Autologous Immune Cell-Based Regenerative Therapies to Treat Vasculogenic Erectile Dysfunction: Is the Immuno-Centric Revolution Ready for the Prime Time?
Abstract
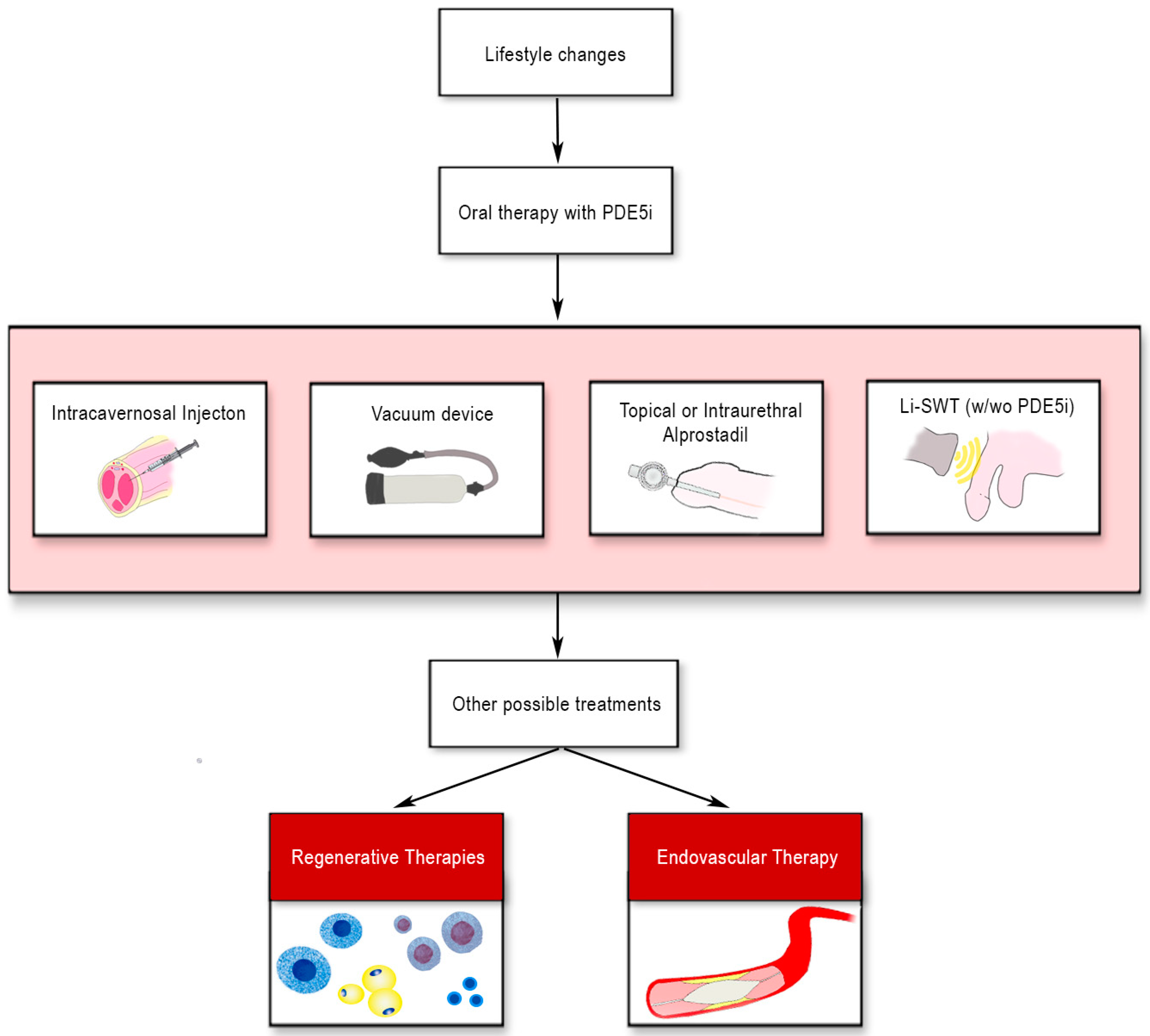
:1. Introduction
2. Erectile Dysfunction in Patients Affected by Diabetes
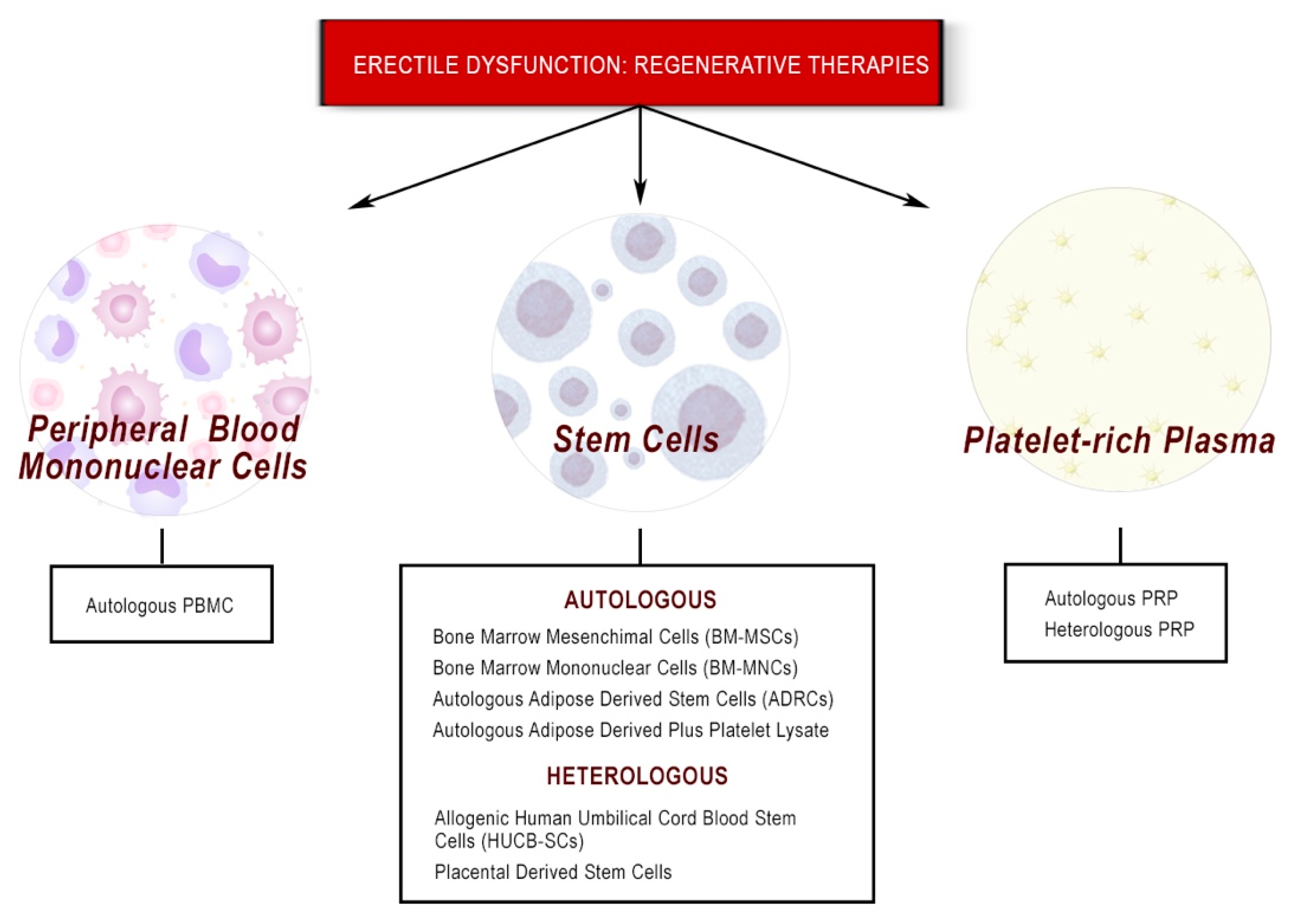
3. Stem Cell Therapy and Erectile Dysfunction
3.1. Heterologous Stem Cell Therapies: Cord Blood/Placenta Derived
3.2. Autologous Stem Cell Therapies: Bone Marrow and Adipose Tissue
4. Platelet-Rich Plasma Therapy
5. Harnessing the Immune System for Tissue Repair and Regeneration: A Lesson from the Heart
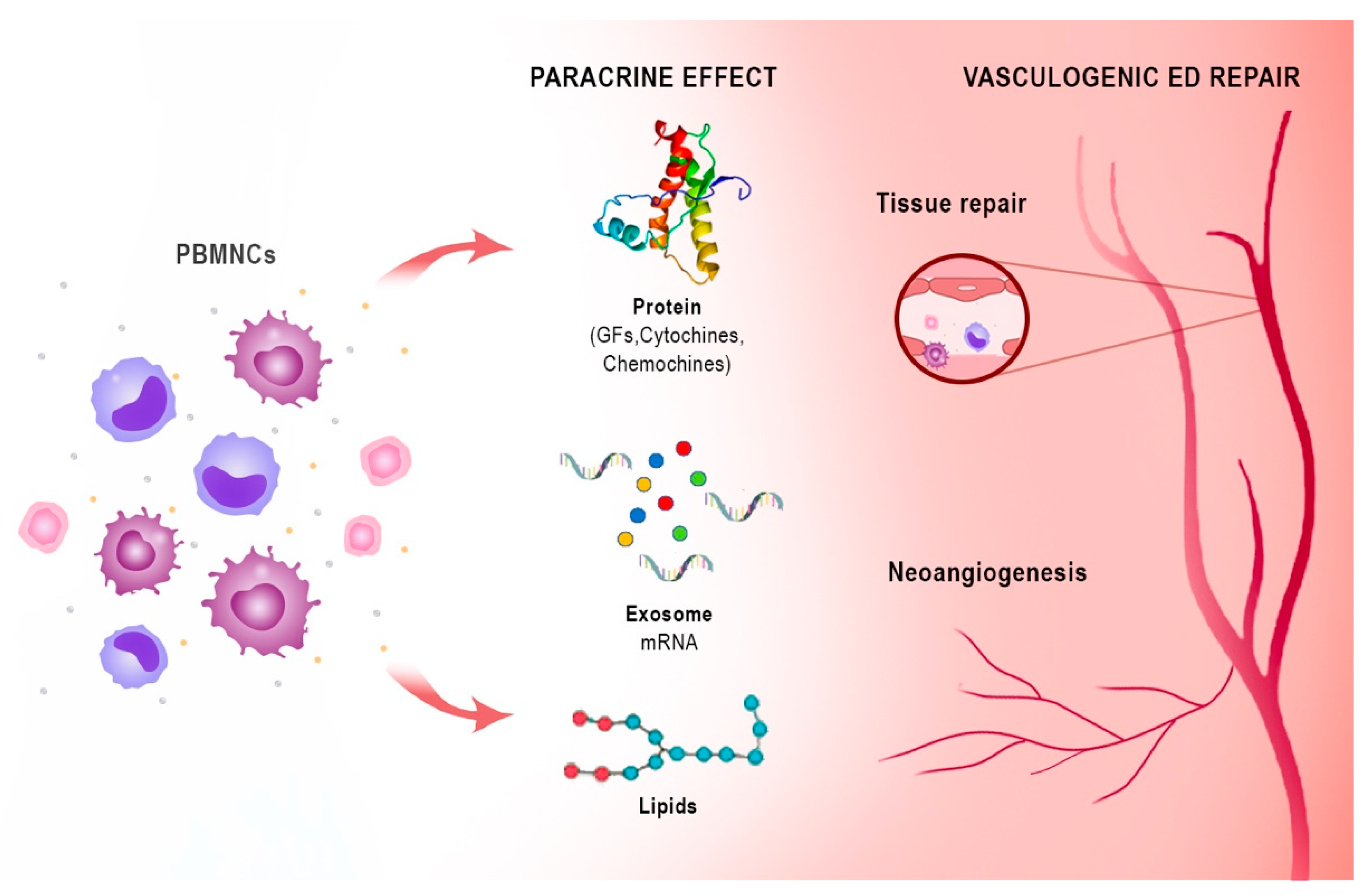
6. Harness Peripheral Blood Mononuclear Cells Angiogenic Potency: From Critical Limb Ischemia to ED
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brotons, F.B.; Campos, J.C.; Gonzalez-Correales, R.; Martín-Morales, A.; Moncada, I.; Pomerol, J.M. Core document on erectile dysfunction: Key aspects in the care of a patient with erectile dysfunction. Int. J. Impot Res. 2004, 16 (Suppl. 2), S26–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropolous, K.; Gül, M.; et al. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 25, 333–357. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Nehra, A.; Breau, R.H.; Culkin, D.J.; Faraday, M.M.; Hakim, L.S.; Heidelbaugh, J.; Khera, M.; McVary, K.T.; Miner, M.M.; et al. Erectile Dysfunction: AUA Guideline. J. Urol. 2018, 200, 633–641. [Google Scholar] [CrossRef]
- McMahon, C.N.; Smith, C.J.; Shabsigh, R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ 2006, 332, 589–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penson, D.F.; Latini, D.M.; Lubeck, D.P.; Wallace, K.L.; Henning, J.M.; Lue, T.F. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care 2003, 26, 1093–1099. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, L.; Marfella, M.A.; Siniscalchi, M.; Marino, L.; Nappo, F.; Giugliano, F.; De Lucia, D.; Giugliano, D. Erectile and endothelial dysfunction in Type II diabetes: A possible link. Diabetologia 2001, 44, 1155–1160. [Google Scholar] [CrossRef] [Green Version]
- Chitaley, K. Type 1 and Type 2 diabetic-erectile dysfunction: Same diagnosis (ICD-9), different disease? J. Sex. Med. 2009, 6 (Suppl. 3), 262–268. [Google Scholar] [CrossRef]
- Chitaley, K.; Kupelian, V.; Subak, L.; Wessells, H. Diabetes, obesity and erectile dysfunction: Field overview and research priorities. J. Urol. 2009, 182 (Suppl. 6), S45–S50. [Google Scholar] [CrossRef] [Green Version]
- Feldman, H.A.; Goldstein, I.; Hatzichristou, D.G.; Krane, R.J.; McKinlay, J.B. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J Urol. 1994, 151, 54–61. [Google Scholar] [CrossRef]
- Lehman, T.P.; Jacobs, J.A. Etiology of diabetic impotence. J. Urol. 1983, 129, 291–294. [Google Scholar] [CrossRef]
- Koncz, L.; Balodimos, M.C. Impotence in diabetes mellitus. Med. Times 1970, 98, 159–170. [Google Scholar] [PubMed]
- Whitehead, E.D.; Klyde, B.J. Diabetes-related impotence in the elderly. Clin. Geriatr. Med. 1990, 6, 771–795. [Google Scholar] [CrossRef]
- Ganz, P.; Vita, J.A. Testing endothelial vasomotor function: Nitric oxide, a multipotent molecule. Circulation 2003, 108, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Azadzoi, K.M.; Goldstein, I.; Saenz de Tejada, I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Investig. 1991, 88, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cellek, S.; Rodrigo, J.; Lobos, E.; Fernández, P.; Serrano, J.; Moncada, S. Selective nitrergic neurodegeneration in diabetes mellitus—A nitric oxide-dependent phenomenon. Br. J. Pharmacol. 1999, 128, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Role of central nitric oxide in the control of penile erection and yawning. Prog. Neuropsychopharmacol. Biol. Psychiatry 1997, 21, 899–922. [Google Scholar] [CrossRef]
- Cartledge, J.J.; Eardley, I.; Morrison, J.F. Nitric oxide-mediated corpus cavernosal smooth muscle relaxation is impaired in ageing and diabetes. BJU Int. 2001, 87, 394–401. [Google Scholar] [CrossRef]
- Saenz de Tejada, I.; Goldstein, I.; Azadzoi, K.; Krane, R.J.; Cohen, R.A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N. Engl. J. Med. 1989, 320, 1025–1030. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Champion, H.C.; Usta, M.F.; Cellek, S.; Chitaley, K.; Webb, R.C.; Lewis, R.L.; Mills, T.M.; Hellstrom, W.J.; Kadowitz, P.J. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: A mechanism for diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. USA 2004, 101, 9121–9126. [Google Scholar] [CrossRef] [Green Version]
- Vita, J.A.; Keaney, J.F., Jr. Endothelial function: A barometer for cardiovascular risk? Circulation 2002, 106, 640–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007, 583 Pt 1, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Cignarelli, A.; Genchi, V.A.; D’Oria, R.; Giordano, F.; Caruso, I.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Role of Glucose-Lowering Medications in Erectile Dysfunction. J. Clin. Med. 2021, 10, 2501. [Google Scholar] [CrossRef]
- Morano, S.; Gatti, A.; Mandosi, E.; Tiberti, C.; Fallarino, M.; Cipriani, R.; Buchetti, B.; Gandini, L.; Sgrò, P.; Jannini, E.A.; et al. Circulating monocyte oxidative activity is increased in patients with type 2 diabetes and erectile dysfunction. J. Urol. 2007, 177, 655–659. [Google Scholar] [CrossRef]
- McMahon, C.G. Current diagnosis and management of erectile dysfunction. Med. J. Aust. 2019, 210, 469–476. [Google Scholar] [CrossRef]
- Walsh, T.J.; Hotaling, J.M.; Smith, A.; Saigal, C.; Wessells, H. Men with diabetes may require more aggressive treatment for erectile dysfunction. Int. J. Impot. Res. 2014, 26, 112–115. [Google Scholar] [CrossRef]
- Hatzimouratidis, K.; Hatzichristou, D.G. Phosphodiesterase type 5 inhibitors: Unmet needs. Curr. Pharm. Des. 2009, 15, 3476–3485. [Google Scholar] [CrossRef]
- Diehm, N.; Marggi, S.; Ueki, Y.; Schumacher, D.; Keo, H.H.; Regli, C.; Do, D.D.; Moeltgen, T.; Grimsehl, P.; Wyler, S.; et al. Endovascular Therapy for Erectile Dysfunction-Who Benefits Most? Insights From a Single-Center Experience. J. Endovasc. Ther. 2019, 26, 181–190. [Google Scholar] [CrossRef]
- Shan, H.; Chen, F.; Zhang, T.; He, S.; Xu, L.; Wei, A. Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0121428. [Google Scholar] [CrossRef]
- Sun, D.Z.; Abelson, B.; Babbar, P.; Damaser, M.S. Harnessing the mesenchymal stem cell secretome for regenerative urology. Nat. Rev. Urol. 2019, 16, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lin, H.; Yu, W.; Li, X.; Chen, Y.; Qiu, X.; Wang, R.; Dai, Y. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int. J. Androl. 2012, 35, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Korf-Klingebiel, M.; Kempf, T.; Sauer, T.; Brinkmann, E.; Fischer, P.; Meyer, G.P.; Ganser, A.; Drexler, H.; Wollert, K.C. Bone marrow cells are a rich source of growth factors and cytokines: Implications for cell therapy trials after myocardial infarction. Eur. Heart J. 2008, 29, 2851–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, L.; Mildner, M.; Gyöngyösi, M.; Ankersmit, H.J. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis 2016, 21, 1336–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.M.; Fandel, T.M.; Lin, G.; Shindel, A.W.; Banie, L.; Lin, C.S.; Lue, T.F. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J. Sex. Med. 2010, 7 Pt 1, 89–98. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Ruan, Y.; Wang, T.; Liu, J. Stem Cell Therapy for Diabetic Erectile Dysfunction in Rats: A Meta-Analysis. PLoS ONE 2016, 11, e0154341. [Google Scholar] [CrossRef]
- Bahk, J.Y.; Jung, J.H.; Han, H.; Min, S.K.; Lee, Y.S. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: Preliminary report of 7 cases. Exp. Clin. Transpl. 2010, 8, 150–160. [Google Scholar]
- Levy, J.A.; Marchand, M.; Iorio, L.; Cassini, W.; Zahalsky, M.P. Determining the Feasibility of Managing Erectile Dysfunction in Humans With Placental-Derived Stem Cells. J. Am. Osteopath. Assoc. 2016, 116, e1–e5. [Google Scholar] [CrossRef] [Green Version]
- Yiou, R.; Hamidou, L.; Birebent, B.; Bitari, D.; Lecorvoisier, P.; Contremoulins, I.; Khodari, M.; Rodriguez, A.M.; Augustin, D.; Roudot-Thoraval, F.; et al. Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postradical Prostatectomy Erectile Dysfunction: An Open Dose-Escalation Pilot Study. Eur. Urol. 2016, 69, 988–991. [Google Scholar] [CrossRef]
- Yiou, R.; Hamidou, L.; Birebent, B.; Bitari, D.; Le Corvoisier, P.; Contremoulins, I.; Rodriguez, A.M.; Augustin, D.; Roudot-Thoraval, F.; de la Taille, A.; et al. Intracavernous Injections of Bone Marrow Mononucleated Cells for Postradical Prostatectomy Erectile Dysfunction: Final Results of the INSTIN Clinical Trial. Eur. Urol. Focus. 2017, 3, 643–645. [Google Scholar] [CrossRef]
- Al Demour, S.; Jafar, H.; Adwan, S.; AlSharif, A.; Alhawari, H.; Alrabadi, A.; Zayed, A.; Jaradat, A.; Awidi, A. Safety and Potential Therapeutic Effect of Two Intracavernous Autologous Bone Marrow Derived Mesenchymal Stem Cells injections in Diabetic Patients with Erectile Dysfunction: An Open Label Phase I Clinical Trial. Urol. Int. 2018, 101, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Bieri, M.; Said, E.; Antonini, G.; Dickerson, D.; Tuma, J.; Bartlett, C.E.; Patel, A.N.; Gershman, A. Phase I and registry study of autologous bone marrow concentrate evaluated in PDE5 inhibitor refractory erectile dysfunction. J. Transl. Med. 2020, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Haahr, M.K.; Harken Jensen, C.; Toyserkani, N.M.; Andersen, D.C.; Damkier, P.; Sørensen, J.A.; Sheikh, S.P.; Lund, L. A 12-Month Follow-up After a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. Urology 2018, 121, 203.e6–203.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protogerou, V.; Michalopoulos, E.; Mallis, P.; Gontika, I.; Dimou, Z.; Liakouras, C.; Stavropoulos-Giokas, C.; Kostakopoulos, N.; Chrisofos, M.; Deliveliotis, C. Administration of Adipose Derived Mesenchymal Stem Cells and Platelet Lysate in Erectile Dysfunction: A Single Center Pilot Study. Bioengineering 2019, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protogerou, V.; Beshari, S.E.; Michalopoulos, E.; Mallis, P.; Chrysikos, D.; Samolis, A.A.; Stavropoulos-Giokas, C.; Troupis, T. The Combined Use of Stem Cells and Platelet Lysate Plasma for the Treatment of Erectile Dysfunction: A Pilot Study-6 Months Results. Medicines 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magenta, A.; Florio, M.C.; Ruggeri, M.; Furgiuele, S. Autologous cell therapy in diabetes-associated critical limb ischemia: From basic studies to clinical outcomes (Review). Int. J. Mol. Med. 2021, 48, 173. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Spinetti, G.; Santopaolo, M.; Madeddu, P. Impaired Regeneration Contributes to Poor Outcomes in Diabetic Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jarajapu, Y.P.; Hazra, S.; Segal, M.; Li Calzi, S.; Jadhao, C.; Qian, K.; Mitter, S.K.; Raizada, M.K.; Boulton, M.E.; Grant, M.B. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS ONE 2014, 9, e93965, Erratum in: PLoS ONE 2014, 9, e103913. LiCalzi, Sergio [corrected to Li Calzi, Sergio]; Jhadao, Chandra [corrected to Jadhao, Chandra].. [Google Scholar] [CrossRef]
- Wang, S.K.; Green, L.A.; Motaganahalli, R.L.; Wilson, M.G.; Fajardo, A.; Murphy, M.P. Rationale and design of the MarrowStim PAD Kit for the Treatment of Critical Limb Ischemia in Subjects with Severe Peripheral Arterial Disease (MOBILE) trial investigating autologous bone marrow cell therapy for critical limb ischemia. J. Vasc. Surg. 2017, 65, 1850–1857.e2. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Jiang, Y.; Deng, W.; Zhang, Y.; Liang, Z.; Wu, Q.; Jiang, X.; Zhang, L.; Gao, F.; Cao, Y.; et al. Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischemia and Foot Ulcer in Patients with Diabetes. Cell Transplant. 2019, 28, 645–652. [Google Scholar] [CrossRef]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Inoue, O.; Usui, S.; Takashima, S.I.; Nomura, A.; Yamaguchi, K.; Takeda, Y.; Goten, C.; Hamaoka, T.; Ootsuji, H.; Murai, H.; et al. Diabetes impairs the angiogenic capacity of human adipose-derived stem cells by reducing the CD271+ subpopulation in adipose tissue. Biochem. Biophys. Res. Commun. 2019, 517, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Rennert, R.C.; Sorkin, M.; Januszyk, M.; Duscher, D.; Kosaraju, R.; Chung, M.T.; Lennon, J.; Radiya-Dixit, A.; Raghvendra, S.; Maan, Z.N.; et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res. Ther. 2014, 5, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 8. [Google Scholar] [CrossRef]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.G.; Li, S.W.; Zheng, X.M.; Hu, L.Q.; Hu, W.L.; Luo, Y. The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian J. Androl. 2009, 11, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Wu, Y.N.; Ho, H.O.; Chen, K.C.; Sheu, M.T.; Chiang, H.S. The neuroprotective effect of platelet-rich plasma on erectile function in bilateral cavernous nerve injury rat model. J. Sex. Med. 2012, 9, 2838–2848. [Google Scholar] [CrossRef]
- Wu, Y.N.; Wu, C.C.; Sheu, M.T.; Chen, K.C.; Ho, H.O.; Chiang, H.S. Optimization of platelet-rich plasma and its effects on the recovery of erectile function after bilateral cavernous nerve injury in a rat model. J. Tissue Eng. Regen. Med. 2016, 10, E294–E304. [Google Scholar] [CrossRef]
- Epifanova, M.V.; Chalyi, M.E.; Krasnov, A.O. Investigation of mechanisms of action of growth factors of autologous platelet-rich plasma used to treat erectile dysfunction. Urologiia 2017, 1, 46–48. (In Russian) [Google Scholar] [CrossRef]
- Matz, E.L.; Pearlman, A.M.; Terlecki, R.P. Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig. Clin. Urol. 2018, 59, 61–65. [Google Scholar] [CrossRef]
- Shanley, L.C.; Mahon, O.R.; Kelly, D.J.; Dunne, A. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater. 2021, 133, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.A. A Time to Press Reset and Regenerate Cardiac Stem Cell Biology. JAMA Cardiol. 2019, 4, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, B.A.; Balkan, W.; Winkler, J.; Gyöngyösi, M.; Goliasch, G.; Fernández-Avilés, F.; Hare, J.M. Pre-clinical Studies of Stem Cell Therapy for Heart Disease. Circ. Res. 2018, 122, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020, 577, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Chasing the recipe for a pro-regenerative immune system. Semin. Cell Dev. Biol. 2017, 61, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godwin, J.W.; Debuque, R.; Salimova, E.; Rosenthal, N.A. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen. Med. 2017, 2, 22. [Google Scholar] [CrossRef]
- Ben-Mordechai, T.; Holbova, R.; Landa-Rouben, N.; Harel-Adar, T.; Feinberg, M.S.; Abd Elrahman, I.; Blum, G.; Epstein, F.H.; Silman, Z.; Cohen, S.; et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 2013, 62, 1890–1901. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.R.; Godwin, J.W.; Rosenthal, N.A. Macrophages in cardiac homeostasis, injury responses and progenitor cell mobilisation. Stem Cell Res. 2014, 13 Pt 3, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.; Marín, S.; Riol, N.; Carbonell-Uberos, F.; Miñana, M.D. Human adipose tissue-resident monocytes exhibit an endothelial-like phenotype and display angiogenic properties. Stem Cell Res. Ther. 2014, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Feng, J.; Liu, K.; Zhou, S.; Lu, F. Early Macrophage Infiltration Improves Fat Graft Survival by Inducing Angiogenesis and Hematopoietic Stem Cell Recruitment. Plast. Reconstr. Surg. 2018, 141, 376–386. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Verboket, R.; Schaible, A.; Marzi, I.; Bonig, H. The osteo-inductive activity of bone-marrow-derived mononuclear cells resides within the CD14+ population and is independent of the CD34+ population. Eur. Cell Mater. 2018, 35, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.J.; Al-Sayegh, H.; Bashir, J.; Goodyear, S.; Freeman, M.D. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 258. [Google Scholar] [CrossRef] [Green Version]
- Chaput, C.D.; Shar, A.; Jupiter, D.; Hubert, Z.; Clough, B.; Krause, U.; Gregory, C.A. How stem cell composition in bone marrow aspirate relates to clinical outcomes when used for cervical spine fusion. PLoS ONE 2018, 13, e0203714. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; Abutaleb, N.; Wang, J.T.; Ye, J.; Shaikh, Z.; Sriworarat, C.; Qian, Y.; Bursac, N. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat. Biomed. Eng. 2018, 2, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Hopper, N.; Wardale, J.; Howard, D.; Brooks, R.; Rushton, N.; Henson, F. Peripheral blood derived mononuclear cells enhance the migration and chondrogenic differentiation of multipotent mesenchymal stromal cells. Stem Cells Int. 2015, 2015, 323454. [Google Scholar] [CrossRef] [PubMed]
- Hopper, N.; Wardale, J.; Brooks, R.; Power, J.; Rushton, N.; Henson, F. Peripheral Blood Mononuclear Cells Enhance Cartilage Repair in in vivo Osteochondral Defect Model. PLoS ONE 2015, 10, e0133937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug. Deliv. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef]
- Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Persiani, F.; Paolini, A.; Camilli, D.; Mascellari, L.; Platone, A.; Magenta, A.; Furgiuele, S. Peripheral Blood Mononuclear Cells Therapy for Treatment of Lower Limb Ischemia in Diabetic Patients: A Single-Center Experience. Ann. Vasc. Surg. 2018, 53, 190–196. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, B.; Gentile, P.; Orlandi, F.; Bocchini, I.; Di Pasquali, C.; Agovino, A.; Gizzi, C.; Patrizi, F.; Scioli, M.G.; Orlandi, A.; et al. Limb rescue: A new autologous-peripheral blood mononuclear cells technology in critical limb ischemia and chronic ulcers. Tissue Eng. Part C Methods 2015, 21, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Fung, E.; Helisch, A. Macrophages in collateral arteriogenesis. Front. Physiol. 2012, 3, 353. [Google Scholar] [CrossRef] [Green Version]
- Barnett, F.H.; Rosenfeld, M.; Wood, M.; Kiosses, W.B.; Usui, Y.; Marchetti, V.; Aguilar, E.; Friedlander, M. Macrophages form functional vascular mimicry channels in vivo. Sci. Rep. 2016, 6, 36659. [Google Scholar] [CrossRef]
- Gurevich, D.B.; Severn, C.E.; Twomey, C.; Greenhough, A.; Cash, J.; Toye, A.M.; Mellor, H.; Martin, P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018, 37, e97786. [Google Scholar] [CrossRef]
- Liu, C.; Wu, C.; Yang, Q.; Gao, J.; Li, L.; Yang, D.; Luo, L. Macrophages Mediate the Repair of Brain Vascular Rupture through Direct Physical Adhesion and Mechanical Traction. Immunity 2016, 44, 1162–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnasamy, K.; Limbourg, A.; Kapanadze, T.; Gamrekelashvili, J.; Beger, C.; Häger, C.; Lozanovski, V.J.; Falk, C.S.; Napp, L.C.; Bauersachs, J.; et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat. Commun. 2017, 8, 952. [Google Scholar] [CrossRef]
- Awad, O.; Dedkov, E.I.; Jiao, C.; Bloomer, S.; Tomanek, R.J.; Schatteman, G.C. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, J.S.; Mallat, Z.; Tedgui, A.; Lévy, B.I. Post-ischaemic neovascularization and inflammation. Cardiovasc. Res. 2008, 78, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabile, E.; Kinnaird, T.; la Sala, A.; Hanson, S.K.; Watkins, C.; Campia, U.; Shou, M.; Zbinden, S.; Fuchs, S.; Kornfeld, H.; et al. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 2006, 113, 118–124, Erratum in: Circulation 2006, 113, e711.. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lužnik, Z.; Anchouche, S.; Dana, R.; Yin, J. Regulatory T Cells in Angiogenesis. J. Immunol. 2020, 205, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Zouggari, Y.; Ait-Oufella, H.; Waeckel, L.; Vilar, J.; Loinard, C.; Cochain, C.; Récalde, A.; Duriez, M.; Levy, B.I.; Lutgens, E.; et al. Regulatory T cells modulate postischemic neovascularization. Circulation 2009, 120, 1415–1425, Erratum in: Circulation 2010, 121, e31. Lutgens, Ester [corrected to Lutgens, Esther]. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seraphim, P.M.; Leal, E.C.; Moura, J.; Gonçalves, P.; Gonçalves, J.P.; Carvalho, E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 2020, 254, 117813. [Google Scholar] [CrossRef] [PubMed]
- Leung, O.M.; Li, J.; Li, X.; Chan, V.W.; Yang, K.Y.; Ku, M.; Ji, L.; Sun, H.; Waldmann, H.; Tian, X.Y.; et al. Regulatory T Cells Promote Apelin-Mediated Sprouting Angiogenesis in Type 2 Diabetes. Cell Rep. 2018, 24, 1610–1626. [Google Scholar] [CrossRef] [Green Version]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef]
- Spaltro, G.; Straino, S.; Gambini, E.; Bassetti, B.; Persico, L.; Zoli, S.; Zanobini, M.; Capogrossi, M.C.; Spirito, R.; Quarti, C.; et al. Characterization of the Pall Celeris system as a point-of-care device for therapeutic angiogenesis. Cytotherapy 2015, 17, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.P.; Yang, X.F.; Li, S.Z.; Wen, J.C.; Zhang, Y.; Han, Z.C. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb. Haemost. 2007, 98, 1335–1342. [Google Scholar] [CrossRef]
- Dubský, M.; Jirkovská, A.; Bem, R.; Fejfarová, V.; Pagacová, L.; Nemcová, A.; Sixta, B.; Chlupac, J.; Peregrin, J.H.; Syková, E.; et al. Comparison of the effect of stem cell therapy and percutaneous transluminal angioplasty on diabetic foot disease in patients with critical limb ischemia. Cytotherapy 2014, 16, 1733–1738. [Google Scholar] [CrossRef]
- Scatena, A.; Petruzzi, P.; Maioli, F.; Lucaroni, F.; Ambrosone, C.; Ventoruzzo, G.; Liistro, F.; Tacconi, D.; Di Filippi, M.; Attempati, N.; et al. Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia. J. Clin. Med. 2021, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Squadrito, M.L.; Iruela-Arispe, M.L.; De Palma, M. Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp. Cell Res. 2013, 319, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Terlizzese, G.; Stubinski, R.; Casini, A.; Clerici, G.; Sangiorgi, G. A case report of pudendal arteries angioplasty with sirolimus drug-coated balloon and drug-eluting stent associated with intracavernous autologous peripheral blood mononuclear cells injection for untreatable vasculogenic erectile dysfunction. Eur. Heart J. Case Rep. 2021, 5, ytab244. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.C.; Oh, P.C.; Lee, K.; Ahn, T.; Byun, K. Increasing injection frequency enhances the survival of injected bone marrow derived mesenchymal stem cells in a critical limb ischemia animal model. Korean J. Physiol. Pharmacol. 2016, 20, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Halim, H.I.A.E.l.; Elbakary, R.H.; Okasha, E.F.; Mousa, A.M.; Salah, E.F. Effect of Peripheral Blood Mononuclear Cells on Induced Ischemia/Reperfusion in Skeletal Muscle of Adult Male Albino Rat: An Immunohistochemical Study. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2133–2144. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, M.; Rehak, L.; Massaro, G.; Benedetto, D.; Matteucci, A.; Russo, G.; Esperto, F.; Federici, M.; Mauriello, A.; Sangiorgi, G.M. Autologous Immune Cell-Based Regenerative Therapies to Treat Vasculogenic Erectile Dysfunction: Is the Immuno-Centric Revolution Ready for the Prime Time? Biomedicines 2022, 10, 1091. https://doi.org/10.3390/biomedicines10051091
Bonanni M, Rehak L, Massaro G, Benedetto D, Matteucci A, Russo G, Esperto F, Federici M, Mauriello A, Sangiorgi GM. Autologous Immune Cell-Based Regenerative Therapies to Treat Vasculogenic Erectile Dysfunction: Is the Immuno-Centric Revolution Ready for the Prime Time? Biomedicines. 2022; 10(5):1091. https://doi.org/10.3390/biomedicines10051091
Chicago/Turabian StyleBonanni, Michela, Laura Rehak, Gianluca Massaro, Daniela Benedetto, Andrea Matteucci, Giulio Russo, Francesco Esperto, Massimo Federici, Alessandro Mauriello, and Giuseppe Massimo Sangiorgi. 2022. "Autologous Immune Cell-Based Regenerative Therapies to Treat Vasculogenic Erectile Dysfunction: Is the Immuno-Centric Revolution Ready for the Prime Time?" Biomedicines 10, no. 5: 1091. https://doi.org/10.3390/biomedicines10051091
APA StyleBonanni, M., Rehak, L., Massaro, G., Benedetto, D., Matteucci, A., Russo, G., Esperto, F., Federici, M., Mauriello, A., & Sangiorgi, G. M. (2022). Autologous Immune Cell-Based Regenerative Therapies to Treat Vasculogenic Erectile Dysfunction: Is the Immuno-Centric Revolution Ready for the Prime Time? Biomedicines, 10(5), 1091. https://doi.org/10.3390/biomedicines10051091







