Alloferon Affects the Chemosensitivity of Pancreatic Cancer by Regulating the Expression of SLC6A14
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunofluorescence Microscopic Analysis
2.3. Western Blot Analysis
2.4. CCK-8 Assay
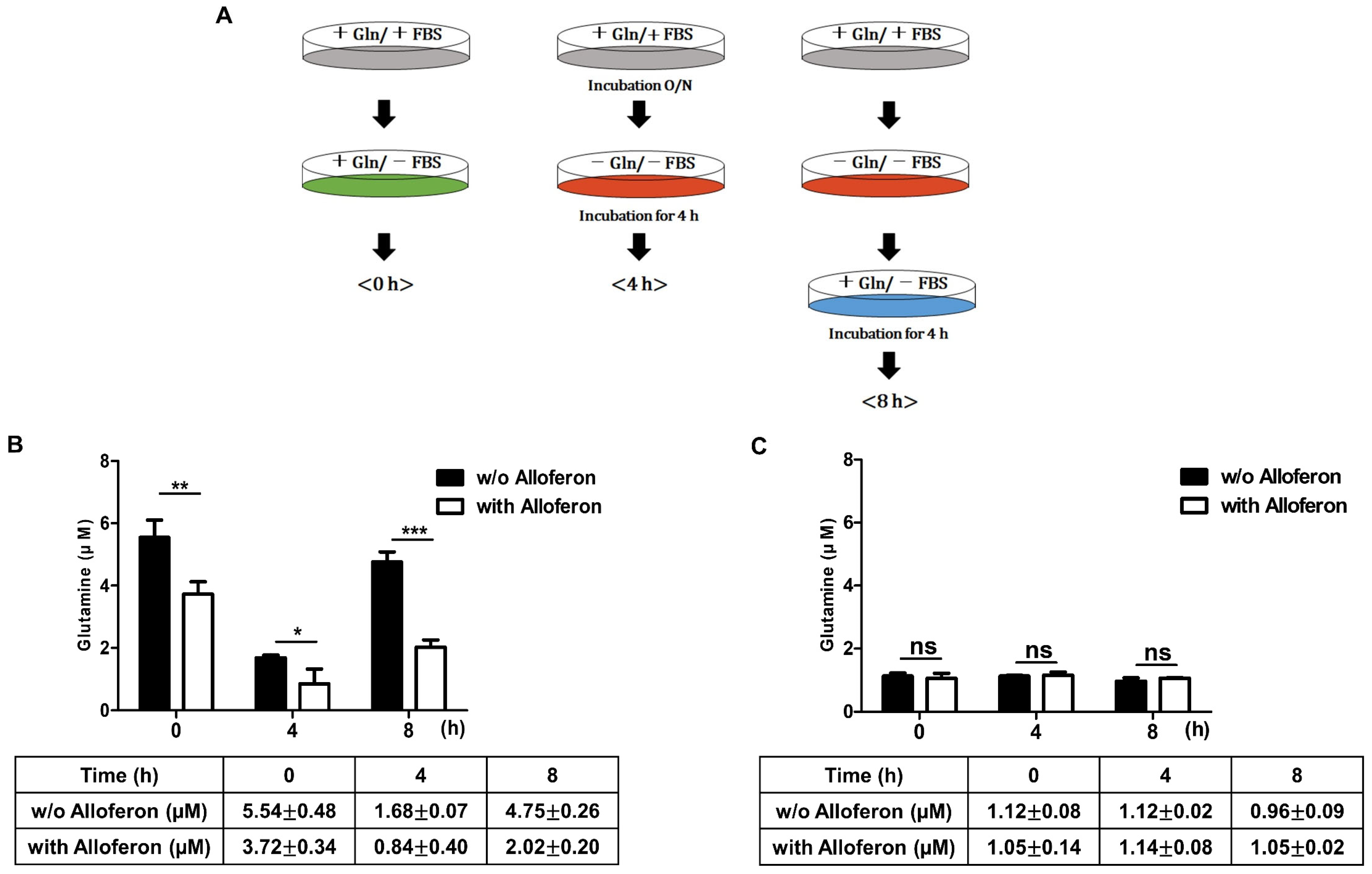
2.5. Glutamine Uptake Assay
2.6. Cell Cycle Analysis
2.7. Statistical Analysis
3. Results
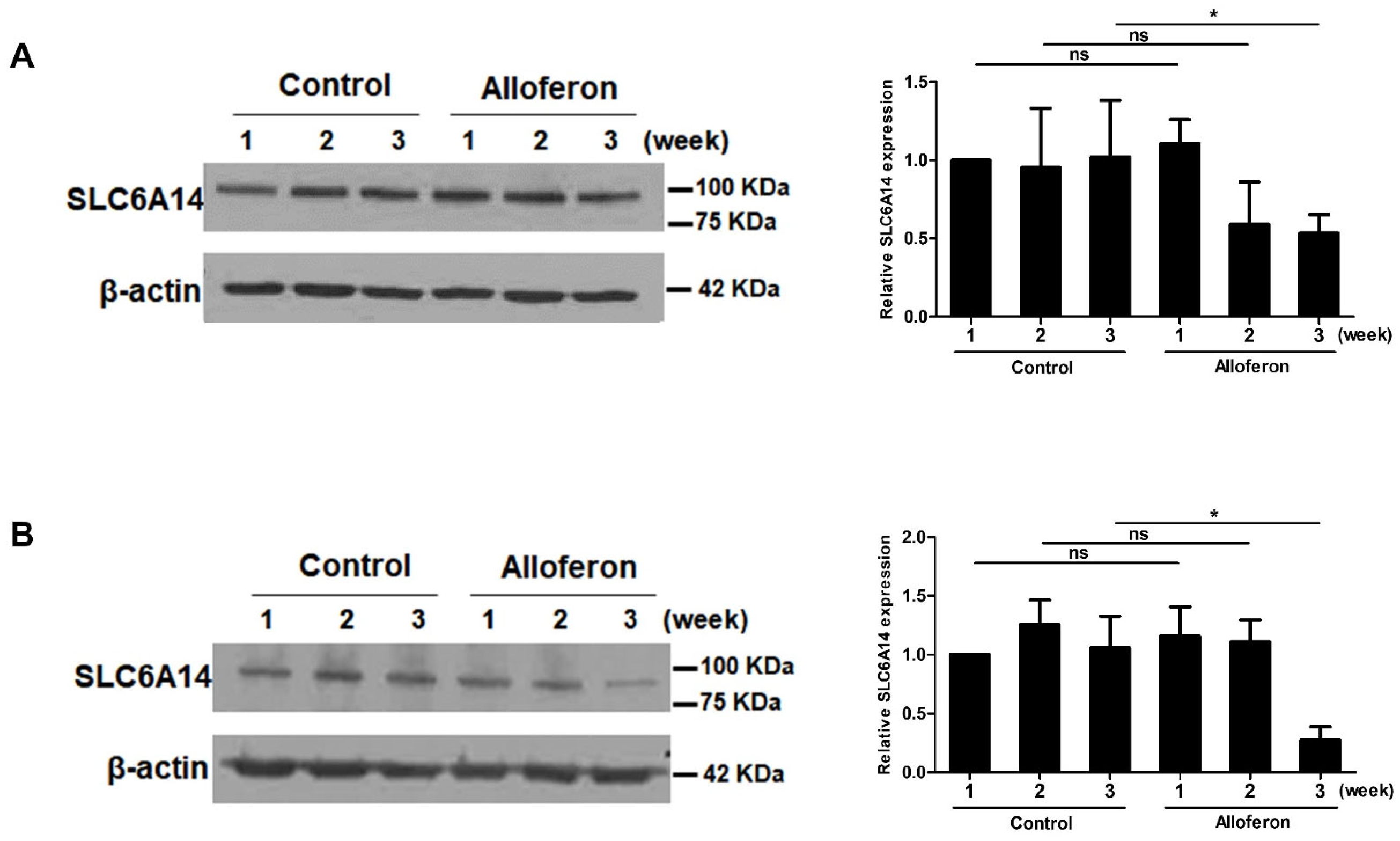
3.1. Alloferon Suppresses the Expression of SLC6A14 in PCa Cell Lines
3.2. Alloferon Increases the Chemosensitivity of Panc-1 and AsPC-1
3.3. Alloferon Inhibits Glutamine Uptake in PCa Cells
3.4. Co-Treatment of PCa Cells with Alloferon and Gemcitabine Alters Cell Cycle Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Available online: www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 2 March 2022).
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Schwarz, L.; Borbath, I.; Henry, A.; Van Laethem, J.-L.; Malka, D.; Ducreux, M.; Conroy, T. An update on treatment options for pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 2019, 11, 1758835919875568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanam, I.; Chung, V. Targeted therapies for pancreatic cancer. Cancers 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, A.G.; Pulido, C.F.; Machado, M.; Brito, M.J.; Couto, N.; Sousa, O.; Melo, S.A.; Mansinho, H. Treatment optimization of locally advanced and metastatic pancreatic cancer. Int. J. Oncol. 2021, 59, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, W. Pancreatic cancer: A review of risk factors, diagnosis, and treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Xia, G.; Lei, S.; Huang, X.; Huang, X. Survival of pancreatic cancer patients is negatively correlated with age at diagnosis: A population-based retrospective study. Sci. Rep. 2020, 10, 7048. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1. [Google Scholar]
- Newsholme, P.; Lima, M.; Procopio, J.; Pithon-Curi, T.; Bazotte, R.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Csibi, A.; Fendt, S.-M.; Li, C.; Poulogiannis, G.; Choo, A.Y.; Chapski, D.J.; Jeong, S.M.; Dempsey, J.M.; Parkhitko, A.; Morrison, T.; et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 2013, 153, 840–854. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.W.; Layden, B.T.; Chakrabarti, P. Inhibition of mTOR complexes protects cancer cells from glutamine starvation induced cell death by restoring Akt stability. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, J.L.; Guan, K.-L. Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 2013, 38, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Kovačević, Z.; Morris, H. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972, 32, 326–333. [Google Scholar]
- Nguyen, T.-L.; Durán, R.V. Glutamine metabolism in cancer therapy. Cancer Drug Resist. 2018, 1, 126–138. [Google Scholar] [CrossRef] [Green Version]
- El Ansari, R.; McIntyre, A.; Craze, M.L.; Ellis, I.O.; Rakha, E.A.; Green, A.R. Altered glutamine metabolism in breast cancer; subtype dependencies and alternative adaptations. Histopathology 2018, 72, 183–190. [Google Scholar] [CrossRef]
- Demas, D.M.; Demo, S.; Fallah, Y.; Clarke, R.; Nephew, K.P.; Althouse, S.; Sandusky, G.; He, W.; Shajahan-Haq, A.N. Glutamine metabolism drives growth in advanced hormone receptor positive breast cancer. Front. Oncol. 2019, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.; Haanstra, J.R.; Engqvist, M.; Gerding, A.; Bakker, B.M.; Klingmüller, U.; Teusink, B.; Nielsen, J. Quantitative analysis of amino acid metabolism in liver cancer links glutamate excretion to nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2020, 117, 10294–10304. [Google Scholar] [CrossRef]
- Vanhove, K.; Derveaux, E.; Graulus, G.-J.; Mesotten, L.; Thomeer, M.; Noben, J.-P.; Guedens, W.; Adriaensens, P. Glutamine addiction and therapeutic strategies in lung cancer. Int. J. Mol. Sci. 2019, 20, 252. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Sheng, X.; Willson, A.K.; Roque, D.R.; Stine, J.E.; Guo, H.; Jones, H.M.; Zhou, C.; Bae-Jump, V.L. Glutamine promotes ovarian cancer cell proliferation through the mTOR/S6 pathway. Endocr.-Relat. Cancer 2015, 22, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Hampton, T. Blocking Glutamine Metabolism Shrinks Kidney Cancers in Mice. JAMA 2015, 314, 16. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine metabolism in cancer: Understanding the heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, R.; Taylor, P.M.; Hundal, H.S. Amino acid transporters: Roles in amino acid sensing and signalling in animal cells. Biochem. J. 2003, 373, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef] [Green Version]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2531–2539. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Babu, E.; Prasad, P.D.; Ganapathy, V. The amino acid transporter SLC6A14 in cancer and its potential use in chemotherapy. Asian J. Pharm. Sci. 2014, 9, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Miyauchi, S.; Martindale, R.G.; Herdman, A.V.; Podolsky, R.; Miyake, K.; Mager, S.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Upregulation of the amino acid transporter ATB0,+(SLC6A14) in colorectal cancer and metastasis in humans. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2005, 1741, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Prasad, P.D.; Ghamande, S.; Moore-Martin, P.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Mager, S.; Ganapathy, M.E.; Ganapathy, V. Up-regulation of the amino acid transporter ATB0,+(SLC6A14) in carcinoma of the cervix. Gynecol. Oncol. 2006, 100, 8–13. [Google Scholar] [CrossRef]
- Karunakaran, S.; Umapathy, N.S.; Thangaraju, M.; Hatanaka, T.; Itagaki, S.; Munn, D.H.; Prasad, P.D.; Ganapathy, V. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem. J. 2008, 414, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, S.; Ramachandran, S.; Coothankandaswamy, V.; Elangovan, S.; Babu, E.; Periyasamy-Thandavan, S.; Gurav, A.; Gnanaprakasam, J.P.; Singh, N.; Schoenlein, P.V.; et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J. Biol. Chem. 2011, 286, 31830–31838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penheiter, A.R.; Erdogan, S.; Murphy, S.J.; Hart, S.N.; Felipe Lima, J.; Rakhshan Rohakhtar, F.; O’Brien, D.R.; Bamlet, W.; Wuertz, R.E.; Smyrk, T.C.; et al. Transcriptomic and immunohistochemical profiling of SLC6A14 in pancreatic ductal adenocarcinoma. BioMed Res. Int. 2015, 2015, 593572. [Google Scholar] [CrossRef] [PubMed]
- Coothankandaswamy, V.; Cao, S.; Xu, Y.; Prasad, P.; Singh, P.; Reynolds, C.; Yang, S.; Ogura, J.; Ganapathy, V.; Bhutia, Y.D. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 2016, 173, 3292–3306. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Cheng, D.; Hou, L.; Zhou, S.; Ying, T.; Yang, Q. SLC3A2 is upregulated in human osteosarcoma and promotes tumor growth through the PI3K/Akt signaling pathway. Oncol. Rep. 2017, 37, 2575–2582. [Google Scholar] [CrossRef] [Green Version]
- Chernysh, S.; Kim, S.; Bekker, G.; Pleskach, V.; Filatova, N.; Anikin, V.; Platonov, V.G.; Bulet, P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 2002, 99, 12628–12632. [Google Scholar] [CrossRef] [Green Version]
- Kuczer, M.; Dziubasik, K.; Midak-Siewirska, A.; Zahorska, R.; Łuczak, M.; Konopińska, D. Studies of insect peptides alloferon, Any-GS and their analogues. Synthesis and antiherpes activity. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2010, 16, 186–189. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.K.; Bae, S.; Kim, H.; Park, Y.; Chu, N.K.; Kim, S.G.; Kim, H.-R.; Hwang, Y.-I.; Kang, J.S.; et al. The anti-inflammatory effect of alloferon on UVB-induced skin inflammation through the down-regulation of pro-inflammatory cytokines. Immunol. Lett. 2013, 149, 110–118. [Google Scholar] [CrossRef]
- Chernysh, S.; Irina, K.; Irina, A. Anti-tumor activity of immunomodulatory peptide alloferon-1 in mouse tumor transplantation model. Int. Immunopharmacol. 2012, 12, 312–314. [Google Scholar] [CrossRef]
- Bae, S.; Oh, K.; Kim, H.; Kim, Y.; Kim, H.-R.; Hwang, Y.-i.; Lee, D.-S.; Kang, J.S.; Lee, W.J. The effect of alloferon on the enhancement of NK cell cytotoxicity against cancer via the up-regulation of perforin/granzyme B secretion. Immunobiology 2013, 218, 1026–1033. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, Y.; Kim, H.; Kang, J.S.; Lee, W.J. Anti-inflammatory effect of alloferon on ovalbumin-induced asthma. Immune Netw. 2015, 15, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, A.; Zheng, H.; Chen, Z.; Lin, X.; Li, C.; Yao, Q.; Bhutia, Y.D.; Ganapathy, V.; Chen, R.; Kou, L. Synergism between SLC6A14 blockade and gemcitabine in pancreactic cancer: A 1H-NMR-based metabolomic study in pancreatic cancer cells. Biochem. J. 2020, 477, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, H.; Scheibling, U.; Bratthäll, C.; Green, H.; Elander, N.O. Real world evidence on gemcitabine and nab-paclitaxel combination chemotherapy in advanced pancreatic cancer. BMC Cancer 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Teng, C.; Li, T. Combination therapy versus gemcitabine monotherapy in the treatment of elderly pancreatic cancer: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2018, 12, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Fang, J.; Luo, G. Meta-analysis of gemcitabine and cisplatin combination chemotherapy versus gemcitabine alone for pancreatic cancer. J. Cancer Res. Ther. 2016, 12, 104. [Google Scholar]
- Palacio, S.; Hosein, P.J.; Reis, I.; Akunyili, I.I.; Ernani, V.; Pollack, T.; Macintyre, J.; Restrepo, M.H.; Merchan, J.R.; Lima, C.M.R. The nab-paclitaxel/gemcitabine regimen for patients with refractory advanced pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2018, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Huanwen, W.; Zhiyong, L.; Xiaohua, S.; Xinyu, R.; Kai, W.; Tonghua, L. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Mol. Cancer 2009, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.-U.; Kim, J.W.; Sung, J.H.; Kang, M.H.; Kim, S.-H.; Chang, H.; Lee, J.-O.; Kim, Y.J.; Lee, K.-W.; Kim, J.H.; et al. p21-activated kinase 4 (PAK4) as a predictive marker of gemcitabine sensitivity in pancreatic cancer cell lines. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2015, 47, 501. [Google Scholar] [CrossRef]
- Bott, A.J.; Maimouni, S.; Zong, W.-X. The pleiotropic effects of glutamine metabolism in cancer. Cancers 2019, 11, 770. [Google Scholar] [CrossRef] [Green Version]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Michalak, K.P.; Maćkowska-Kędziora, A.; Sobolewski, B.; Woźniak, P. Key roles of glutamine pathways in reprogramming the cancer metabolism. Oxidative Med. Cell. Longev. 2015, 2015, 964321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikder, M.O.; Sivaprakasam, S.; Brown, T.P.; Thangaraju, M.; Bhutia, Y.D.; Ganapathy, V. SLC6A14, a Na+/Cl−-coupled amino acid transporter, functions as a tumor promoter in colon and is a target for Wnt signaling. Biochem. J. 2020, 477, 1409–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikder, M.O.F.; Yang, S.; Ganapathy, V.; Bhutia, Y.D. The Na+/Cl−-coupled, broad-specific, amino acid transporter SLC6A14 (ATB 0,+): Emerging roles in multiple diseases and therapeutic potential for treatment and diagnosis. AAPS J. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Babu, E.; Bhutia, Y.D.; Ramachandran, S.; Gnanaprakasam, J.P.; Prasad, P.D.; Thangaraju, M.; Ganapathy, V. Deletion of the amino acid transporter Slc6a14 suppresses tumour growth in spontaneous mouse models of breast cancer. Biochem. J. 2015, 469, 17–23. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Lee, D.; Go, C.; Jang, Y.; Bae, S.; Agura, T.; Hong, J.; Kang, D.; Kim, Y.; Kang, J.S. Alloferon Affects the Chemosensitivity of Pancreatic Cancer by Regulating the Expression of SLC6A14. Biomedicines 2022, 10, 1113. https://doi.org/10.3390/biomedicines10051113
Jo H, Lee D, Go C, Jang Y, Bae S, Agura T, Hong J, Kang D, Kim Y, Kang JS. Alloferon Affects the Chemosensitivity of Pancreatic Cancer by Regulating the Expression of SLC6A14. Biomedicines. 2022; 10(5):1113. https://doi.org/10.3390/biomedicines10051113
Chicago/Turabian StyleJo, Hyejung, Dahae Lee, Cheolhyeon Go, Yoojin Jang, Suhyun Bae, Tomoyo Agura, Jiye Hong, Dongmin Kang, Yejin Kim, and Jae Seung Kang. 2022. "Alloferon Affects the Chemosensitivity of Pancreatic Cancer by Regulating the Expression of SLC6A14" Biomedicines 10, no. 5: 1113. https://doi.org/10.3390/biomedicines10051113
APA StyleJo, H., Lee, D., Go, C., Jang, Y., Bae, S., Agura, T., Hong, J., Kang, D., Kim, Y., & Kang, J. S. (2022). Alloferon Affects the Chemosensitivity of Pancreatic Cancer by Regulating the Expression of SLC6A14. Biomedicines, 10(5), 1113. https://doi.org/10.3390/biomedicines10051113






