Skeletal Muscle and COVID-19: The Potential Involvement of Bioactive Sphingolipids
Abstract
:1. Introduction
2. Skeletal Muscle Manifestations in COVID-19 Patients
2.1. Acute Manifestations
2.2. Long Persistent Manifestations
3. Mechanisms of SARS-CoV-2 Infection of Skeletal Muscle Damage
3.1. Direct Effect: SARS-CoV-2 Infection of Skeletal Muscle Cells
3.2. Indirect Effect: Cytokine Storm and SkM Remodeling
4. COVID-19 May Be a Risk for Patients Affected by Neuromuscular Diseases
4.1. Myasthenia Gravis
4.2. Multiple Sclerosis
4.3. Amyotrophic Lateral Sclerosis (ALS)
5. Sphingolipids as Biomarkers and Mediators of Virus Pathogenicity
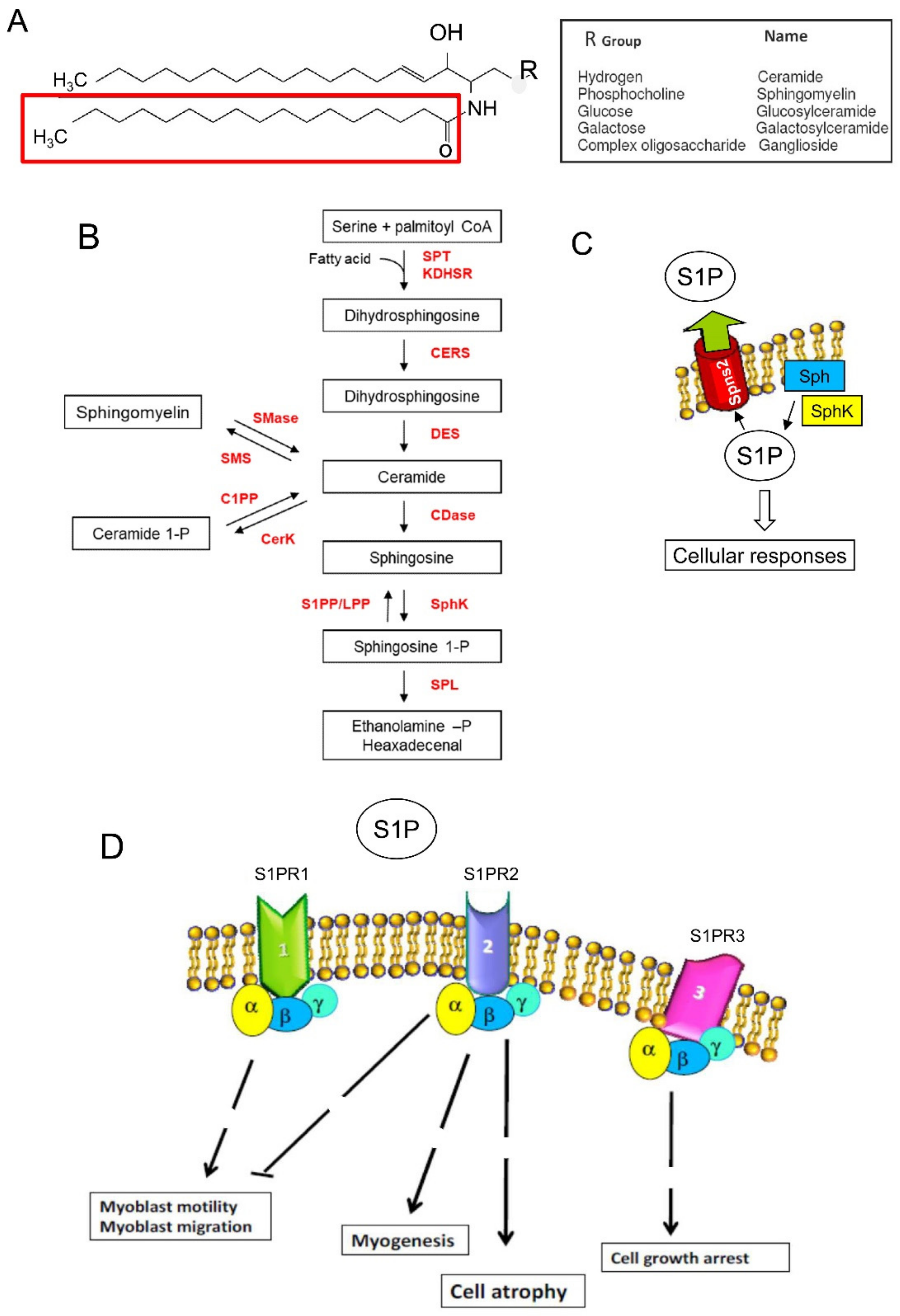
5.1. Sphingolipid Metabolism and Sphingosine 1-Phosphate-Mediated Signaling
5.2. Sphingolipids and Skeletal Muscle Remodeling
5.3. Sphingolipids and Viral Replication
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Christophers, B.; Gallo Marin, B.; Oliva, R.; Powell, W.T.; Savage, T.J.; Michelow, I.C. Trends in clinical presentation of children with COVID-19: A systematic review of individual participant data. Pediatr. Res. 2022, 91, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef]
- Al-Awwal, N.; Dweik, F.; Mahdi, S.; El-Dweik, M.; Anderson, S.H. A Review of SARS-CoV-2 Disease (COVID-19): Pandemic in Our Time. Pathogens 2022, 11, 368. [Google Scholar] [CrossRef]
- Zhao, W.; Li, H.; Li, J.; Xu, B.; Xu, J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J. Med. Virol. 2022, 94, 1886–1892. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Long, B.; Carius, B.M.; Chavez, S.; Liang, S.Y.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022, 54, 46–57. [Google Scholar] [CrossRef]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA 2021, 325, 2015–2016. [Google Scholar] [CrossRef]
- Ge, E.; Li, Y.; Wu, S.; Candido, E.; Wei, X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLoS ONE 2021, 16, e0258154. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Tran, V.-T.; Porcher, R.; Pane, I.; Ravaud, P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat. Commun. 2022, 13, 1812. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Griffith, J.F. Musculoskeletal complications of severe acute respiratory syndrome. Semin. Musculoskelet. Radiol. 2011, 15, 554–560. [Google Scholar] [CrossRef]
- Leung, T.W.; Wong, K.S.; Hui, A.C.; To, K.F.; Lai, S.T.; Ng, W.F.; Ng, H.K. Myopathic changes associated with severe acute respiratory syndrome: A postmortem case series. Arch. Neurol. 2005, 62, 1113–1117. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Lagodzinski, A.; Berezinska, M. COVID-19: From the structure and replication cycle of SARS-CoV-2 to its disease symptoms and treatment. J. Physiol. Pharmacol. 2021, 72, 479–501. [Google Scholar] [CrossRef]
- De Giorgio, M.R.; Di Noia, S.; Morciano, C.; Conte, D. The impact of SARS-CoV-2 on skeletal muscles. Acta Myol. 2020, 39, 307–312. [Google Scholar] [CrossRef]
- Suh, J.; Amato, A.A. Neuromuscular complications of coronavirus disease-19. Curr. Opin. Neurol. 2021, 34, 669–674. [Google Scholar] [CrossRef]
- Paliwal, V.K.; Garg, R.K.; Gupta, A.; Tejan, N. Neuromuscular presentations in patients with COVID-19. Neurol. Sci. 2020, 41, 3039–3056. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanganeh, S.; Goodarzi, N.; Doroudian, M.; Movahed, E. Potential COVID-19 therapeutic approaches targeting angiotensin-converting enzyme 2; An updated review. Rev. Med. Virol. 2021, e2321. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Alketbi, E.H.; Hamdy, R.; El-Kabalawy, A.; Juric, V.; Pignitter, M.; Mosa, M.A.; Almehdi, A.M.; El-Keblawy, A.; Soliman, S.S. Lipid-based therapies against SARS-CoV-2 infection. Rev. Med. Virol. 2021, 31, 1–13. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Gripp, B.; Hoffmann, M.; Pöhlmann, S.; Hoertel, N.; Edwards, M.J.; Kamler, M.; Kornhuber, J.; Becker, K.A.; Gulbins, E. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J. Biol. Chem. 2021, 296, 100701. [Google Scholar] [CrossRef]
- Khan, S.A.; Goliwas, K.F.; Deshane, J.S. Sphingolipids in Lung Pathology in the Coronavirus Disease Era: A Review of Sphingolipid Involvement in the Pathogenesis of Lung Damage. Front. Physiol. 2021, 12, 760638. [Google Scholar] [CrossRef]
- Meacci, E.; Garcia-Gil, M. S1P/S1P Receptor Signaling in Neuromuscolar Disorders. Int. J. Mol. Sci. 2019, 20, 6364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, S.; Maczis, M.A.; Maceyka, M.; Milstien, S. New insights into functions of the sphingosine-1-phosphate transporter SPNS2. J. Lipid Res. 2019, 60, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, K.; Aoki, J.; Hla, T. Lysophospholipid Mediators in Health and Disease. Annu. Rev. Pathol. 2022, 17, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell. Res. 2015, 333, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Khodadoust, M.M. Inferring a causal relationship between ceramide levels and COVID-19 respiratory distress. Sci. Rep. 2021, 11, 20866. [Google Scholar] [CrossRef]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef]
- McGowan, E.M.; Lin, Y.; Chen, S. Targeting Chronic Inflammation of the Digestive System in Cancer Prevention: Modulators of the Bioactive Sphingolipid Sphingosine-1-Phosphate Pathway. Cancers 2022, 14, 535. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, D.; Saxel, O. A myogenic cell line with altered serum requirements for differentiation. Differentiation 1977, 7, 159–166. [Google Scholar] [CrossRef]
- Meacci, E.; Cencetti, F.; Donati, C.; Nuti, F.; Farnararo, M.; Kohno, T.; Igarashi, Y.; Bruni, P. Down-regulation of EDG5/S1P2 during myogenic differentiation results in the specific uncoupling of sphingosine 1-phosphate signalling to phospholipase D. Biochim. Biophys. Acta 2003, 1633, 133–142. [Google Scholar] [CrossRef]
- Donati, C.; Meacci, E.; Nuti, F.; Becciolini, L.; Farnararo, M.; Bruni, P. Sphingosine 1-phosphate regulates myogenic differentiation: A major role for S1P2 receptor. FASEB J. 2005, 19, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Nuti, F.; Donati, C.; Cencetti, F.; Farnararo, M.; Bruni, P. Sphingosine kinase activity is required for myogenic differentiation of C2C12 myoblasts. J. Cell. Physiol. 2008, 214, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Danieli-Betto, D.; Peron, S.; Germinario, E.; Zanin, M.; Sorci, G.; Franzoso, S.; Sandonà, D.; Betto, R. Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2010, 298, C550–C558. [Google Scholar] [CrossRef] [Green Version]
- Fortier, M.; Figeac, N.; White, R.B.; Knopp, P.; Zammit, P.S. Sphingosine-1-phosphate receptor 3 influences cell cycle progression in muscle satellite cells. Dev. Biol. 2013, 382, 504–516. [Google Scholar] [CrossRef]
- Pierucci, F.; Frati, A.; Battistini, C.; Matteini, F.; Iachini, M.C.; Vestri, A.; Penna, F.; Costelli, P.; Meacci, E. Involvement of released sphingosine 1-phosphate/sphingosine 1-phosphate receptor axis in skeletal muscle atrophy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3598–3614. [Google Scholar] [CrossRef]
- Sassoli, C.; Pierucci, F.; Zecchi-Orlandini, S.; Meacci, E. Sphingosine 1-Phosphate (S1P)/ S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration. Int. J. Mol. Sci. 2019, 20, 5545. [Google Scholar] [CrossRef] [Green Version]
- Sassoli, C.; Formigli, L.; Bini, F.; Tani, A.; Squecco, R.; Battistini, C.; Zecchi-Orlandini, S.; Francini, F.; Meacci, E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J. Cell. Mol. Med. 2011, 15, 2498–2511. [Google Scholar] [CrossRef] [Green Version]
- Zanin, M.; Germinario, E.; Dalla Libera, L.; Sandonà, D.; Sabbadini, R.A.; Betto, R.; Danieli-Betto, D. Trophic action of sphingosine 1-phosphate in denervated rat soleus muscle. Am. J. Physiol. Cell Physiol. 2008, 294, C36–C46. [Google Scholar] [CrossRef]
- Cowart, L.A. A novel role for sphingolipid metabolism in oxidant-mediated skeletal muscle fatigue. Focus on “Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue”. Am. J. Physiol. Cell Physiol. 2010, 299, C549–C551. [Google Scholar] [CrossRef]
- Danieli-Betto, D.; Germinario, E.; Esposito, A.; Megighian, A.; Midrio, M.; Ravara, B.; Damiani, E.; Libera, L.D.; Sabbadini, R.A.; Betto, R. Sphingosine 1-phosphate protects mouse extensor digitorum longus skeletal muscle during fatigue. Am. J. Physiol. Cell Physiol. 2005, 288, C1367–C1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strle, K.; Broussard, S.R.; McCusker, R.H.; Shen, W.H.; Johnson, R.W.; Freund, G.G.; Dantzer, R.; Kelley, K.W. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 2004, 145, 4592–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mebarek, S.; Komati, H.; Naro, F.; Zeiller, C.; Alvisi, M.; Lagarde, M.; Prigent, A.-F.; Némoz, G. Inhibition of de novo ceramide synthesis upregulates phospholipase D and enhances myogenic differentiation. J. Cell Sci. 2007, 120, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Larichaudy, J.; Zufferli, A.; Serra, F.; Isidori, A.M.; Naro, F.; Dessalle, K.; Desgeorges, M.; Piraud, M.; Cheillan, D.; Vidal, H.; et al. TNF-α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet. Muscle 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierucci, F.; Frati, A.; Battistini, C.; Penna, F.; Costelli, P.; Meacci, E. Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase. Cancers 2021, 13, 3285. [Google Scholar] [CrossRef]
- Teo, T.H.; Chan, Y.H.; Lee, W.W.; Lum, F.M.; Amrun, S.N.; Her, Z.; Rajarethinam, R.; Merits, A.; Rötzschke, O.; Rénia, L.; et al. Fingolimod treatment abrogates chikungunya virus-induced arthralgia. Sci. Transl. Med. 2017, 9, eaal1333. [Google Scholar] [CrossRef]
- Yousefi, H.; Mashouri, L.; Okpechi, S.C.; Alahari, N.; Alahari, S.K.; Alahari, S.K. Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: A review describing drug mechanisms of action. Biochem. Pharmacol. 2021, 183, 114296. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Guidon, A.C.; Amato, A.A. COVID-19 and neuromuscular disorders. Neurology 2020, 94, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Madia, F.; Merico, B.; Primiano, G.; Cutuli, S.L.; De Pascale, G.; Servidei, S. Acute myopathic quadriplegia in patients with COVID-19 in the intensive care unit. Neurology 2020, 95, 492–494. [Google Scholar] [CrossRef]
- Islam, B.; Ahmed, M.; Islam, Z.; Begum, S.M. Severe acute myopathy following SARS-CoV-2 infection: A case report and review of recent literature. Skelet. Muscle 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Versace, V.; Sebastianelli, L.; Ferrazzoli, D.; Saltuari, L.; Kofler, M.; Löscher, W.; Uncini, A. Case Report: Myopathy in Critically Ill COVID-19 Patients: A Consequence of Hyperinflammation? Front. Neurol. 2021, 12, 625144. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.M.; Ng, G.Y.; Jones, A.Y.; Lee, E.W.; Siu, E.H.; Hui, D.S. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust. J. Physiother. 2005, 51, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Ortmeyer, H.K.; Ryan, A.S.; Hafer-Macko, C.; Oursler, K.K. Skeletal muscle cellular metabolism in older HIV-infected men. Physiol. Rep. 2016, 4, e12794. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.A.; Olugasa, B.O.; Emikpe, B.O. Molecular detection of influenza A(H1N1)pdm09 viruses with M genes from human pandemic strains among Nigerian pigs 2013–2015: Implications and associated risk factors. Epidemiol. Infect. 2017, 145, 3345–3360. [Google Scholar] [CrossRef] [Green Version]
- Aschman, T.; Schneider, J.; Greuel, S.; Meinhardt, J.; Streit, S.; Goebel, H.H.; Büttnerova, I.; Elezkurtaj, S.; Scheibe, F.; Radke, J.; et al. Association Between SARS-CoV-2 Infection and Immune-Mediated Myopathy in Patients Who Have Died. JAMA Neurol. 2021, 78, 948–960. [Google Scholar] [CrossRef]
- Perez-Bermejo, J.A.; Kang, S.; Rockwood, S.J.; Simoneau, C.R.; Joy, D.A.; Silva, A.C.; Ramadoss, G.N.; Flanigan, W.R.; Fozouni, P.; Li, H.; et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci. Transl. Med. 2021, 13, eabf7872. [Google Scholar] [CrossRef]
- Ma, C.; Tu, D.; Gu, J.; Xu, Q.; Hou, P.; Wu, H.; Guo, Z.; Bai, Y.; Zhao, X.; Li, P. The Predictive Value of Myoglobin for COVID-19-Related Adverse Outcomes: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 757799. [Google Scholar] [CrossRef]
- Mageriu, V.; Zurac, S.; Bastian, A.; Staniceanu, F.; Manole, E. Histological findings in skeletal muscle of SARS-CoV2 infected patient. J. Immunoass. Immunochem. 2020, 41, 1000–1009. [Google Scholar] [CrossRef]
- Trinity, J.D.; Craig, J.C.; Fermoyle, C.C.; McKenzie, A.I.; Lewis, M.T.; Park, S.H.; Rondina, M.T.; Richardson, R.S. Impact of presymptomatic COVID-19 on vascular and skeletal muscle function: A case study. J. Appl. Physiol. 2021, 130, 1961–1970. [Google Scholar] [CrossRef]
- Hooper, J.E.; Uner, M.; Priemer, D.S.; Rosenberg, A.; Chen, L. Muscle Biopsy Findings in a Case of SARS-CoV-2-Associated Muscle Injury. J. Neuropathol. Exp. Neurol. 2021, 80, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.P.; Baumeier, C.; Pietsch, H.; Bock, C.T.; Poller, W.; Escher, F. Cardiovascular consequences of viral infections: From COVID to other viral diseases. Cardiovasc. Res. 2021, 117, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef] [PubMed]
- Pitscheider, L.; Karolyi, M.; Burkert, F.R.; Helbok, R.; Wanschitz, J.V.; Horlings, C.; Pawelka, E.; Omid, S.; Traugott, M.; Seitz, T.; et al. Muscle involvement in SARS-CoV-2 infection. Eur. J. Neurol. 2020, 28, 3411–3417. [Google Scholar] [CrossRef] [PubMed]
- Barberis, E.; Timo, S.; Amede, E.; Vanella, V.V.; Puricelli, C.; Cappellano, G.; Raineri, D.; Cittone, M.G.; Rizzi, E.; Pedrinelli, A.R.; et al. Large-Scale Plasma Analysis Revealed New Mechanisms and Molecules Associated With the Host Response to SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 8623. [Google Scholar] [CrossRef]
- Lepore, E.; Lauretta, R.; Bianchini, M.; Mormando, M.; Di Lorenzo, C.; Unfer, V. Inositols Depletion and Resistance: Principal Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 6796. [Google Scholar] [CrossRef]
- Hocaoglu, E.; Ors, S.; Yildiz, O.; Inci, E. Correlation of Pectoralis Muscle Volume and Density with Severity of COVID-19 Pneumonia in Adults. Acad. Radiol. 2021, 28, 166–172. [Google Scholar] [CrossRef]
- Ufuk, F.; Demirci, M.; Sagtas, E.; Akbudak, I.H.; Ugurlu, E.; Sari, T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur. J. Radiol. 2020, 131, 109271. [Google Scholar] [CrossRef]
- Moctezuma-Velázquez, P.; Miranda-Zazueta, G.; Ortiz-Brizuela, E.; González-Lara, M.F.; Tamez-Torres, K.M.; Román-Montes, C.M.; Díaz-Mejía, B.A.; Pérez-García, E.; Villanueva-Reza, M.; Tovar-Méndez, V.H.; et al. Low Thoracic Skeletal Muscle Area Is Not Associated With Negative Outcomes in Patients with COVID-19. Am. J. Phys. Med. Rehabil. 2021, 100, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Cabañes-Martínez, L.; Villadóniga, M.; González-Rodríguez, L.; Araque, L.; Díaz-Cid, A.; Ruz-Caracuel, I.; Pian, H.; Sánchez-Alonso, S.; Fanjul, S.; Del Álamo, M.; et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin. Neurophysiol. 2020, 131, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Fietsam, A.C.; Deters, J.R.; Bryant, A.D.; Kamholz, J. Post-COVID-19 Fatigue: Potential Contributing Factors. Brain Sci. 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, F.; Demircioğlu Güneri, F.; Kardeş, S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: Prospective follow-up by phone interviews. Rheumatol. Int. 2021, 41(7), 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef] [PubMed]
- Simani, L.; Ramezani, M.; Darazam, I.A.; Sagharichi, M.; Aalipour, M.A.; Ghorbani, F.; Pakdaman, H. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J. Neurovirol. 2021, 27, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Van Gassel, R.; Bels, J.; Remij, L.; van Bussel, B.; Posthuma, R.; Gietema, H.A.; Verbunt, J.; van der Horst, I.; Olde Damink, S.; van Santen, S.; et al. Functional Outcomes and Their Association With Physical Performance in Mechanically Ventilated Coronavirus Disease 2019 Survivors at 3 Months Following Hospital Discharge: A Cohort Study. Crit. Care Med. 2021, 49, 1726–1738. [Google Scholar] [CrossRef]
- Tanriverdi, A.; Savci, S.; Kahraman, B.O.; Ozpelit, E. Extrapulmonary features of post-COVID-19 patients: Muscle function, physical activity, mood, and sleep quality. Ir. J. Med. Sci. 2021, 1–7. [Google Scholar] [CrossRef]
- Farr, E.; Wolfe, A.R.; Deshmukh, S.; Rydberg, L.; Soriano, R.; Walter, J.M.; Boon, A.J.; Wolfe, L.F.; Franz, C.K. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann. Clin. Transl. Neurol. 2021, 8, 1745–1749. [Google Scholar] [CrossRef]
- Shanbehzadeh, S.; Tavahomi, M.; Zanjari, N.; Ebrahimi-Takamjani, I.; Amiri-Arimi, S. Physical and mental health complications post-COVID-19: Scoping review. J. Psychosom. Res. 2021, 147, 110525. [Google Scholar] [CrossRef]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients Without Previous Disabilities Recovering From COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.B.; Minton, H.C.; Morrow, M.; Johnson, C.; Moore, M.A.; O’Keefe, G.A.D.; Benameur, K.; Higdon, J.; Fairley, J.K. Postacute Sequelae of SARS-CoV-2 Infection and Impact on Quality of Life 1–6 Months After Illness and Association with Initial Symptom Severity. Open Forum Infect. Dis. 2021, 8, ofab352. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Screening for Sarcopenia (Physical Frailty) in the COVID-19 Era. Int. J. Endocrinol. 2021, 2021, 5563960. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Yoon, J.S.; Kim, E.J.; Hong, H.L.; Kwon, H.H.; Jung, C.Y.; Kim, K.C.; Sung, Y.S.; Park, S.H.; Kim, S.K.; et al. Prognostic Implication of Baseline Sarcopenia for Length of Hospital Stay and Survival in Patients With Coronavirus Disease 2019. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, e110–e116. [Google Scholar] [CrossRef] [PubMed]
- Campi, I.; Gennari, L.; Merlotti, D.; Mingiano, C.; Frosali, A.; Giovanelli, L.; Torlasco, C.; Pengo, M.F.; Heilbron, F.; Soranna, D.; et al. Vitamin D and COVID-19 severity and related mortality: A prospective study in Italy. BMC Infect. Dis. 2021, 21, 566. [Google Scholar] [CrossRef]
- Karaarslan, F.; Güneri, F.D.; Kardeş, S. Long COVID: Rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin. Rheumatol. 2022, 41, 289–296. [Google Scholar] [CrossRef]
- Nasuelli, N.A.; Pettinaroli, R.; Godi, L.; Savoini, C.; De Marchi, F.; Mazzini, L.; Crimaldi, F.; Pagni, A.; Pompa, C.P.; Colombo, D. Critical illness neuro-myopathy (CINM) and focal amyotrophy in intensive care unit (ICU) patients with SARS-CoV-2: A case series. Neurol. Sci. 2021, 42, 1119–1121. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Shen, H.; Li, Z.; Geng, J.; Han, H.; Cai, J.; Li, X.; Kang, W.; Weng, D.; et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J. Pathol. 2003, 200, 282–289. [Google Scholar] [CrossRef]
- McCray, P.B., Jr.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Ozden, S.; Huerre, M.; Riviere, J.P.; Coffey, L.L.; Afonso, P.V.; Mouly, V.; de Monredon, J.; Roger, J.C.; El Amrani, M.; Yvin, J.L.; et al. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS ONE 2007, 2, e527. [Google Scholar] [CrossRef]
- Miller, F.W.; Lamb, J.A.; Schmidt, J.; Nagaraju, K. Risk factors and disease mechanisms in myositis. Nat. Rev. Rheumatol. 2018, 14, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Inflammatory myopathies: Update on diagnosis, pathogenesis and therapies, and COVID-19-related implications. Acta Myol. 2020, 39, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, P.J.; Always, S.E.; Mohamed, J.S. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J. Appl. Physiol. 2020, 129, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, M.; Pirani, F.; Tagliabracci, A.; Valsecchi, M.; Procopio, A.D.; Busardò, F.P.; Graciotti, L. SARS-CoV-2 identification in lungs, heart and kidney specimens by transmission and scanning electron microscopy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5186–5188. [Google Scholar] [CrossRef] [PubMed]
- Bullock, H.A.; Goldsmith, C.S.; Zaki, S.R.; Martines, R.B.; Miller, S.E. Difficulties in Differentiating Coronaviruses from Subcellular Structures in Human Tissues by Electron Microscopy. Emerg. Infect. Dis. 2021, 27, 1023–1031. [Google Scholar] [CrossRef]
- Shi, Z.; de Vries, H.J.; Vlaar, A.P.J.; van der Hoeven, J.; Boon, R.A.; Heunks, L.M.A.; Ottenheijm, C.A.C.; Dutch COVID-19 Diaphragm Investigators. Diaphragm Pathology in Critically Ill Patients With COVID-19 and Postmortem Findings from 3 Medical Centers. JAMA Intern. Med. 2021, 181, 122–124. [Google Scholar] [CrossRef]
- Suh, J.; Mukerji, S.S.; Collens, S.I.; Padera, R.F., Jr.; Pinkus, G.S.; Amato, A.A.; Solomon, I.H. Skeletal Muscle and Peripheral Nerve Histopathology in COVID-19. Neurology 2021, 97, e849–e858. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F. SARS-CoV-2 associated rhabdomyolysis in 32 patients. Turk. J. Med. Sci. 2021, 51, 1598–1601. [Google Scholar] [CrossRef]
- Rosato, C.; Bolondi, G.; Russo, E.; Oliva, A.; Scognamiglio, G.; Mambelli, E.; Longoni, M.; Rossi, G.; Agnoletti, V. Clinical, electromyographical, histopathological characteristics of COVID-19 related rhabdomyolysis. SAGE Open Med. Case Rep. 2020, 8, 2050313X20983132. [Google Scholar] [CrossRef]
- Winslow, M.A.; Hall, S.E. Muscle wasting: A review of exercise, classical and non-classical RAS axes. J. Cell. Mol. Med. 2019, 23, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Aravena, J.; Abrigo, J.; Gonzalez, F.; Aguirre, F.; Gonzalez, A.; Simon, F.; Cabello-Verrugio, C. Angiotensin (1–7) Decreases Myostatin-Induced NF-kappaB Signaling and Skeletal Muscle Atrophy. Int. J. Mol.Sci. 2020, 21, 1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Bois, P.; Pablo Tortola, C.; Lodka, D.; Kny, M.; Schmidt, F.; Song, K.; Schmidt, S.; Bassel-Duby, R.; Olson, E.N.; Fielitz, J. Angiotensin II Induces Skeletal Muscle Atrophy by Activating TFEB-Mediated MuRF1 Expression. Circ. Res. 2015, 117, 424–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozato, S.; Yamamoto, K.; Takeshita, H.; Nozato, Y.; Imaizumi, Y.; Fujimoto, T.; Yokoyama, S.; Nagasawa, M.; Takeda, M.; Hongyo, K.; et al. Angiotensin 1–7 alleviates aging-associated muscle weakness and bone loss, but is not associated with accelerated aging in ACE2-knockout mice. Clin. Sci. 2019, 133, 2005–2018. [Google Scholar] [CrossRef]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Takeda, M.; Fukada, S.I.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Nozato, Y.; Fujimoto, T.; et al. Angiotensin-converting enzyme 2 deficiency accelerates and angiotensin 1-7 restores age-related muscle weakness in mice. J. Cachexia Sarcopenia Muscle 2018, 9, 975–986. [Google Scholar] [CrossRef]
- Frier, B.C.; Williams, D.B.; Wright, D.C. The effects of apelin treatment on skeletal muscle mitochondrial content. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1761–R1768. [Google Scholar] [CrossRef] [Green Version]
- Baig, S.; Paik, J.J. Inflammatory muscle disease—An update. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101484. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020, 41, 81–492. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Jt. Surg. Am. 2020, 102, 1197–1204. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Gąsowski, J.; Michel, J.P.; Veronese, N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef]
- Morley, J.E. Editorial: COVID-19—The Long Road to Recovery. J. Nutr. Health Aging 2020, 24, 917–919. [Google Scholar] [CrossRef]
- Chiu, M.N.; Bhardwaj, M.; Sah, S.P. Safety profile of COVID-19 drugs in a real clinical setting. Eur. J. Clin. Pharmacol. 2022, 78, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Maqbool, I.; Madni, A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur. J. Pharmacol. 2021, 894, 173854. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Chen, T.H. Care for Patients With Neuromuscular Disorders in the COVID-19 Pandemic Era. Front. Neurol. 2021, 12, 607790. [Google Scholar] [CrossRef] [PubMed]
- Natera-de Benito, D.; Aguilera-Albesa, S.; Costa-Comellas, L.; García-Romero, M.; Miranda-Herrero, M.C.; Rúbies Olives, J.; García-Campos, Ó.; Martínez Del Val, E.; Martinez Garcia, M.J.; Medina Martínez, I.; et al. COVID-19 in children with neuromuscular disorders. J. Neurol. 2021, 268, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Pisella, L.I.; Fernandes, S.; Solé, G.; Stojkovic, T.; Tard, C.; Chanson, J.B.; Bouhour, F.; Salort-Campana, E.; Beaudonnet, G.; Debergé, L.; et al. A multicenter cross-sectional French study of the impact of COVID-19 on neuromuscular diseases. Orphanet J. Rare Dis. 2021, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Cajamarca-Baron, J.; Guavita-Navarro, D.; Buitrago-Bohorquez, J.; Gallego-Cardona, L.; Navas, A.; Cubides, H.; Arredondo, A.M.; Escobar, A.; Rojas-Villarraga, A. SARS-CoV-2 (COVID-19) in patients with some degree of immunosuppression. Reumatol. Clin. (Engl. Ed.) 2021, 17, 408–419. [Google Scholar] [CrossRef]
- Levine, H.; Prais, D.; Aharoni, S.; Nevo, Y.; Katz, J.; Rahmani, E.; Goldberg, L.; Scheuerman, O. COVID-19 in advanced Duchenne/Becker muscular dystrophy patients. Neuromuscul. Disord. 2021, 31, 607–611. [Google Scholar] [CrossRef]
- Quinlivan, R.; Desikan, M.; Cruces, F.; Pietrusz, A.; Savvatis, K. Clinical outcome of SARS-CoV-2 infection in 7 adults with Duchenne muscular dystrophy attending a specialist neuromuscular centre. Neuromuscul. Disord. 2021, 31, 603–606. [Google Scholar] [CrossRef]
- Hannah, J.R.; Ali, S.S.; Nagra, D.; Adas, M.A.; Buazon, A.D.; Galloway, J.B.; Gordon, P.A. Skeletal muscles and Covid-19: A systematic review of rhabdomyolysis and myositis in SARS-CoV-2 infection. Clin. Exp. Rheumatol. 2022, 40, 329–338. [Google Scholar] [CrossRef]
- Borges, L.S.; Richman, D.P. Muscle-Specific Kinase Myasthenia Gravis. Front Immunol. 2020, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.S.; Hardy, N.; Ghozy, S.; Dibas, M.; Paranjape, G.; Evanson, K.W.; Reierson, N.L.; Cowie, K.; Kamrowski, S.; Schmidt, S.; et al. Characteristics, treatment, and outcomes of Myasthenia Gravis in COVID-19 patients: A systematic review. Clin. Neurol. Neurosurg. 2022, 213, 107140. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Kovvuru, S.; Nalleballe, K.; Onteddu, S.R.; Nowak, R.J. Electronic health record derived-impact of COVID-19 on myasthenia gravis. J. Neurol. Sci. 2021, 423, 117362. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Rzepiński, Ł.; Zawadka-Kunikowska, M. COVID-19 pandemic year in a sample of Polish myasthenia gravis patients: An observational study. Neurol. Neurochir. Pol. 2022, 56, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Županić, S.; Perić Šitum, M.; Majdak, M.; Karakaš, M.; Bašić, S.; Sporiš, D. Case series of COVID-19 in patients with myasthenia gravis: A single institution experience. Acta Neurol. Belg. 2021, 121, 1039–1044. [Google Scholar] [CrossRef]
- Solé, G.; Mathis, S.; Friedman, D.; Salort-Campana, E.; Tard, C.; Bouhour, F.; Magot, A.; Annane, D.; Clair, B.; Le Masson, G.; et al. Impact of Coronavirus Disease 2019 in a French Cohort of Myasthenia Gravis. Neurology 2021, 96, e2109–e2120. [Google Scholar] [CrossRef]
- Businaro, P.; Vaghi, G.; Marchioni, E.; Diamanti, L.; Arceri, S.; Bini, P.; Colombo, E.; Cosentino, G.; Alfonsi, E.; Costa, A.; et al. COVID-19 in patients with myasthenia gravis: Epidemiology and disease course. Muscle Nerve 2021, 64, 206–211. [Google Scholar] [CrossRef]
- Saied, Z.; Rachdi, A.; Thamlaoui, S.; Nabli, F.; Jeridi, C.; Baffoun, N.; Kaddour, C.; Belal, S.; Ben Sassi, S. Myasthenia gravis and COVID-19: A case series and comparison with literature. Acta Neurol. Scand. 2021, 144, 334–340. [Google Scholar] [CrossRef]
- Jakubíková, M.; Týblová, M.; Tesař, A.; Horáková, M.; Vlažná, D.; Ryšánková, I.; Nováková, I.; Dolečková, K.; Dušek, P.; Piťha, J.; et al. Predictive factors for a severe course of COVID-19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. Eur. J. Neurol. 2021, 28, 3418–3425. [Google Scholar] [CrossRef]
- Karimi, N.; Okhovat, A.A.; Ziaadini, B.; Haghi Ashtiani, B.; Nafissi, S.; Fatehi, F. Myasthenia gravis associated with novel coronavirus 2019 infection: A report of three cases. Clin. Neurol. Neurosur. 2021, 208, 106834. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.M.; Kumar, B.S.; Osman, S.; Murthy, J.M.K. Temporal association between SARS-CoV-2 and new-onset myasthenia gravis: Is it causal or coincidental? BMJ Case Rep. 2021, 14, e244146. [Google Scholar] [CrossRef] [PubMed]
- Sriwastava, S.; Tandon, M.; Kataria, S.; Daimee, M.; Sultan, S. New onset of ocular myasthenia gravis in a patient with COVID-19: A novel case report and literature review. J. Neurol. 2021, 268, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, L.; Baheerathan, A.; Cao, M.; Leite, M.I.; Viegas, S. MuSK Antibody-Associated Myasthenia Gravis with SARS-CoV-2 Infection: A Case Report. Ann. Intern. Med. 2021, 174, 872–873. [Google Scholar] [CrossRef]
- Assini, A.; Gandoglia, I.; Damato, V.; Rikani, K.; Evoli, A.; Del Sette, M. Myasthenia gravis associated with anti-MuSK antibodies developed after SARS-CoV-2 infection. Eur. J. Neurol. 2021, 28, 3537–3539. [Google Scholar] [CrossRef]
- Tagliaferri, A.R.; Narvaneni, S.; Azzam, M.H.; Grist, W. A Case of COVID-19 Vaccine Causing a Myasthenia Gravis Crisis. Cureus 2021, 13, e15581. [Google Scholar] [CrossRef]
- Chavez, A.; Pougnier, C. A Case of COVID-19 Vaccine Associated New Diagnosis Myasthenia Gravis. J. Prim. Care Community Health 2021, 12, 21501327211051933. [Google Scholar] [CrossRef]
- Ruan, Z.; Tang, Y.; Li, C.; Sun, C.; Zhu, Y.; Li, Z.; Chang, T. COVID-19 Vaccination in Patients with Myasthenia Gravis: A Single-Center Case Series. Vaccines 2021, 9, 1112. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, R.; Yang, H.; Yang, H. To Be or Not To Be Vaccinated: That Is a Question in Myasthenia Gravis. Front. Immunol. 2021, 12, 733418. [Google Scholar] [CrossRef]
- Luo, Y.T.; Liang, Y.F.; He, H.; Zhang, M.T.; Wang, R.; Li, H.L. The immunosuppressant fingolimod ameliorates experimental autoimmune myasthenia gravis by regulating T-cell balance and cytokine secretion. Am. J. Transl. Res. 2020, 12, 2600–2613. [Google Scholar]
- Liu, Y.; Yang, C.L.; Yang, B.; Du, T.; Li, X.L.; Zhang, P.; Ge, M.R.; Lian, Y.; Li, H.; Liu, Y.D.; et al. Prophylactic administration of fingolimod (FTY720) ameliorated experimental autoimmune myasthenia gravis by reducing the number of dendritic cells, follicular T helper cells and antibody-secreting cells. Int. Immunopharmacol. 2021, 96, 107511. [Google Scholar] [CrossRef] [PubMed]
- Pelz, A.; Schaffert, H.; Diallo, R.; Hiepe, F.; Meisel, A.; Kohler, S. S1P receptor antagonists fingolimod and siponimod do not improve the outcome of experimental autoimmune myasthenia gravis mice after disease onset. Eur. J. Immunol. 2018, 48, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunkhorst, R.; Vutukuri, R.; Pfeilschifter, W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front. Cell. Neurosci. 2014, 8, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarwar, S.; Rogers, S.; Mohamed, A.S.; Ogula, E.; Ayantayo, R.A.; Ahmed, A.; Shahzadi, I.; Kataria, S.; Singh, R. Multiple Sclerosis Following SARS-CoV-2 Infection: A Case Report and Literature Review. Cureus 2021, 13, e19036. [Google Scholar] [CrossRef] [PubMed]
- Karsidag, S.; Sahin, S.; Ates, M.F.; Cinar, N.; Kendirli, S. Demyelinating Disease of the Central Nervous System Concurrent with COVID-19. Cureus 2021, 13, e17297. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, A.; Aprile, M.; Gagliardo, C.; Giammanco, G.M.; D’Amelio, M.; Aridon, P.; La Tona, G.; Salemi, G.; Ragonese, P. Clinical Onset and Multiple Sclerosis Relapse after SARS-CoV-2 Infection. Neurol. Int. 2021, 13, 695–700. [Google Scholar] [CrossRef]
- Barzegar, M.; Vaheb, S.; Mirmosayyeb, O.; Afshari-Safavi, A.; Nehzat, N.; Shaygannejad, V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult. Scler. Relat. Disord. 2021, 52, 102947. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sedaghat, N.; Aghababaee, A.; Kargaran, P.K.; Maracy, M.R.; Ganjalikhani-Hakemi, M.; Rayani, M.; Abhari, A.P.; Khorvash, R.; Salari, M.; et al. COVID-19 and the Risk of Relapse in Multiple Sclerosis Patients: A Fight with No Bystander Effect? Mult. Scler. Relat. Disord. 2021, 51, 102915. [Google Scholar] [CrossRef]
- Sedaghat, N. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult. Scler. Relat. Disord. 2021, 53, 103054. [Google Scholar] [CrossRef]
- Zabalza, A.; Cárdenas-Robledo, S.; Tagliani, P.; Arrambide, G.; Otero-Romero, S.; Carbonell-Mirabent, P.; Rodriguez-Barranco, M.; Rodríguez-Acevedo, B.; Restrepo Vera, J.L.; Resina-Salles, M.; et al. COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021, 28, 3384–3395. [Google Scholar] [CrossRef]
- Möhn, N.; Konen, F.F.; Pul, R.; Kleinschnitz, C.; Prüss, H.; Witte, T.; Stangel, M.; Skripuletz, T. Experience in Multiple Sclerosis Patients with COVID-19 and Disease-Modifying Therapies: A Review of 873 Published Cases. J. Clin. Med. 2020, 9, 4067. [Google Scholar] [CrossRef] [PubMed]
- Louapre, C.; Collongues, N.; Stankoff, B.; Giannesini, C.; Papeix, C.; Bensa, C.; Deschamps, R.; Créange, A.; Wahab, A.; Pelletier, J.; et al. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020, 77, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Saraceno, L.; Susani, E.L.; Marazzi, M.R.; Moioli, M.C.; Agostoni, E.C.; Protti, A. SARS-CoV-2 infection after alemtuzumab in a multiple sclerosis patient: Milder disease symptoms in comparison with coinfected relatives: A case report and review of the literature. Neurol. Sci. 2021, 42, 4881–4884. [Google Scholar] [CrossRef] [PubMed]
- Czarnowska, A.; Brola, W.; Zajkowska, O.; Rusek, S.; Adamczyk-Sowa, M.; Kubicka-Bączyk, K.; Kalinowska-Łyszczarz, A.; Kania, K.; Słowik, A.; Wnuk, M.; et al. Clinical course and outcome of SARS-CoV-2 infection in multiple sclerosis patients treated with disease-modifying therapies—The Polish experience. Neurol. Neurochir. Pol. 2021, 55, 212–222. [Google Scholar] [CrossRef]
- Habek, M.; Jakob Brecl, G.; Bašić Kes, V.; Rogić, D.; Barun, B.; Gabelić, T.; Emeršič, A.; Horvat Ledinek, A.; Grbić, N.; Lapić, I.; et al. Humoral immune response in convalescent COVID-19 people with multiple sclerosis treated with high-efficacy disease-modifying therapies: A multicenter, case-control study. J. Neuroimmunol. 2021, 359, 577696. [Google Scholar] [CrossRef]
- Hughes, R.; Whitley, L.; Fitovski, K.; Schneble, H.M.; Muros, E.; Sauter, A.; Craveiro, L.; Dillon, P.; Bonati, U.; Jessop, N.; et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 49, 102725. [Google Scholar] [CrossRef]
- Van Kempen, Z.; Strijbis, E.; Al, M.; Steenhuis, M.; Uitdehaag, B.; Rispens, T.; Killestein, J. SARS-CoV-2 Antibodies in Adult Patients With Multiple Sclerosis in the Amsterdam MS Cohort. JAMA Neurol. 2021, 78, 880–882. [Google Scholar] [CrossRef]
- Conte, W.L. Attenuation of antibody response to SARS-CoV-2 infection in patients with multiple sclerosis on ocrelizumab: A case-control study. Mult. Scler. Relat. Disord. 2021, 52, 103014. [Google Scholar] [CrossRef]
- Achiron, A.; Gurevich, M.; Falb, R.; Dreyer-Alster, S.; Sonis, P.; Mandel, M. SARS-CoV-2 antibody dynamics and B-cell memory response over time in COVID-19 convalescent subjects. Clin. Microbiol. Infect. 2021, 27, 1349.e1–1349.e6. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, M.; Wang, J.; Li, J.; Hu, G.; Wang, L. Over 1-year duration and age difference of SARS-CoV-2 antibodies in convalescent COVID-19 patients. J. Med. Virol. 2021, 93, 6506–6511. [Google Scholar] [CrossRef]
- Rammohan, K.W.; Williams, M.J. Expert Perspectives on COVID-19 Vaccination for People Living with Multiple Sclerosis. Neurol. Ther. 2021, 10, 415–425. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Pikatza-Menoio, O.; Elicegui, A.; Bengoetxea, X.; Naldaiz-Gastesi, N.; López de Munain, A.; Gerenu, G.; Gil-Bea, F.J.; Alonso-Martín, S. The Skeletal Muscle Emerges as a New Disease Target in Amyotrophic Lateral Sclerosis. J. Pers. Med. 2021, 11, 671. [Google Scholar] [CrossRef]
- Digala, L.P.; Prasanna, S.; Rao, P.; Govindarajan, R.; Qureshi, A.I. Impact of COVID-19 Infection Among Hospitalized Amyotrophic Lateral Sclerosis Patients. J. Clin. Neuromuscul. Dis. 2021, 22, 180–181. [Google Scholar] [CrossRef]
- Bertran Recasens, B.; Povedano Panadés, M.; Rubio, M.A. Impact of the COVID-19 pandemic on a cohort of ALS patients in Catalonia. Impacto de la pandemia de COVID-19 en una cohorte de pacientes con ELA en Cataluña. Neurologia 2021, 36, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Galea, M.D.; Galea, V.P.; Eberhart, A.C.; Patwa, H.S.; Howard, I.; Fournier, C.N.; Bedlack, R.S. Infection rate, mortality and characteristics of veterans with amyotrophic lateral sclerosis with COVID-19. Muscle Nerve 2021, 64, E18–E20. [Google Scholar] [CrossRef] [PubMed]
- Zanella, I.; Zacchi, E.; Piva, S.; Filosto, M.; Beligni, G.; Alaverdian, D.; Amitrano, S.; Fava, F.; Baldassarri, M.; Frullanti, E.; et al. C9orf72 Intermediate Repeats Confer Genetic Risk for Severe COVID-19 Pneumonia Independently of Age. Int. J. Mol. Sci. 2021, 22, 6991. [Google Scholar] [CrossRef]
- Li, X.; Bedlack, R. COVID-19-accelerated disease progression in two patients with amyotrophic lateral sclerosis. Muscle Nerve 2021, 64, E13–E15. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef] [Green Version]
- Tani, M.; Ito, M.; Igarashi, Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Tasaki, T.; Ninomiya, H.; Ueda, Y.; Kuremoto, K.I.; Mitsutake, S.; Igarashi, Y.; Okazaki, T.; Takegami, T. Sphingomyelin generated by sphingomyelin synthase 1 is involved in attachment and infection with Japanese encephalitis virus. Sci. Rep. 2016, 6, 37829. [Google Scholar] [CrossRef] [PubMed]
- D’Aprile, C.; Prioni, S.; Mauri, L.; Prinetti, A.; Grassi, S. Lipid rafts as platforms for sphingosine 1-phosphate metabolism and signalling. Cell Signal. 2021, 80, 109929. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Lessons from S1P receptor targeting in multiple sclerosis. Pharmacol. Ther. 2022, 230, 107971. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Tan-Chen, S.; Guitton, J.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism and Signaling in Skeletal Muscle: From Physiology to Physiopathology. Front. Endocrinol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Loehr, J.A.; Abo-Zahrah, R.; Pal, R.; Rodney, G.G. Sphingomyelinase promotes oxidant production and skeletal muscle contractile dysfunction through activation of NADPH oxidase. Front. Physiol. 2014, 5, 530. [Google Scholar] [CrossRef] [Green Version]
- Babenko, N.A.; Garkavenko, V.V.; Storozhenko, G.V.; Timofiychuk, O.A. Role of acid sphingomyelinase in the age-dependent dysregulation of sphingolipids turnover in the tissues of rats. Gen. Physiol. Biophys. 2016, 35, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Hassouneh, L.K.; Timofiychuk, O.A.; Babenko, N.A. Acid sphingomyelinase inhibitors, imipramine and zoledronic acid, increase skeletal muscle tissue sensitivity to insulin action at old age. Gen. Physiol. Biophys. 2018, 37, 163–674. [Google Scholar] [CrossRef]
- Nagata, Y.; Partridge, T.A.; Matsuda, R.; Zammit, P.S. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J. Cell Biol. 2006, 174, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Olivera, A.; Allende, M.L.; Proia, R.L. Shaping the landscape: Metabolic regulation of S1P gradients. Biochim. Biophys. Acta 2013, 1831, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoja, M.; Ruohola-Baker, H. Drosophila as a starting point for developing therapeutics for the rare disease Duchenne Muscular Dystrophy. Rare Dis. 2013, 1, e24995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, K.C.; Baldwin, D.; Saba, J.D. Sphingolipid signaling and hematopoietic malignancies: To the rheostat and beyond. Anticancer Agents Med. Chem. 2011, 11, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Ieronimakis, N.; Pantoja, M.; Hays, A.L.; Dosey, T.L.; Qi, J.; Fischer, K.A.; Hoofnagle, A.N.; Sadilek, M.; Chamberlain, J.S.; Ruohola-Baker, H.; et al. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet. Muscle 2013, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germinario, E.; Peron, S.; Toniolo, L.; Betto, R.; Cencetti, F.; Donati, C.; Bruni, P.; Danieli-Betto, D. S1P2 receptor promotes mouse skeletal muscle regeneration. J. Appl. Physiol. 2012, 113, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Kelley, R.C.; Ferreira, L.F. Diaphragm abnormalities in heart failure and aging: Mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail. Rev. 2017, 22, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Jin, Y.; Zhao, P.; Wu, J.; Ren, Z. Lipid droplets contribute myogenic differentiation in C2C12 by promoting the remodeling of the acstin-filament. Cell Death Dis. 2021, 12, 1102. [Google Scholar] [CrossRef]
- Rivas, D.A.; McDonald, D.J.; Rice, N.P.; Haran, P.H.; Dolnikowski, G.G.; Fielding, R.A. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R561–R569. [Google Scholar] [CrossRef] [Green Version]
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of sphingolipids in senescence: Implication in aging and age-related diseases. J. Clin. Investig. 2018, 128, 2702–2712. [Google Scholar] [CrossRef]
- Baranowski, M.; Błachnio-Zabielska, A.U.; Charmas, M.; Helge, J.W.; Dela, F.; Książek, M.; Długołęcka, B.; Klusiewicz, A.; Chabowski, A.; Górski, J. Exercise increases sphingoid base-1-phosphate levels in human blood and skeletal muscle in a time- and intensity-dependent manner. Eur. J. Appl. Physiol. 2015, 115, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- Perreault, L.; Newsom, S.A.; Strauss, A.; Kerege, A.; Kahn, D.E.; Harrison, K.A.; Snell-Bergeon, J.K.; Nemkov, T.; D’Alessandro, A.; Jackman, M.R.; et al. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight 2018, 3, e96805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodun, K.; Chabowski, A.; Baranowski, M. Sphingosine-1-phosphate in acute exercise and training. Scand. J. Med. Sci. Sports 2021, 31, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar] [PubMed]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Filgueiram, T.O.; Castoldi, A.; Santos, L.E.R.; de Amorim, G.J.; de Sousa Fernandes, M.S.; Anastácio, W.L.D.N.; Campos, E.Z.; Santos, T.M.; Souto, F.O. The Relevance of a Physical Active Lifestyle and Physical Fitness on Immune Defense: Mitigating Disease Burden, with Focus on COVID-19 Consequences. Front. Immunol. 2021, 12, 587146. [Google Scholar] [CrossRef]
- Schneider-Schaulies, S.; Schumacher, F.; Wigger, D.; Schöl, M.; Waghmare, T.; Schlegel, J.; Seibel, J.; Kleuser, B. Sphingolipids: Effectors and Achilles Heals in Viral Infections? Cells 2021, 10, 2175. [Google Scholar] [CrossRef]
- Meacci, E.; Garcia-Gil, M.; Pierucci, F. SARS-CoV-2 Infection: A Role for S1P/S1P Receptor Signaling in the Nervous System? Int. J. Mol. Sci. 2020, 21, 6773. [Google Scholar] [CrossRef]
- Wang, Y.; Perlman, S. COVID-19: Inflammatory Profile. Annu. Rev. Med. 2022, 73, 65–80. [Google Scholar] [CrossRef]
- Sorice, M.; Misasi, R.; Riitano, G.; Manganelli, V.; Martellucci, S.; Longo, A.; Garofalo, T.; Mattei, V. Targeting Lipid Rafts as a Strategy Against Coronavirus. Front. Cell Dev. Biol. 2021, 8, 618296. [Google Scholar] [CrossRef]
- Fantini, J.; Chahinian, H.; Yahi, N. Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal. Int. J. Antimicrob. Agents 2020, 56, 106020. [Google Scholar] [CrossRef] [PubMed]
- Strating, J.R.; van Kuppeveld, F.J. Viral rewiring of cellular lipid metabolism to create membranous repl1ication compartments. Curr. Opin. Cell Biol. 2017, 47, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, X.; Fu, Z.; Chen, J.; Chen, S.; Tan, Y. PathogenTrack and Yeskit: Tools for identifying intracellular pathogens from single-cell RNA-sequencing datasets as illustrated by application to COVID-19. Front. Med. 2022, 16, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Kolesnick, R. Raft ceramide in molecular medicine. Oncogene 2003, 22, 7070–7077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassmé, H.; Riehle, A.; Wilker, B.; Gulbins, E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J. Biol. Chem. 2005, 280, 26256–26262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezgovsek, J.; Gulbins, E.; Friedrich, S.K.; Lang, K.S.; Duhan, V. Sphingolipids in early viral replication and innate immune activation. Biol. Chem. 2018, 399, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Muehlbacher, M.; Trapp, S.; Pechmann, S.; Friedl, A.; Reichel, M.; Mühle, C.; Terfloth, L.; Groemer, T.W.; Spitzer, G.M.; et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE 2011, 6, e23852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Schloer, S.; Brunotte, L.; Goretzko, J.; Mecate-Zambrano, A.; Korthals, N.; Gerke, V.; Ludwig, S.; Rescher, U. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg. Microbes Infect. 2020, 9, 2245–2255. [Google Scholar] [CrossRef]
- Zimniak, M.; Kirschner, L.; Hilpert, H.; Geiger, N.; Danov, O.; Oberwinkler, H.; Steinke, M.; Sewald, K.; Seibel, J.; Bodem, J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. J. Sci. Rep. 2021, 11, 5890. [Google Scholar] [CrossRef]
- Proto, M.C.; Fiore, D.; Piscopo, C.; Pagano, C.; Galgani, M.; Bruzzaniti, S.; Laezza, C.; Gazzerro, P.; Bifulco, M. Lipid homeostasis and mevalonate pathway in COVID-19: Basic concepts and potential therapeutic targets. Prog. Lipid Res. 2021, 82, 101099. [Google Scholar] [CrossRef] [PubMed]
- Moolamalla, S.T.R.; Balasubramanian, R.; Chauhan, R.; Priyakumar, U.D.; Vinod, P.K. Host metabolic reprogramming in response to SARS-CoV-2 infection: A systems biology approach. Microb. Pathog. 2021, 158, 105114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Thomas, T.; Akpan, I.J.; Reisz, J.A.; Cendali, F.I.; Gamboni, F.; Nemkov, T.; Thangaraju, K.; Katneni, U.; Tanaka, K.; et al. Biological and Clinical Factors Contributing to the Metabolic Heterogeneity of Hospitalized Patients with and without COVID-19. Cells 2021, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Vitner, E.B.; Achdout, H.; Avraham, R.; Politi, B.; Cherry, L.; Tamir, H.; Yahalom-Ronen, Y.; Paran, N.; Melamed, S.; Erez, N.; et al. Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus. J. Biol. Chem. 2021, 296, 100470. [Google Scholar] [CrossRef]
- Janneh, A.H.; Kassir, M.F.; Dwyer, C.J.; Chakraborty, P.; Pierce, J.S.; Flume, P.A.; Li, H.; Nadig, S.N.; Mehrotra, S.; Ogretmen, B. Alterations of lipid metabolism provide serologic biomarkers for the detection of asymptomatic versus symptomatic COVID-19 patients. Sci. Rep. 2021, 11, 14232. [Google Scholar] [CrossRef]
- Edwards, M.J.; Becker, K.A.; Gripp, B.; Hoffmann, M.; Keitsch, S.; Wilker, B.; Soddemann, M.; Gulbins, A.; Carpinteiro, E.; Patel, S.H.; et al. Sphingosine prevents binding of SARS-CoV-2 spike to its cellular receptor ACE2. J. Biol. Chem. 2020, 295, 15174–15182. [Google Scholar] [CrossRef]
- Wolf, J.J.; Studstill, C.J.; Hahm, B. Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections. Viruses 2019, 11, 1097. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.S.; Märtz, K.B.; Nierhaus, A.; Daum, G.; Schwedhelm, E.; Kluge, S.; Gräler, M.H. Loss of sphingosine 1-phosphate (S1P) in septic shock is predominantly caused by decreased levels of high-density lipoproteins (HDL). J. Intensive Care 2019, 7, 23. [Google Scholar] [CrossRef]
- Torretta, E.; Garziano, M.; Poliseno, M.; Capitanio, D.; Biasin, M.; Santantonio, T.A.; Clerici, M.; Lo Caputo, S.; Trabattoni, D.; Gelfi, C. Severity of COVID-19 Patients Predicted by Serum Sphingolipids Signature. Int. J. Mol. Sci. 2021, 22, 10198. [Google Scholar] [CrossRef]
- Marfia, G.; Navone, S.; Guarnaccia, L.; Campanella, R.; Mondoni, M.; Locatelli, M.; Barassi, A.; Fontana, L.; Palumbo, F.; Garzia, E.; et al. Decreased serum level of sphingosine-1-phosphate: A novel predictor of clinical severity in COVID-19. EMBO Mol. Med. 2021, 13, e13424. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ottolenghi, S.; Morano, C.; Rinaldo, R.; Roda, G.; Chiumello, D.; Centanni, S.; Samaja, M.; Paroni, R. Link between serum lipid signature and prognostic factors in COVID-19 patients. Sci. Rep. 2021, 11, 21633. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, C.; Bizkarguenaga, M.; Gil-Redondo, R.; Diercks, T.; Arana, E.; García de Vicuña, A.; Seco, M.; Bosch, A.; Palazón, A.; San Juan, I.; et al. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience 2020, 23, 101645. [Google Scholar] [CrossRef] [PubMed]
- Meoni, G.; Ghini, V.; Maggi, L.; Vignoli, A.; Mazzoni, A.; Salvati, L.; Capone, M.; Vanni, A.; Tenori, L.; Fontanari, P.; et al. Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog. 2021, 17, e1009243. [Google Scholar] [CrossRef] [PubMed]
- Castañé, H.; Iftimie, S.; Baiges-Gaya, G.; Rodríguez-Tomàs, E.; Jiménez-Franco, A.; López-Azcona, A.F.; Garrido, P.; Castro, A.; Camps, J.; Joven, J. Machine learning and semi-targeted lipidomics identify distinct serum lipid signatures in hospitalized COVID-19-positive and COVID-19-negative patients. Metabolism 2022, 131, 155197. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Moreno, L.O.; Rello, S.R.; Orduña, A.; Bernardo, D.; Cifuentes, A. Metabolomics study of COVID-19 patients in four different clinical stages. Sci. Rep. 2022, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Z.; Feng, G.; Chen, M.; Wan, Q.; Lin, J.; Wu, L.; Nie, W.; Chen, S. Distinct lipid metabolic dysregulation in asymptomatic COVID-19. iScience 2021, 24, 102974. [Google Scholar] [CrossRef]
- Li, Y.; Hou, G.; Zhou, H.; Wang, Y.; Tun, H.M.; Zhu, A.; Zhao, J.; Xiao, F.; Lin, S.; Liu, D.; et al. Multi-platform omics analysis reveals molecular signature for COVID-19 pathogenesis, prognosis and drug target discovery. Signal Transduct. Target. Ther. 2021, 6, 155. [Google Scholar] [CrossRef]
- Marín-Corral, J.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Pascual-Guardia, S.; Muñoz-Bermúdez, R.; Salazar-Degracia, A.; Pérez-Terán, P.; Restrepo, M.I.; Khymenets, O.; Haro, N.; et al. Metabolic Signatures Associated with Severity in Hospitalized COVID-19 Patients. Int. J. Mol. Sci. 2021, 22, 4794. [Google Scholar] [CrossRef]
- Caterino, M.; Costanzo, M.; Fedele, R.; Cevenini, A.; Gelzo, M.; Di Minno, A.; Andolfo, I.; Capasso, M.; Russo, R.; Annunziata, A.; et al. The Serum Metabolome of Moderate and Severe COVID-19 Patients Reflects Possible Liver Alterations Involving Carbon and Nitrogen Metabolism. Int. J. Mol. Sci. 2021, 22, 9548. [Google Scholar] [CrossRef]
- Masoodi, M.; Peschka, M.; Schmiedel, S.; Haddad, M.; Frye, M.; Maas, C.; Lohse, A.; Huber, S.; Kirchhof, P.; Nofer, J.R.; et al. Disturbed lipid and amino acid metabolisms in COVID-19 patients. J. Mol. Med. 2022, 100, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Caterino, M.; Fedele, R.; Cevenini, A.; Pontillo, M.; Barra, L.; Ruoppolo, M. COVIDomics: The Proteomic and Metabolomic Signatures of COVID-19. Int. J. Mol. Sci. 2022, 23, 2414. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Shalagina, M.N.; Protopopov, V.A.; Sergeev, V.G.; Ovechkin, S.V.; Ovchinina, N.G.; Sekunov, A.V.; Zefirov, A.L.; Zakirjanova, G.F.; Bryndina, I.G. Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse. Int. J. Mol. Sci. 2019, 20, 4860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef] [Green Version]
- Kornhuber, J.; Hoertel, N.; Gulbins, E. The acid sphingomyelinase/ceramide system in COVID-19. Mol. Psychiatry 2022, 27, 307–314. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Abellán, M.; de la Muela, P.; Vernet, R.; Beeker, N.; Neuraz, A.; et al. Association between FIASMA psychotropic medications and reduced risk of intubation or death in individuals with psychiatric disorders hospitalized for severe COVID-19: An observational multicenter study. Transl. Psychiatry 2022, 12, 90. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meacci, E.; Pierucci, F.; Garcia-Gil, M. Skeletal Muscle and COVID-19: The Potential Involvement of Bioactive Sphingolipids. Biomedicines 2022, 10, 1068. https://doi.org/10.3390/biomedicines10051068
Meacci E, Pierucci F, Garcia-Gil M. Skeletal Muscle and COVID-19: The Potential Involvement of Bioactive Sphingolipids. Biomedicines. 2022; 10(5):1068. https://doi.org/10.3390/biomedicines10051068
Chicago/Turabian StyleMeacci, Elisabetta, Federica Pierucci, and Mercedes Garcia-Gil. 2022. "Skeletal Muscle and COVID-19: The Potential Involvement of Bioactive Sphingolipids" Biomedicines 10, no. 5: 1068. https://doi.org/10.3390/biomedicines10051068
APA StyleMeacci, E., Pierucci, F., & Garcia-Gil, M. (2022). Skeletal Muscle and COVID-19: The Potential Involvement of Bioactive Sphingolipids. Biomedicines, 10(5), 1068. https://doi.org/10.3390/biomedicines10051068





