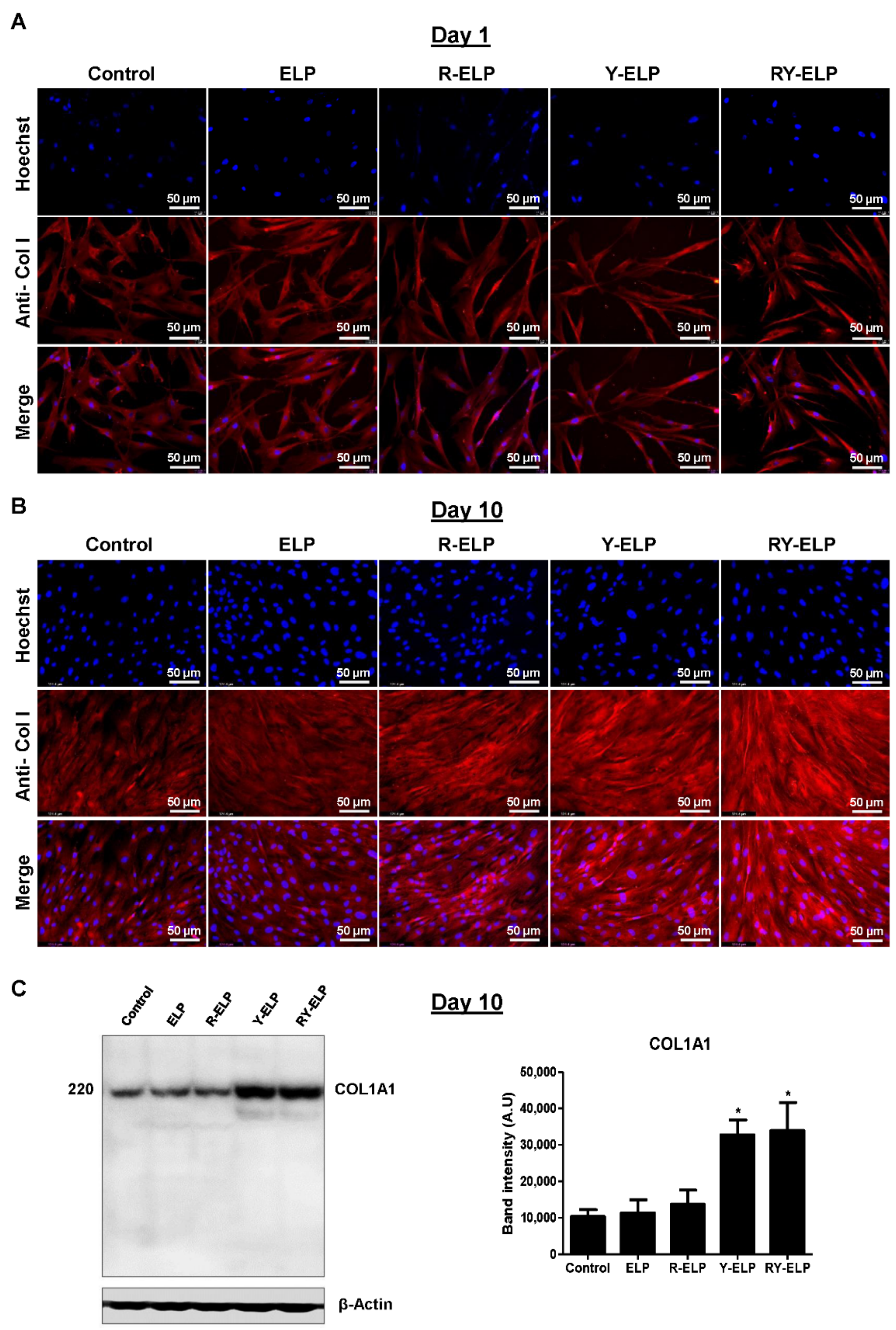
3.8. Effect of Fusion ELP on ECM Secretion
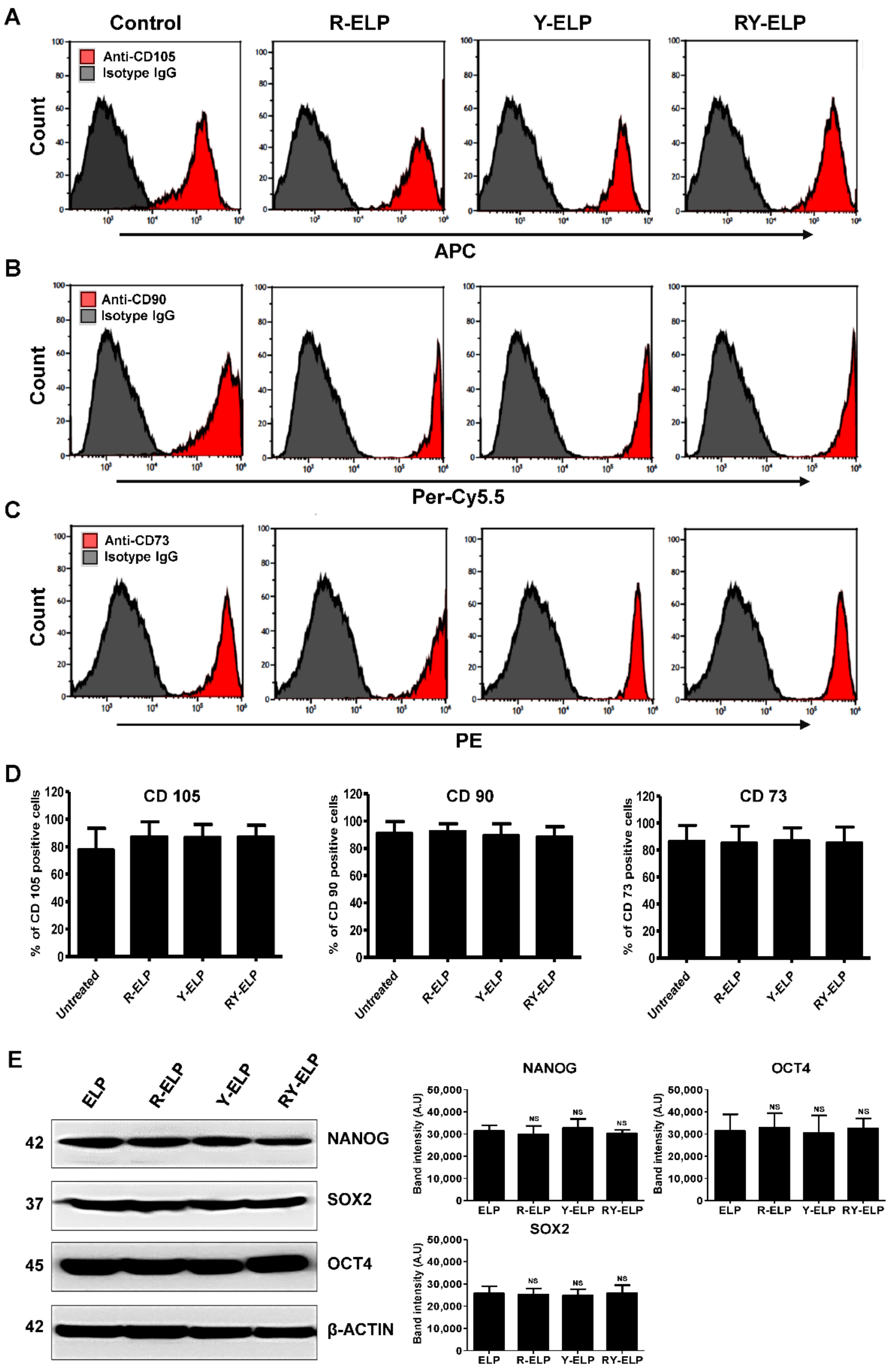
3.9. Effect of Fusion ELP on Stemness Property of MSCs
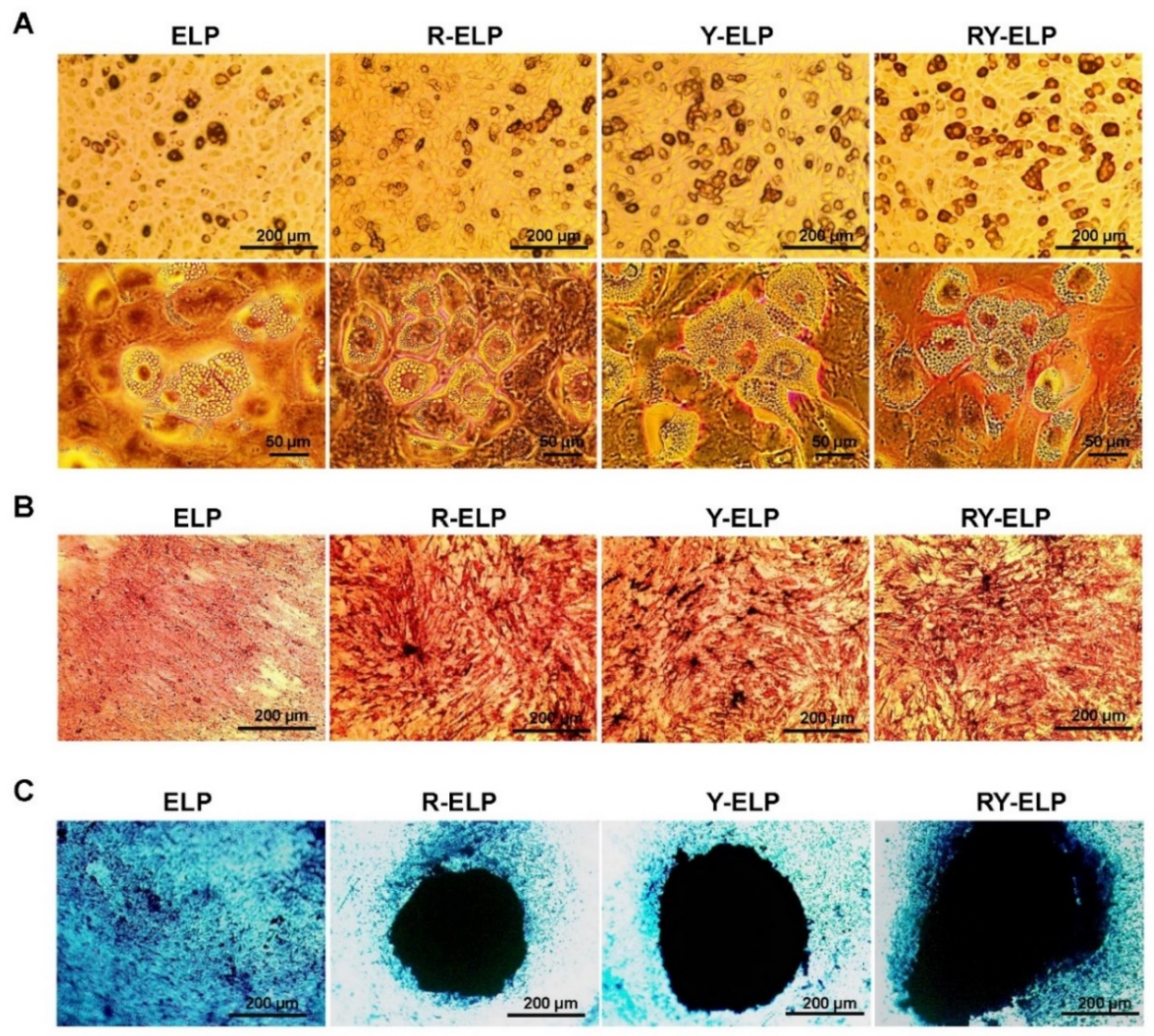
3.10. Effect of Fusion ELP on Trilineage Differentiation of MSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadereit, S.; Udolph, G. Umbilical Cord Blood: A Future for Regenerative Medicine? World Scientific Publishing Company: Singapore, 2010. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Song, I.-H. Adult mesenchymal stem cells for cell therapy in clinical application. Yeungnam Univ. J. Med. 2009, 26, 1–14. [Google Scholar] [CrossRef][Green Version]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in mesenchymal stem cells: Functional alterations, molecular mechanisms, and rejuvenation strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Qiu, L.; Ma, J.; Zhang, H.; Cheng, M.; Li, W.; Zhao, X.; Liu, K. Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol. Biol. Rep. 2011, 38, 5161–5168. [Google Scholar] [CrossRef]
- Kozhevnikova, M.; Mikaelyan, A.; Payushina, O.; Starostin, V. Comparative characterization of mesenchymal bone marrow stromal cells at early and late stages of culturing. Biol. Bull. 2008, 35, 132–138. [Google Scholar] [CrossRef]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y.-l. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 2009, 15, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Ifkovits, J.L.; Burdick, J.A. Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef]
- Kim, B.-S.; Park, I.-K.; Hoshiba, T.; Jiang, H.-L.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Design of artificial extracellular matrices for tissue engineering. Prog. Polym. Sci. 2011, 36, 238–268. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Chilkoti, A.; Christensen, T.; MacKay, J.A. Stimulus responsive elastin biopolymers: Applications in medicine and biotechnology. Curr. Opin. Chem. Biol. 2006, 10, 652–657. [Google Scholar] [CrossRef]
- Johnson, T.; Koria, P. Expression and purification of neurotrophin-elastin-like peptide fusion proteins for neural regeneration. BioDrugs 2016, 30, 117–127. [Google Scholar] [CrossRef]
- McCarthy, B.; Yuan, Y.; Koria, P. Elastin-like-polypeptide based fusion proteins for osteogenic factor delivery in bone healing. Biotechnol. Prog. 2016, 32, 1029–1037. [Google Scholar] [CrossRef]
- Koria, P.; Yagi, H.; Kitagawa, Y.; Megeed, Z.; Nahmias, Y.; Sheridan, R.; Yarmush, M.L. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc. Natl. Acad. Sci. USA 2011, 108, 1034–1039. [Google Scholar] [CrossRef]
- Ryu, J.S.; Raucher, D. Elastin-like polypeptide for improved drug delivery for anticancer therapy: Preclinical studies and future applications. Expert Opin. Drug Deliv. 2015, 12, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.B. Application of elastin-mimetic recombinant proteins in chemotherapeutics delivery, cellular engineering, and regenerative medicine. Bioengineered 2013, 4, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Thomas, D.; Monaghan, M.; Carroll, O.; Chen, X.; Woodhouse, K.; O’Brien, T.; Pandit, A. An injectable elastin-based gene delivery platform for dose-dependent modulation of angiogenesis and inflammation for critical limb ischemia. Biomaterials 2015, 65, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Devalliere, J.; Uygun, B.E.; Yarmush, M.L. Functionalized biopolymer particles enhance performance of a tissue-protective peptide under proteolytic and thermal stress. Biomacromolecules 2016, 17, 2073–2079. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, A.; Cohen, R.I.; Faulknor, R.; Schloss, R.; Yarmush, M.L.; Berthiaume, F. The development and characterization of SDF1α-elastin-like-peptide nanoparticles for wound healing. J. Control. Release 2016, 232, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Legate, K.R.; Wickström, S.A.; Fässler, R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009, 23, 397–418. [Google Scholar] [CrossRef]
- Won, J.-I.; Barron, A.E. A new cloning method for the preparation of long repetitive polypeptides without a sequence requirement. Macromolecules 2002, 35, 8281–8287. [Google Scholar] [CrossRef]
- Sarangthem, V.; Cho, E.A.; Bae, S.M.; Singh, T.D.; Kim, S.J.; Kim, S.; Jeon, W.B.; Lee, B.H.; Park, R.W. Construction and application of elastin like polypeptide containing IL-4 receptor targeting peptide. PLoS ONE 2013, 8, e81891. [Google Scholar] [CrossRef]
- Trabbic-Carlson, K.; Meyer, D.E.; Liu, L.; Piervincenzi, R.; Nath, N.; LaBean, T.; Chilkoti, A. Effect of protein fusion on the transition temperature of an environmentally responsive elastin-like polypeptide: A role for surface hydrophobicity? Protein Eng. Des. Sel. PEDS 2004, 17, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Nettles, D.L.; Chilkoti, A.; Setton, L.A. Applications of elastin-like polypeptides in tissue engineering. Adv. Drug Deliv. Rev. 2010, 62, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Gurd, F.B. A Histological Study of the Skin Lesions of Pellagra. J. Exp. Med. 1911, 13, 98–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [PubMed]
- Sabatini, F.; Petecchia, L.; Tavian, M.; Jodon de Villeroche, V.; Rossi, G.A.; Brouty-Boye, D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab. Investig. 2005, 85, 962–971. [Google Scholar] [CrossRef]
- Lorenz, K.; Sicker, M.; Schmelzer, E.; Rupf, T.; Salvetter, J.; Schulz-Siegmund, M.; Bader, A. Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp. Dermatol. 2008, 17, 925–932. [Google Scholar] [CrossRef]
- Covas, D.T.; Panepucci, R.A.; Fontes, A.M.; Silva, W.A., Jr.; Orellana, M.D.; Freitas, M.C.; Neder, L.; Santos, A.R.; Peres, L.C.; Jamur, M.C.; et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008, 36, 642–654. [Google Scholar] [CrossRef]
- Haniffa, M.A.; Wang, X.N.; Holtick, U.; Rae, M.; Isaacs, J.D.; Dickinson, A.M.; Hilkens, C.M.; Collin, M.P. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J. Immunol. 2007, 179, 1595–1604. [Google Scholar] [CrossRef]
- Chang, Y.; Li, H.; Guo, Z. Mesenchymal stem cell-like properties in fibroblasts. Cell. Physiol. Biochem. 2014, 34, 703–714. [Google Scholar] [CrossRef]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.D.; McAnany, C.E.; Mura, C.; Lampe, K.J. Toward a Designable Extracellular Matrix: Molecular Dynamics Simulations of an Engineered Laminin-Mimetic, Elastin-Like Fusion Protein. Biomacromolecules 2016, 17, 3222–3233. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, A.; Sonnenberg, A. Function and interactions of integrins. Cell Tissue Res. 2001, 305, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 2004, 5, 383–388. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Meirelles Lda, S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Williams, A.R.; Hare, J.M. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef]
- Hoch, A.I.; Leach, J.K. Concise review: Optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl. Med. 2014, 3, 643–652. [Google Scholar] [CrossRef]
- Wang, H.; Luo, X.; Leighton, J. Extracellular Matrix and Integrins in Embryonic Stem Cell Differentiation. Biochem. Insights 2015, 8 (Suppl. 2), 15–21. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarangthem, V.; Sharma, H.; Mendiratta, M.; Sahoo, R.K.; Park, R.-W.; Kumar, L.; Singh, T.D.; Mohanty, S. Application of Bio-Active Elastin-like Polypeptide on Regulation of Human Mesenchymal Stem Cell Behavior. Biomedicines 2022, 10, 1151. https://doi.org/10.3390/biomedicines10051151
Sarangthem V, Sharma H, Mendiratta M, Sahoo RK, Park R-W, Kumar L, Singh TD, Mohanty S. Application of Bio-Active Elastin-like Polypeptide on Regulation of Human Mesenchymal Stem Cell Behavior. Biomedicines. 2022; 10(5):1151. https://doi.org/10.3390/biomedicines10051151
Chicago/Turabian StyleSarangthem, Vijaya, Harshita Sharma, Mohini Mendiratta, Ranjit Kumar Sahoo, Rang-Woon Park, Lalit Kumar, Thoudam Debraj Singh, and Sujata Mohanty. 2022. "Application of Bio-Active Elastin-like Polypeptide on Regulation of Human Mesenchymal Stem Cell Behavior" Biomedicines 10, no. 5: 1151. https://doi.org/10.3390/biomedicines10051151
APA StyleSarangthem, V., Sharma, H., Mendiratta, M., Sahoo, R. K., Park, R.-W., Kumar, L., Singh, T. D., & Mohanty, S. (2022). Application of Bio-Active Elastin-like Polypeptide on Regulation of Human Mesenchymal Stem Cell Behavior. Biomedicines, 10(5), 1151. https://doi.org/10.3390/biomedicines10051151






