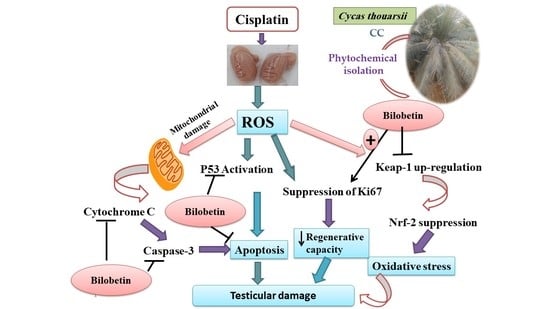
The Mechanistic Perspective of Bilobetin Protective Effects against Cisplatin-Induced Testicular Toxicity: Role of Nrf-2/Keap-1 Signaling, Inflammation, and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extraction and Bilobetin Isolation
2.2. Animals
2.3. Chemicals and Spectral Techniques
2.4. Experimental Design
2.5. Sample Collection
2.6. Determination of Testis Body Weight Ratio
2.7. Assessment of Serum Testosterone and Cytochrome-c
2.8. Measurement of Lipid Peroxidation
2.9. Measurement of SOD Activity
2.10. qRT-PCR forVCAM, NrF-2, Keap-1, IL-0, α-SMA, and P53 Genes
2.11. Histopathological Examination of Testis Sections
2.12. Immunohistochemical Staining of Ki67 and Caspase-3
2.13. Statistical Analysis
3. Results
3.1. Phytochemical Investigation
Structure Elucidation of Bilobetin
3.2. Biological Investigation
3.2.1. Effects of Bilobetin on Testicular Weight Changes
3.2.2. Effects on Serum Testosterone Level
3.2.3. Effects on Cytochrome-C Release in the Cytosol
3.2.4. Effects on Testicular Oxidative Stress Markers
3.2.5. Effects on Testicular Nrf2 Gene Expression
3.2.6. Effects on Testicular Keap-1 Gene Expression
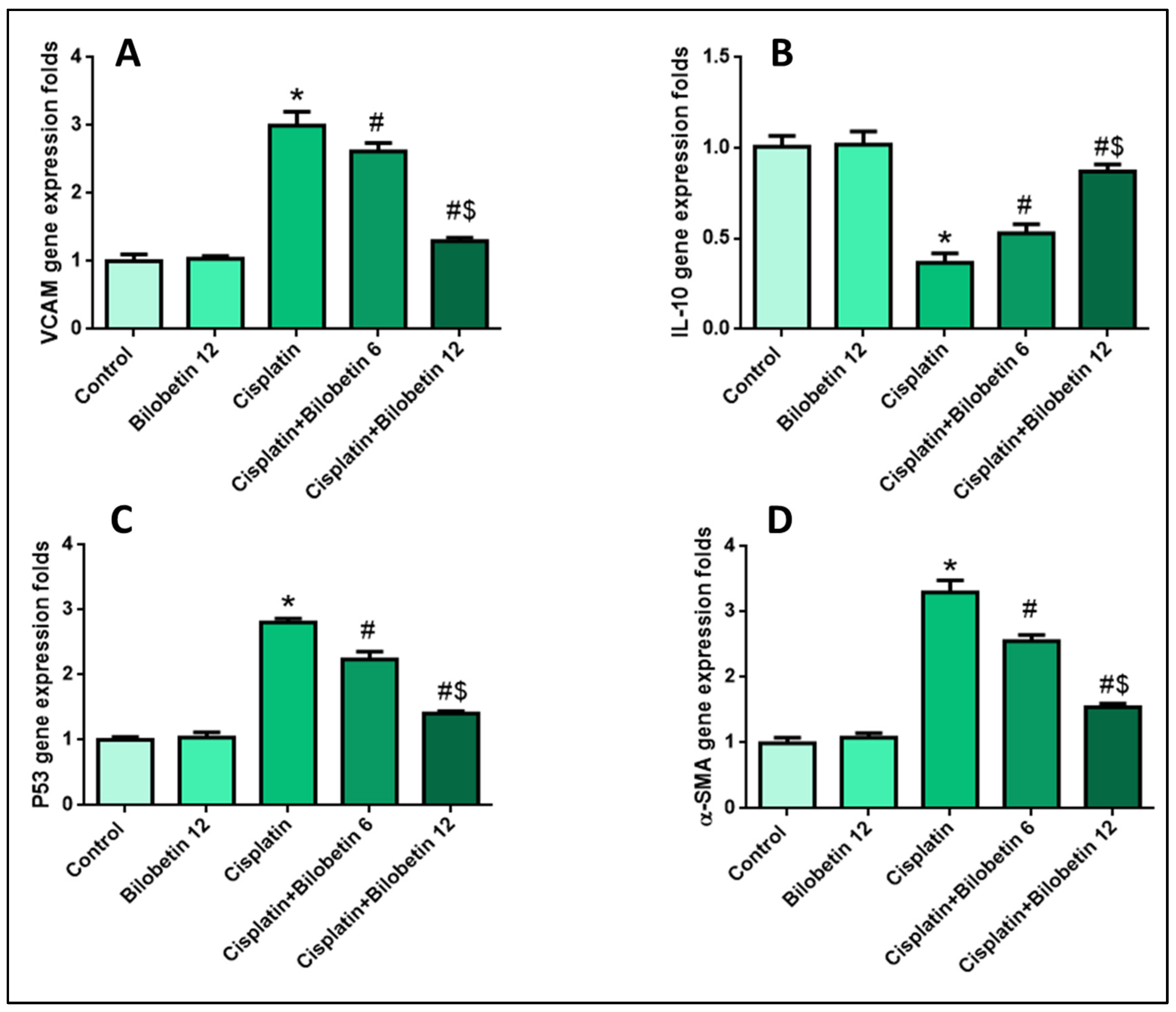
3.2.7. Effects on Testicular VCAM Gene Expression
3.2.8. Effects on Testicular IL-10 Gene Expression
3.2.9. Effects on Testicular P53 Gene Expression
3.2.10. Effects on Testicular α-SMA Gene Expression
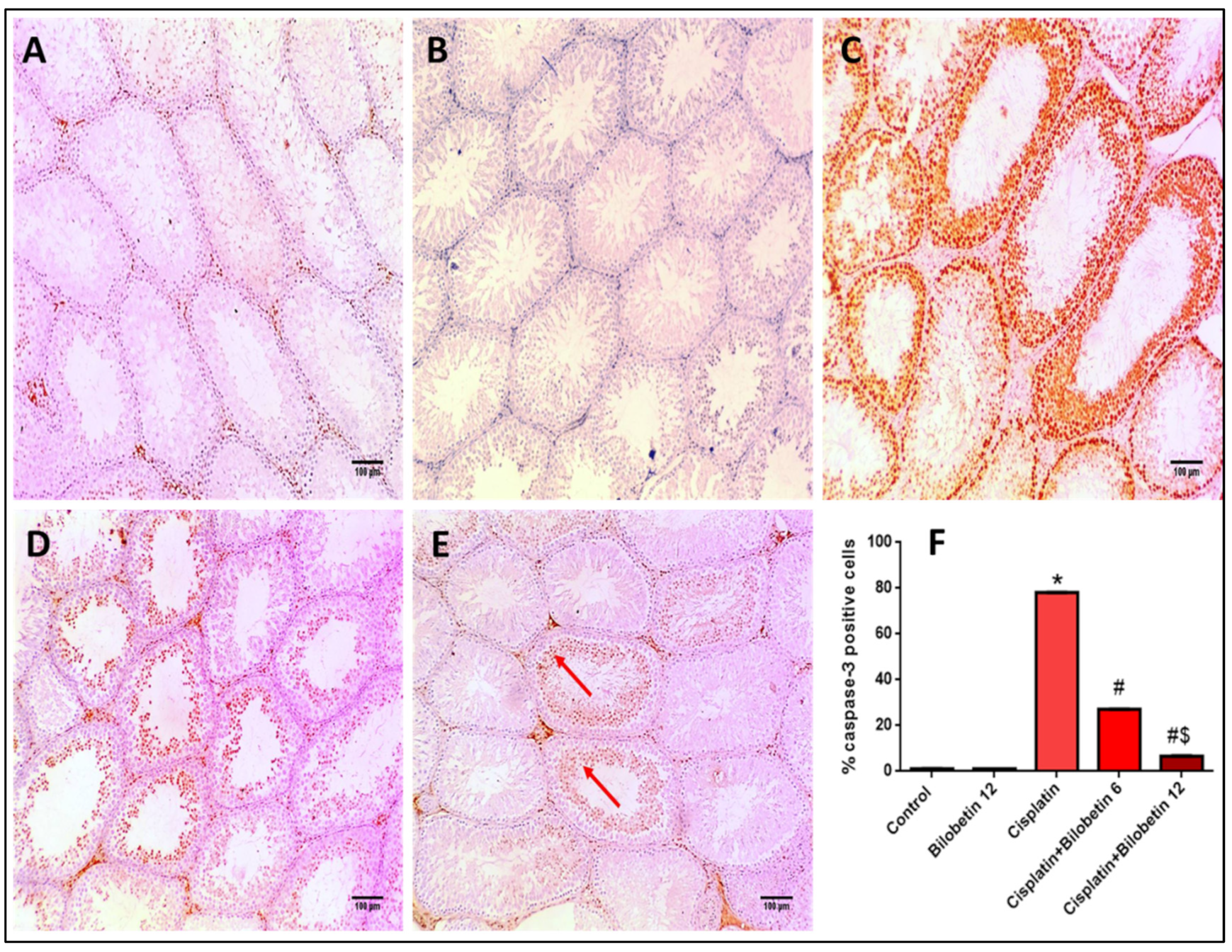
3.2.11. Effects on Immunohistochemical Staining of Ki67
3.2.12. Effects on Immunohistochemical Staining of Caspase-3
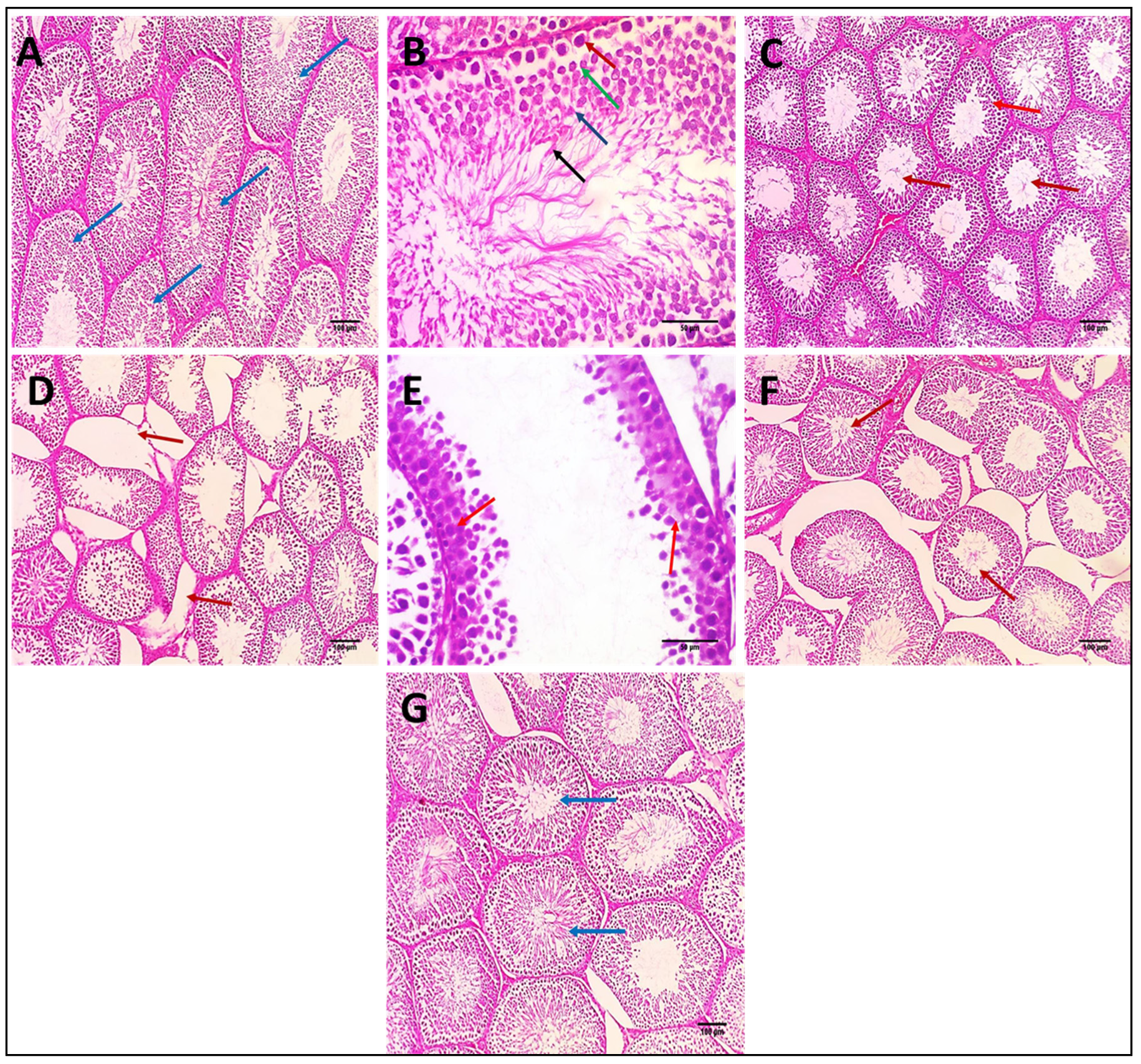
3.2.13. Effects on Histopathological Examination of Testicular Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.-Y.; Zhang, Q.-Y.; Zheng, G.-J.; Feng, B. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother. 2019, 110, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Elrashidy, R.A.; Hasan, R.A. Stromal cell-derived factor-1α predominantly mediates the ameliorative effect of linagliptin against cisplatin-induced testicular injury in adult male rats. Cytokine 2020, 136, 155260. [Google Scholar] [CrossRef] [PubMed]
- Mesbahzadeh, B.; Hassanzadeh-Taheri, M.; Aliparast, M.-s.; Baniasadi, P.; Hosseini, M. The protective effect of crocin on cisplatin-induced testicular impairment in rats. BMC Urol. 2021, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Eren, H.; Mercantepe, T.; Tumkaya, L.; Mercantepe, F.; Horsanali, M.O.; Yilmaz, A. Evaluation of the protective effects of amifostine and melatonin against cisplatin induced testis injury via oxidative stress and apoptosis in rats. Exp. Mol. Pathol. 2020, 112, 104324. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Walbi, I.A.; Albarqi, H.A.; Ali, F.E.; Hassanein, E.H. Roflumilast protects from cisplatin-induced testicular toxicity in male rats and enhances its cytotoxicity in prostate cancer cell line. Role of NF-κB-p65, cAMP/PKA and Nrf2/HO-1, NQO1 signaling. Food Chem. Toxicol. 2021, 151, 112133. [Google Scholar] [CrossRef]
- Wang, T.-E.; Lai, Y.-H.; Yang, K.-C.; Lin, S.-J.; Chen, C.-L.; Tsai, P.-S. Counteracting cisplatin-induced testicular damages by natural polyphenol constituent honokiol. Antioxidants 2020, 9, 723. [Google Scholar] [CrossRef]
- Sherif, I.O.; Abdel-Aziz, A.; Sarhan, O.M. Cisplatin-induced testicular toxicity in rats: The protective effect of arjunolic acid. J. Biochem. Mol. Toxicol. 2014, 28, 515–521. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.; Yildirim, S.; Kandemir, F.M.; Aksu, E.H.; Guler, M.C.; Kiziltunc Ozmen, H.; Kucukler, S.; Eser, G. The antiapoptotic and antioxidant effects of eugenol against cisplatin-induced testicular damage in the experimental model. Andrologia 2019, 51, e13353. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, J.; Zhao, C.; Lu, S.; Qiao, J.; Han, M. The combinatory effects of natural products and chemotherapy drugs and their mechanisms in breast cancer treatment. Phytochem. Rev. 2020, 19, 1179–1197. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Hou, Y.; Tian, Y.; Chen, L.; Liu, S.; Zhang, N.; Dong, J. Anti-inflammatory effects of chemical components from Ginkgo biloba L. male flowers on lipopolysaccharide-stimulated RAW264. 7 macrophages. Phytother. Res. 2019, 33, 989–997. [Google Scholar] [CrossRef]
- Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Elekhnawy, E. Investigation of the Antibacterial Activity and Efflux Pump Inhibitory Effect of Cycas thouarsii R.Br. Extract against Klebsiella pneumoniae Clinical Isolates. Pharmaceuticals 2021, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.A.; Abo El-Seoud, K.A.; Kabbash, A.; Kassab, A.A.; El-Aasr, M. Hepatoprotective, cytotoxic, antimicrobial and antioxidant activities of Dioon spinulosum leaves Dyer Ex Eichler and its isolated secondary metabolites. Nat. Prod. Res. 2020, 35, 5166–5176. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.A.; Ibrahim, A.E.-R.S.; El-Seoud, K.A.; Attia, G.I.; Ragab, A.E. A new cytotoxic and antioxidant Amentoflavone Monoglucoside from Cycas revoluta Thunb growing in Egypt. J. Pharm. Sci. Res. 2016, 8, 343. [Google Scholar]
- Patel, D.K. Biological importance of a biflavonoids‘ bilobetin’ in the medicine: Medicinal importance, pharmacological activities and analytical aspects. Infect. Disord. Drug Targets 2022. [Google Scholar] [CrossRef]
- Elekhnawy, E.; Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Alqarni, M.; Batiha, G.E.-S.; Obaidullah, A.J.; Fawzy, H.M. Histological assessment, anti-quorum sensing, and anti-biofilm activities of Dioon spinulosum extract: In vitro and in vivo approach. Sci. Rep. 2022, 12, 180. [Google Scholar] [CrossRef]
- Tao, Y.; Zhu, F.; Pan, M.; Liu, Q.; Wang, P. Pharmacokinetic, Metabolism, and Metabolomic Strategies Provide Deep Insight Into the Underlying Mechanism of Ginkgo biloba Flavonoids in the Treatment of Cardiovascular Disease. Front. Nutr. 2022, 9, 857370. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, X.; Chen, Y.; Li, L.; Sun, Q.; Zhang, L. Identification of bilobetin metabolites, in vivo and in vitro, based on an efficient ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry strategy. J. Sep. Sci. 2020, 43, 3408–3420. [Google Scholar] [CrossRef]
- Elmongy, E.I.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Attallah, N.G.; Altwaijry, N.; Batiha, G.E.-S.; El-Sherbeni, S.A. Antidiarrheal and Antibacterial Activities of Monterey Cypress Phytochemicals: In Vivo and In Vitro Approach. Molecules 2022, 27, 346. [Google Scholar] [CrossRef]
- Demir, M.; Altındağ, F. Sinapic acid ameliorates cisplatin-induced disruptions in testicular steroidogenesis and spermatogenesis by modulating androgen receptor, proliferating cell nuclear antigen and apoptosis in male rats. Andrologia 2022, 54, e14369. [Google Scholar] [CrossRef]
- Ilbey, Y.O.; Ozbek, E.; Cekmen, M.; Simsek, A.; Otunctemur, A.; Somay, A. Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: Mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum. Reprod. 2009, 24, 1717–1725. [Google Scholar] [CrossRef] [Green Version]
- Saad, K.M.; Abdelrahman, R.S.; Said, E. Mechanistic perspective of protective effects of nilotinib against cisplatin-induced testicular injury in rats: Role of JNK/caspase-3 signaling inhibition. Environ. Toxicol. Pharmacol. 2020, 76, 103334. [Google Scholar] [CrossRef]
- Harakeh, S.; Qari, M.; Rajeh, N.; Ali, S.; El-Shitany, N.; Hassan, S.; Abd-Allah, E.A.; Tashkandi, H.; Malik, M.F.A.; Aljabri, F.K. Ellagic acid nanoparticles attenuate oxidative stress and testicular damage in high fat Diet/Streptozotocin-Induced diabetic rats. J. King Saud Univ.-Sci. 2022, 34, 101720. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Attallah, N.G.; Mokhtar, F.A.; Elekhnawy, E.; Heneidy, S.Z.; Ahmed, E.; Magdeldin, S.; Negm, W.A.; El-Kadem, A.H. Mechanistic Insights on the In Vitro Antibacterial Activity and In Vivo Hepatoprotective Effects of Salvinia auriculata Aubl against Methotrexate-Induced Liver Injury. Pharmaceuticals 2022, 15, 549. [Google Scholar] [CrossRef]
- Sherif, I.O.; Sarhan, O.M. Candesartan in a rat model of testicular toxicity: New insight on its protective mechanism. Exp. Biol. Med. 2019, 244, 593–601. [Google Scholar] [CrossRef]
- Riddell, I.A.; Lippard, S.J. Cisplatin and oxaliplatin: Our current understanding of their actions. Met. Ions Life Sci. 2018, 18, 1–42. [Google Scholar]
- Hassanzadeh_Taheri, M.; Hosseini, M.; Dorranipour, D.; Afshar, M.; Moodi, H.; Salimi, M. The Oleo-Gum-Resin of Commiphora myrrha ameliorates male reproductive dysfunctions in streptozotocin-induced hyperglycemic rats. Pharm. Sci. 2019, 25, 294–302. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Almajwal, A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer 2017, 17, 883. [Google Scholar] [CrossRef] [Green Version]
- Eid, A.H.; Abdelkader, N.F.; El-Raouf, A.; Ola, M.; Fawzy, H.M.; El-Denshary, E.-E.-D.S. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Arch. Pharmacal Res. 2016, 39, 1693–1702. [Google Scholar] [CrossRef]
- Hassanzadeh-Taheri, M.; Hosseini, M. Comments on “The improvement Effects of Gordonia bronchialis on Male Fertility of Rats with Diabetes Mellitus Induced by Streptozotocin”. Pharm. Sci. 2020, 26, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh-Taheri, M.; Hassanpour-Fard, M.; Doostabadi, M.; Moodi, H.; Vazifeshenas-Darmiyan, K.; Hosseini, M. Co-administration effects of aqueous extract of turnip leaf and metformin in diabetic rats. J. Tradit. Complementary Med. 2018, 8, 178–183. [Google Scholar] [CrossRef]
- Tian, M.; Liu, F.; Liu, H.; Zhang, Q.; Li, L.; Hou, X.; Zhao, J.; Li, S.; Chang, X.; Sun, Y. Grape seed procyanidins extract attenuates Cisplatin-induced oxidative stress and testosterone synthase inhibition in rat testes. Syst. Biol. Reprod. Med. 2018, 64, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Munawar, A.; Razak, S.; Anam, S.; Ain, Q.U.; Ullah, H.; Afsar, T.; Abulmeaty, M.; Almajwal, A. Ameliorative effects of rutin against cisplatin-induced reproductive toxicity in male rats. BMC Urol. 2018, 18, 107. [Google Scholar] [CrossRef]
- Rehman, M.U.; Ali, N.; Rashid, S.; Jain, T.; Nafees, S.; Tahir, M.; Khan, A.Q.; Lateef, A.; Khan, R.; Hamiza, O.O. Alleviation of hepatic injury by chrysin in cisplatin administered rats: Probable role of oxidative and inflammatory markers. Pharmacol. Rep. 2014, 66, 1050–1059. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rather, I.A. Myricetin abrogates cisplatin-induced oxidative stress, inflammatory response, and goblet cell disintegration in colon of wistar rats. Plants 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Kou, X.H.; Zhu, M.F.; Chen, D.; Lu, Y.; Song, H.Z.; Ye, J.L.; Yue, L.F. Bilobetin ameliorates insulin resistance by PKA-mediated phosphorylation of PPARα in rats fed a high-fat diet. Br. J. Pharmacol. 2012, 165, 2692–2706. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, B.; Xia, Z.-M.; Tian, Y.; Zhang, D.; Rui, W.-J.; Dong, J.-X.; Xiao, F.-J. Anticancer effects of five biflavonoids from ginkgo biloba l. Male flowers in vitro. Molecules 2019, 24, 1496. [Google Scholar] [CrossRef] [Green Version]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of Nrf2 in cardiovascular function and disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef]
- Eggler, A.L.; Liu, G.; Pezzuto, J.M.; Van Breemen, R.B.; Mesecar, A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA 2005, 102, 10070–10075. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, E.H.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxidative Med. Cell. Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, S.; Mohammadi, A.T.; Gholami, M.H.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Makvandi, P.; Samec, M.; Liskova, A. Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance. Pharmacol. Res. 2021, 167, 105575. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Alkahtani, S.A.; Elagab, E.A. Tadalafil alleviates cisplatin-induced reproductive toxicity through the activation of the Nrf2/HO-1 pathway and the inhibition of oxidative stress and apoptosis in male rats. Reprod. Toxicol. 2020, 96, 165–174. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Abd El-Twab, S.M.; Hozayen, W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019, 10, 4593–4607. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- ALHaithloul, H.A.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 2019, 111, 676–685. [Google Scholar] [CrossRef]
- Tadagavadi, R.K.; Reeves, W.B. Endogenous IL-10 Attenuates Cisplatin. J. Immunol. 2010, 185, 4904–4911. [Google Scholar] [CrossRef] [Green Version]
- Humanes, B.; Camaño, S.; Lara, J.M.; Sabbisetti, V.; González-Nicolás, M.Á.; Bonventre, J.V.; Tejedor, A.; Lázaro, A. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol. Dial. Transplant. 2017, 32, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Goodarzi, N.; Faramarzi, A.; Cheraghi, H.; Hashemian, A.H.; Jalili, C. Melatonin protects rats testes against bleomycin, etoposide, and cisplatin-induced toxicity via mitigating nitro-oxidative stress and apoptosis. Biomed. Pharmacother. 2021, 138, 111481. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.; Eid, B.G. Cisplatin induced testicular damage through mitochondria mediated apoptosis, inflammation and oxidative stress in rats: Impact of resveratrol. Endocr. J. 2020, 67, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Qutub, H.O.; Fouad, A.E.A.; Audeh, A.M.; Al-Melhim, W.N. Epigallocatechin-3-gallate counters cisplatin toxicity of rat testes. Pharm. Biol. 2017, 55, 1710–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabe, R.F.; Luedde, T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Y.; Wang, C.; Fu, Z.; Liu, W.; Gan, J. Induction of macrophage apoptosis by an organochlorine insecticide acetofenate. Chem. Res. Toxicol. 2009, 22, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hameed, A.M.; Mahmoud, H.S. Cypermethrin induced apoptosis and testicular toxicity by upregulation of p53 in the brain and testis of male rats is alleviated by Sesame oil. J. Taibah Univ. Sci. 2020, 14, 1342–1349. [Google Scholar] [CrossRef]
- Wang, T.; Chen, F.; Chen, Z.; Wu, Y.-F.; Xu, X.-L.; Zheng, S.; Hu, X. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J. Gastroenterol. 2004, 10, 2205. [Google Scholar] [CrossRef] [PubMed]
- Sobecki, M.; Mrouj, K.; Colinge, J.; Gerbe, F.; Jay, P.; Krasinska, L.; Dulic, V.; Fisher, D. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res. 2017, 77, 2722–2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkmann, J.; Müller, D.; Feuerstacke, C.; Kliesch, S.; Bergmann, M.; Mühlfeld, C.; Middendorff, R. Disturbed spermatogenesis associated with thickened lamina propria of seminiferous tubules is not caused by dedifferentiation of myofibroblasts. Hum. Reprod. 2011, 26, 1450–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Compound I | ||
|---|---|---|
| δ-H | δ-C | |
| 2 | 163.5 | |
| 3 | 6.93 (1H, s) | 103.6 |
| 4 | 181.9 | |
| 5 | 161.4 | |
| 6 | 6.19 (1H, d, J = 2.5 Hz) | 98.6 |
| 7 | 163.4 | |
| 8 | 6.49 (1H, d, J = 2.5) | 94.2 |
| 9 | 157.5 | |
| 10 | 103.6 | |
| 1′ | 122.6 | |
| 2′ | 8.07 (1H, d, J =2.5) | 128.3 |
| 3′ | 121. 6 | |
| 4′ | 160.6 | |
| 5′ | 7.48 (1H, d, J = 8.5) | 111.7 |
| 6′ | 8.18 (1H, dd, J = 2.5, 8.5) | 130.9 |
| 2″ | 164.3 | |
| 3″ | 6.80 (1H, s) | 102.5 |
| 4″ | 182.1 | |
| 5″ | 160.6 | |
| 6″ | 6.38 (1H, s) | 98.9 |
| 7″ | 161.8 | |
| 8″ | 103.7 | |
| 9″ | 154.3 | |
| 10″ | 103.7 | |
| 1′′′ | 121.2 | |
| 2′′′ | 7.51 (2H, d, J = 8.5), | 128.0 |
| 3′′′ | 6.71 (2H, d, J = 8.5), | 115.8 |
| 4′′′ | 161.1 | |
| 5′′′ | 6.71 (2H, d, J = 8.5), | 115.8 |
| 6′′′ | 7.51 (2H, d, J = 8.5) | 128.0 |
| 4′-O-CH3 | 3.76 | 55.9 |
| Body Weight (gm) | Testis Weight (gm) | Testis/Body Weight Ratio | ||
|---|---|---|---|---|
| Initial | Final | |||
| Control | 182.6 ± 1.95 | 198 ± 2.55 | 2.776 ± 126.0 | 1.397 ± 0.072 |
| Bilobetin 12 | 180.5 ± 1.14 | 184.2 ± 9.03 | 2.57 ± 0.219 | 1.392 ± 0.1 |
| Cisplatin | 183.6 ± 1.82 | 167.4 ± 11.8 * | 1.782 ± 0.168 * | 1.038 ± 0.063 * |
| CP+ Bilobetin 6 | 182.4 ± 1.67 | 169.6 ± 12.1 | 2.1 ± 0.327 | 1.228 ± 0.125 # |
| CP+ Bilobetin 12 | 183.5 ± 1.3 | 186.2 ± 6.38 # | 2.49 ± 0.105 #$ | 1.338 ± 0.057 # |
| Testicular MDA Content (nm/gm Tissue) | Testicular SOD Activity (U/mg Tissue) | |
|---|---|---|
| Control | 139.8 ± 2.86 | 2.81 ± 0.135 |
| Bilobetin 12 | 140.8 ± 2.28 | 2.79 ± 0.09 |
| Cisplatin | 252 ± 5.33 * | 1.38 ± 0.06 * |
| CP+ Bilobetin 6 | 183 ± 6.55 # | 1.724 ± 0.084 # |
| CP+ Bilobetin 12 | 146.8 ± 3.03 #$ | 2.58 ± 0.83 #$ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negm, W.A.; El-Kadem, A.H.; Hussein, I.A.; Alqahtani, M.J. The Mechanistic Perspective of Bilobetin Protective Effects against Cisplatin-Induced Testicular Toxicity: Role of Nrf-2/Keap-1 Signaling, Inflammation, and Apoptosis. Biomedicines 2022, 10, 1134. https://doi.org/10.3390/biomedicines10051134
Negm WA, El-Kadem AH, Hussein IA, Alqahtani MJ. The Mechanistic Perspective of Bilobetin Protective Effects against Cisplatin-Induced Testicular Toxicity: Role of Nrf-2/Keap-1 Signaling, Inflammation, and Apoptosis. Biomedicines. 2022; 10(5):1134. https://doi.org/10.3390/biomedicines10051134
Chicago/Turabian StyleNegm, Walaa A., Aya H. El-Kadem, Ismail A. Hussein, and Moneerah J. Alqahtani. 2022. "The Mechanistic Perspective of Bilobetin Protective Effects against Cisplatin-Induced Testicular Toxicity: Role of Nrf-2/Keap-1 Signaling, Inflammation, and Apoptosis" Biomedicines 10, no. 5: 1134. https://doi.org/10.3390/biomedicines10051134
APA StyleNegm, W. A., El-Kadem, A. H., Hussein, I. A., & Alqahtani, M. J. (2022). The Mechanistic Perspective of Bilobetin Protective Effects against Cisplatin-Induced Testicular Toxicity: Role of Nrf-2/Keap-1 Signaling, Inflammation, and Apoptosis. Biomedicines, 10(5), 1134. https://doi.org/10.3390/biomedicines10051134