Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. HCT116 Cell Line Culture
2.3. MTT Assay
2.4. Animal Experiment
2.5. Assessing Colon Tissue for Aberrant crypt foci (ACF)
2.6. Colon Histological Assessment
2.7. Measurement of Oxidative Stress and Lipid Peroxidation
2.8. DNA Extraction and 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. F. prausnitzii Cell-Free Supernatant Inhibits Cancer Cell Proliferation
3.2. F. prausnitzii Reduces ACF Formation in Rats
3.3. Measurement of Oxidative Stress and Lipid Peroxidation
3.4. Microscopical and Histological Findings
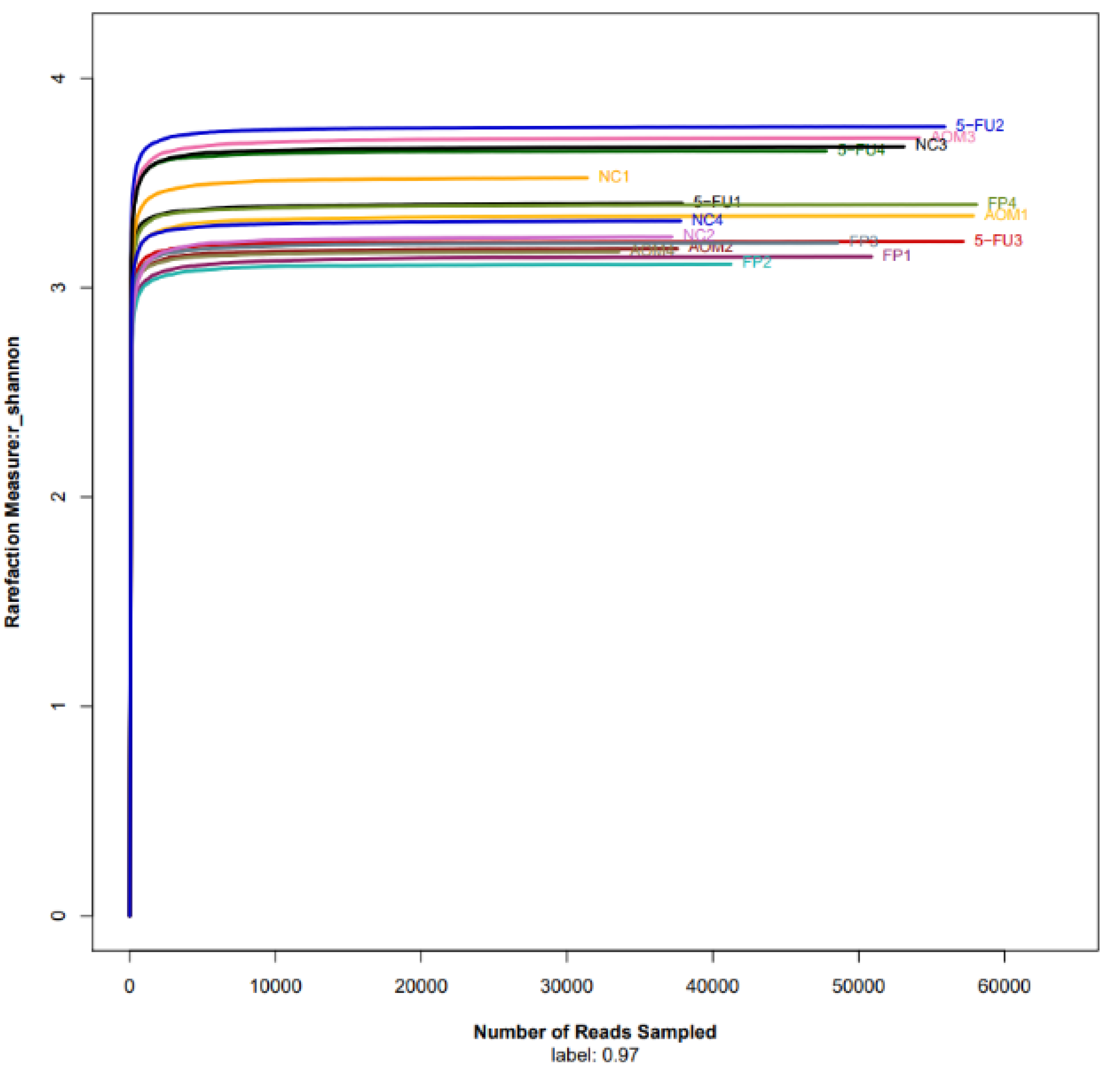
3.5. F. prausnitzii Influences Microbial Diversity of the Gut Microbiota in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, M.P.; Lansdorp-Vogelaar, I.; Goede, L.S.; Kuipers, E.J.; Dekker, E.; Stoker, J.; van Ballegooijen, M. Colorectal Cancer: Cost-Effectiveness of Colonoscopy Versus Ct Colonography Screening with Participation Rates and Costs. Radiology 2018, 287, 901–911. [Google Scholar] [CrossRef]
- Gandomani, H.S.; Aghajani, M.; Mohammadian-Hafshejani, A.; Tarazoj, A.A.; Pouyesh, V.; Salehiniya, H. Colorectal Cancer in the World: Incidence, Mortality and Risk Factors. Biomed. Res. Ther. 2017, 4, 1656–1675. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.G.; J Ahnen, D.J. Colorectal Cancer in the Young. Curr. Gastroenterol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [Green Version]
- Guandalini, S.; Sansotta, N. Probiotics in the Treatment of Inflammatory Bowel Disease. In Probiotics and Child Gastrointestinal Health; Springer: Berlin/Heidelberg, Germany, 2019; pp. 101–107. [Google Scholar]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Basile, M.; Nicolosi, D.; Genovese, C.; Libra, M.; Salmeri, M. Benefits of Using Probiotics as Adjuvants in Anticancer Therapy (Review). World Acad. Sci. J. 2019, 1, 125–135. [Google Scholar] [CrossRef]
- Ding, S.; Hu, C.; Fang, J.; Liu, G. The Protective Role of Probiotics against Colorectal Cancer. Oxidative Med. Cell. Longev. 2020, 2020, 8884583. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Wu, W.; Lv, L.; Bian, X.; Yang, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; et al. Administration of Bifidobacterium Bifidum Cgmcc 15068 Modulates Gut Microbiota and Metabolome in Azoxymethane (Aom)/Dextran Sulphate Sodium (Dss)-Induced Colitis-Associated Colon Cancer (Cac) in Mice. Appl. Microbiol. Biotechnol. 2020, 104, 5915–5928. [Google Scholar] [CrossRef]
- Yue, Y.; Ye, K.; Lu, J.; Wang, X.; Zhang, S.; Liu, L.; Yang, B.; Nassar, K.; Xu, X.; Pang, X. Probiotic Strain Lactobacillus Plantarum Yyc-3 Prevents Colon Cancer in Mice by Regulating the Tumour Microenvironment. Biomed. Pharmacother. 2020, 127, 110159. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, J.I.; Al-Kabsi, A.M.; Eid, E.E.M.; Hussin, S.; Alshawsh, M.A. Probiotics Supplementation in Patients with Colorectal Cancer: A Systematic Review of Randomized Controlled Trials. Nutr. Rev. 2021, 80, 22–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Wu, C.; Yu, J. The Role of Gut Microbiota in Cancer Treatment: Friend or Foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Saus, E.; Iraola-Guzmán, S.; Willis, J.R.; Brunet-Vega, A.; Gabaldón, T. Microbiome and Colorectal Cancer: Roles in Carcinogenesis and Clinical Potential. Mol. Asp. Med. 2019, 69, 93–106. [Google Scholar] [CrossRef]
- Ma, W.; Mao, Q.; Xia, W.; Dong, G.; Yu, C.; Jiang, F. Gut Microbiota Shapes the Efficiency of Cancer Therapy. Front. Microbiol. 2019, 10, 1050. [Google Scholar] [CrossRef] [Green Version]
- Fong, W.; Li, Q.; Yu, J. Gut Microbiota Modulation: A Novel Strategy for Prevention and Treatment of Colorectal Cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- He, X.; Zhao, S.; Li, Y. Faecalibacterium Prausnitzii: A Next-Generation Probiotic in Gut Disease Improvement. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6666114. [Google Scholar] [CrossRef]
- Rabiei, N.; Badi, S.A.; Marvasti, F.E.; Sattari, T.N.; Vaziri, F.; Siadat, S.D. Induction Effects of Faecalibacterium Prausnitzii and Its Extracellular Vesicles on Toll-Like Receptor Signaling Pathway Gene Expression and Cytokine Level in Human Intestinal Epithelial Cells. Cytokine 2019, 121, 154718. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium Prausnitzii: From Microbiology to Diagnostics and Prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Leylabadlo, E.H.; Ghotaslou, R.; Feizabadi, M.M.; Farajnia, S.; Moaddab, S.Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B. The Critical Role of Faecalibacterium Prausnitzii in Human Health: An Overview. Microb. Pathog. 2020, 149, 104344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium Prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, F.M.; Bahl, M.I.; Licht, T.R. Settlers of Our Inner Surface–Factors Shaping the Gut Microbiota from Birth to Toddlerhood. FEMS Microbiol. Rev. 2021, 45, fuab001. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, H.A.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, Å.V.; Söderholm, J.D. Faecalibacterium Prausnitzii Supernatant Improves Intestinal Barrier Function in Mice Dss Colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium Prausnitzii and Human Intestinal Health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the Anti-Cancer Potential of Probiotic Pediococcus Pentosaceus Gs4 against Colon Cancer: In Vitro and in Vivo Approaches. J. Funct. Foods 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Bird, R.P. Role of Aberrant Crypt Foci in Understanding the Pathogenesis of Colon Cancer. Cancer Lett. 1995, 93, 55–71. [Google Scholar] [CrossRef]
- Almagrami, A.A.; Alshawsh, M.A.; Saif-Ali, R.; Shwter, A.; Salem, S.D.; Abdulla, M.A. Evaluation of Chemopreventive Effects of Acanthus Ilicifolius against Azoxymethane-Induced Aberrant Crypt Foci in the Rat Colon. PLoS ONE 2014, 9, e96004. [Google Scholar] [CrossRef] [Green Version]
- Shwter, N.A.; Abdullah, N.A.; Alshawsh, M.A.; Alsalahi, A.; Hajrezaei, M.; Almaqrami, A.A.; Salem, S.D.; Abdulla, M.A. Chemoprevention of Colonic Aberrant Crypt Foci by Gynura Procumbens in Rats. J. Ethnopharmacol. 2014, 151, 1194–1201. [Google Scholar] [CrossRef]
- Chen, P.; Yang, S.; Hu, C.; Zhao, Z.; Liu, J.; Cheng, Y.; Wang, S.; Chen, Q.; Yu, P.; Zhang, X. Sargassum Fusiforme Polysaccharide Rejuvenat Es the Small Intestine in Mice through Altering Its Physiol Ogy and Gut Microbiota Composition. Curr. Mol. Med. 2017, 17, 350–358. [Google Scholar] [CrossRef]
- Kuugbee, D.E.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y.; et al. Structural Change in Microbiota by a Probiotic Cocktail Enhances the Gut Barrier and Reduces Cancer Via Tlr2 Signaling in a Rat Model of Colon Cancer. Dig. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Lo, S.N.; Wang, Y.; Li, L.; Christie, M.; Lee, M.; Wong, R.; Kosmider, S.; Skinner, I. Prognostic Significance of Postsurgery Circulating Tumor DNA in Nonmetastatic Colorectal Cancer: Individual Patient Pooled Analysis of Three Cohort Studies. Int. J. Cancer 2021, 148, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Hadjipetrou, A.; Anyfantakis, D.; Galanakis, C.G.; Kastanakis, M.; Kastanakis, S. Colorectal Cancer, Screening and Primary Care: A Mini Literature Review. World J. Gastroenterol. 2017, 23, 6049. [Google Scholar] [CrossRef] [PubMed]
- Hendler, R.; Zhang, Y. Probiotics in the Treatment of Colorectal Cancer. Medicines 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Prz. Gastroenterol. 2019, 14, 89. [Google Scholar] [CrossRef]
- Venegas, P.D.; de la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (Scfas)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B. Identification of an Anti-Inflammatory Protein from Faecalibacterium Prausnitzii, a Commensal Bacterium Deficient in Crohn’s Disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Jafari, B.; Nejad, R.A.K.; Vaziri, F.; Siadat, S.D. Evaluation of the effects of extracellular vesicles derived from Faecalibacterium prausnitzii on lung cancer cell line. Biologia 2019, 74, 889–898. [Google Scholar] [CrossRef]
- Ma, J.; Sun, L.; Liu, Y.; Ren, H.; Shen, Y.; Bi, F.; Wang, X. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 2020, 20, 82. [Google Scholar] [CrossRef]
- Clarke, J.M.; Young, G.P.; Topping, D.L.; Bird, A.R.; Cobiac, L.; Scherer, B.L.; Winkler, J.G.; Lockett, T.J. Butyrate delivered by butyrylated starch increases distal colonic epithelial apoptosis in carcinogen-treated rats. Carcinogenesis 2012, 33, 197–202. [Google Scholar] [CrossRef]
- Zhang, Y.-K.; Zhang, Q.; Wang, Y.-L.; Zhang, W.-Y.; Hu, H.-Q.; Wu, H.-Y.; Sheng, X.-Z.; Luo, K.-J.; Zhang, H.; Wang, M.; et al. A Comparison study of age and colorectal cancer-related gut bacteria. Front. Cell. Infect. Microbiol. 2021, 11, 606490. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.M.; Cha, J.M.; Shin, H.P.; Jeon, J.W.; Yoon, J.Y. Development of a Novel Metagenomic Biomarker for Prediction of Upper Gastrointestinal Tract Involvement in Patients with Crohn’s Disease. Front. Microbiol. 2020, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low Counts of Faecalibacterium Prausnitzii in Colitis Microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef] [PubMed]

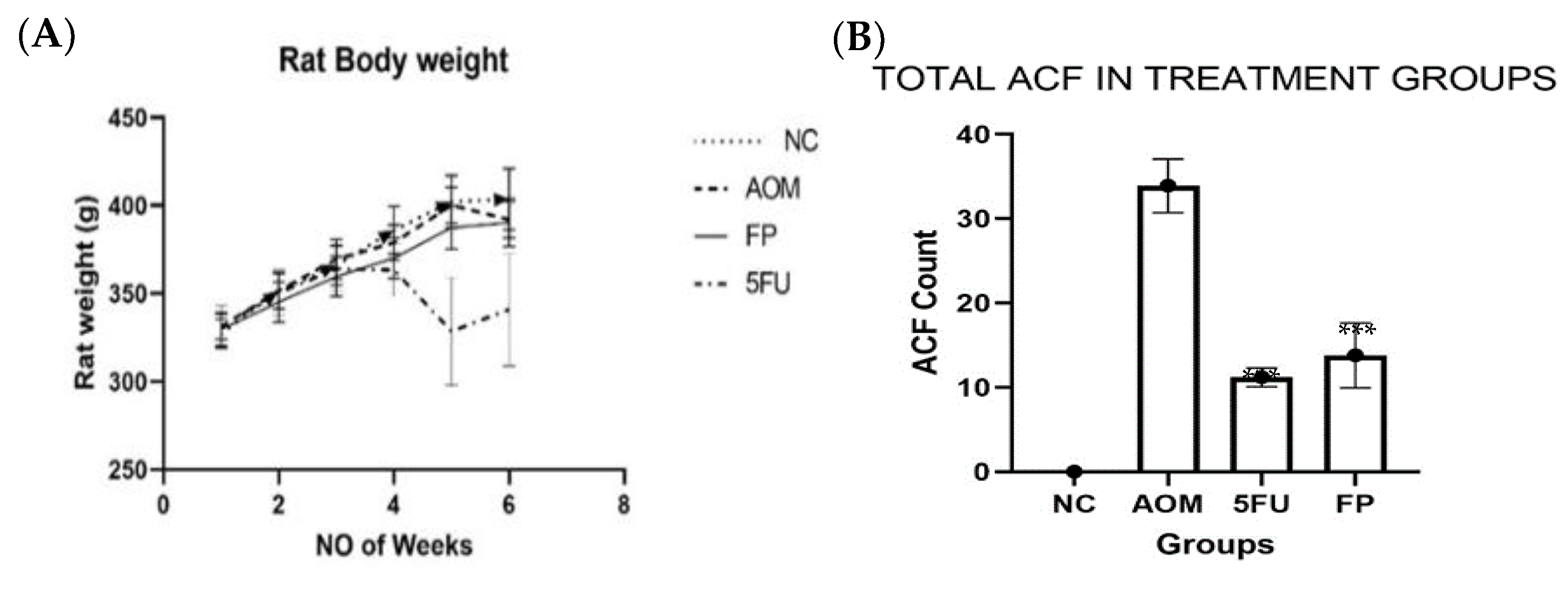
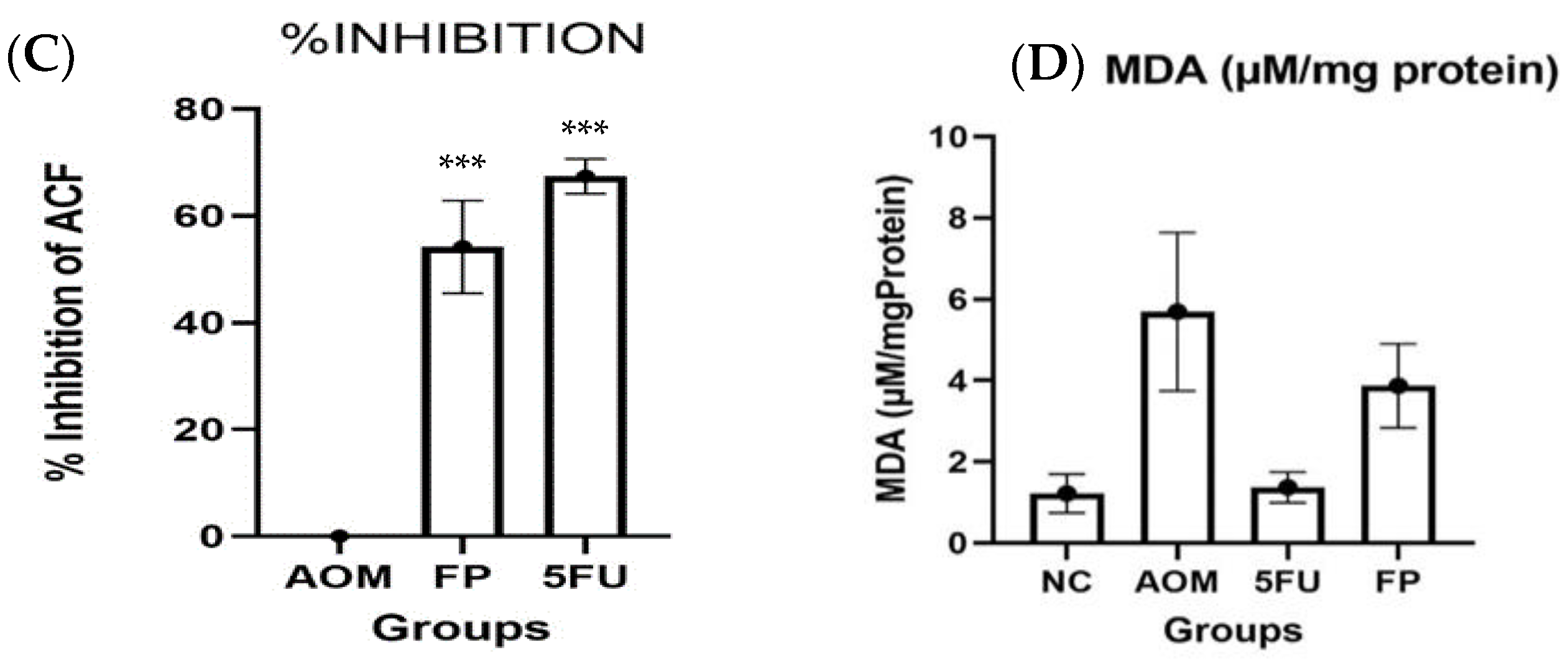
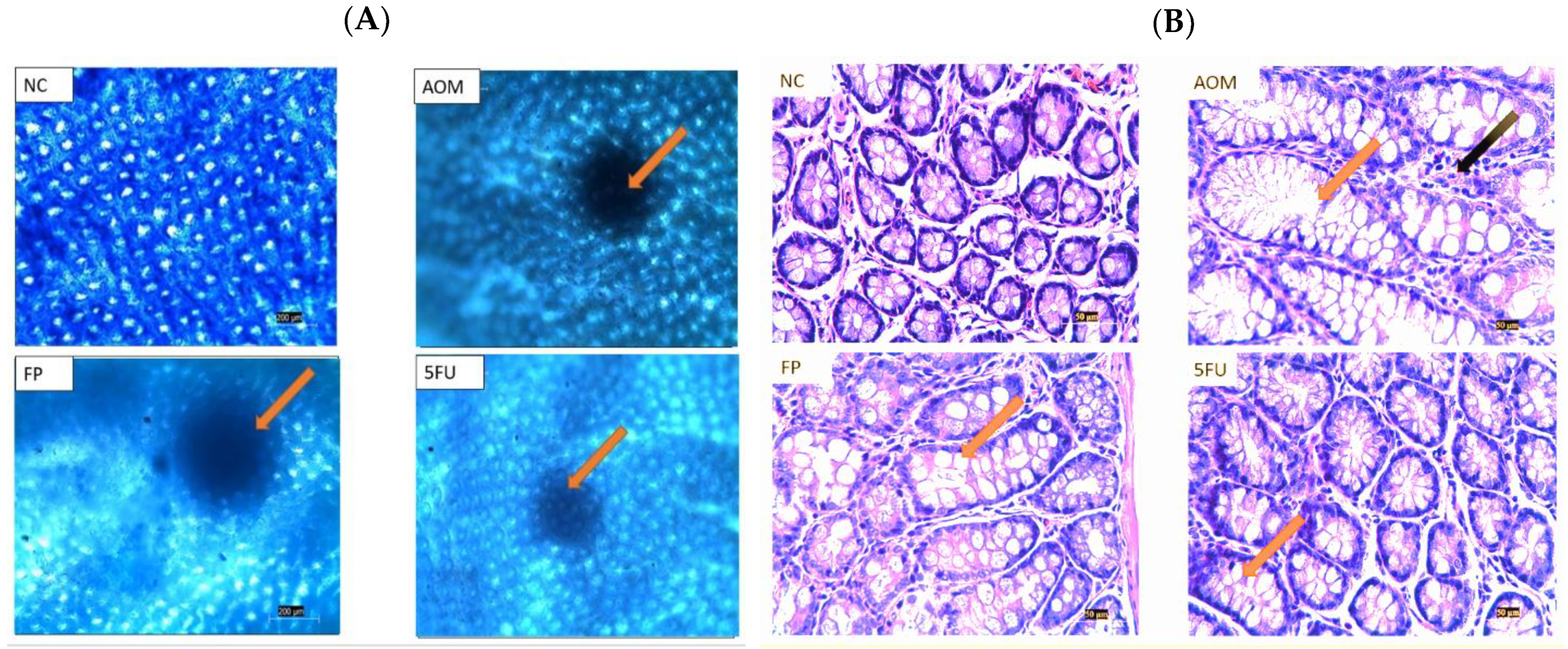


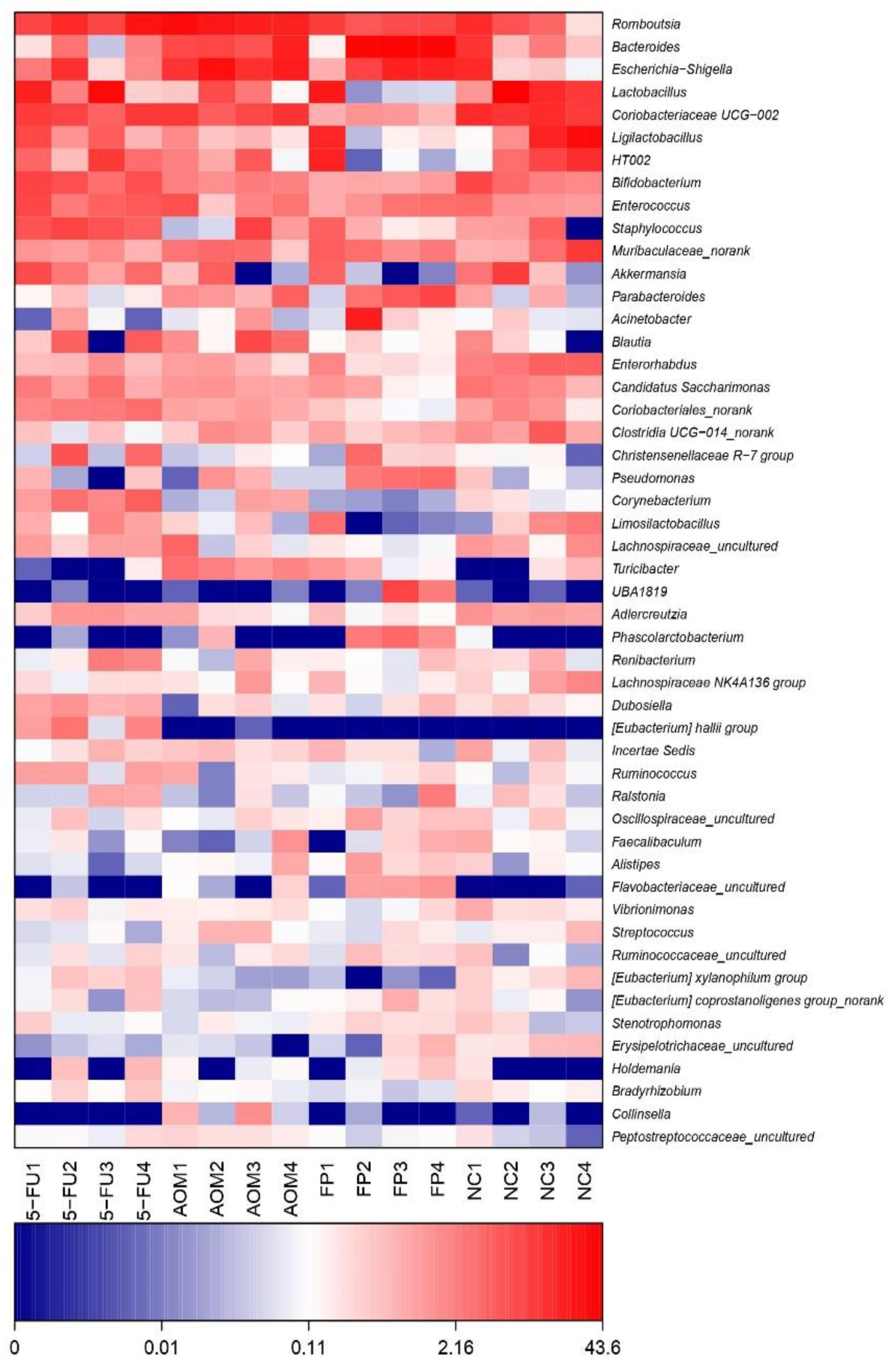

| Groups | Total ACF | % Inhibition |
|---|---|---|
| Normal control | 0 | 0 |
| AOM group | 33.9 ± 3.18 | 0 |
| F. prausnitzii group | 14.8 ± 3.86 *** | 56.3 *** |
| 5-FU group | 11.0 ± 1.10 *** | 67.5 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.-T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. https://doi.org/10.3390/biomedicines10051128
Dikeocha IJ, Al-Kabsi AM, Chiu H-T, Alshawsh MA. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines. 2022; 10(5):1128. https://doi.org/10.3390/biomedicines10051128
Chicago/Turabian StyleDikeocha, Ifeoma Julieth, Abdelkodose Mohammed Al-Kabsi, Hsien-Tai Chiu, and Mohammed Abdullah Alshawsh. 2022. "Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells" Biomedicines 10, no. 5: 1128. https://doi.org/10.3390/biomedicines10051128
APA StyleDikeocha, I. J., Al-Kabsi, A. M., Chiu, H.-T., & Alshawsh, M. A. (2022). Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines, 10(5), 1128. https://doi.org/10.3390/biomedicines10051128







