Tumor-Specific Imaging with Angiostamp800 or Bevacizumab-IRDye 800CW Improves Fluorescence-Guided Surgery over Indocyanine Green in Peritoneal Carcinomatosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fluorescent Probes and Drugs
2.2. Cell Lines
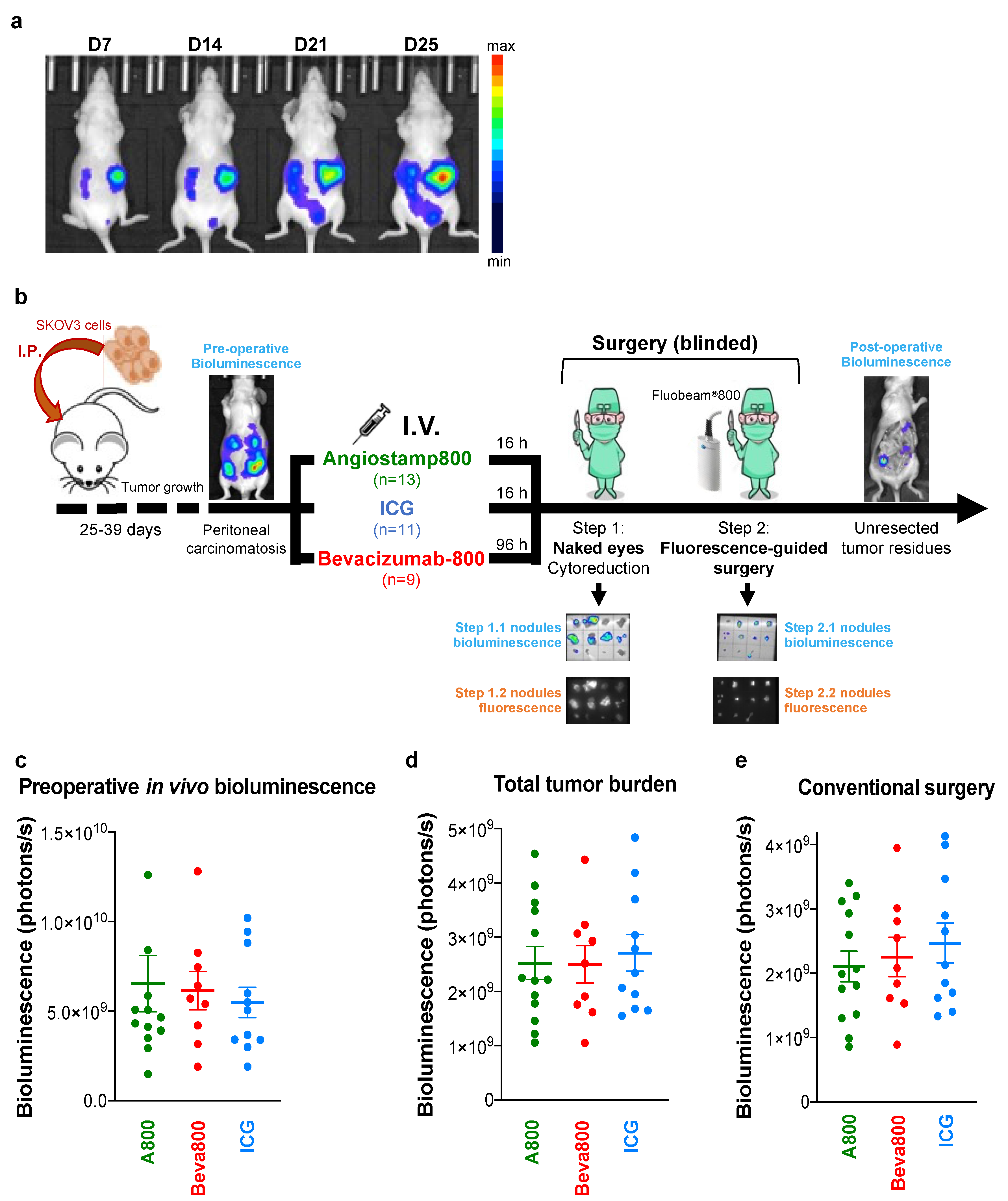
2.3. In Vivo Tumor Models
2.4. Fluorescence-Guided Surgery in Mice with Orthotopic Peritoneal Carcinomatosis
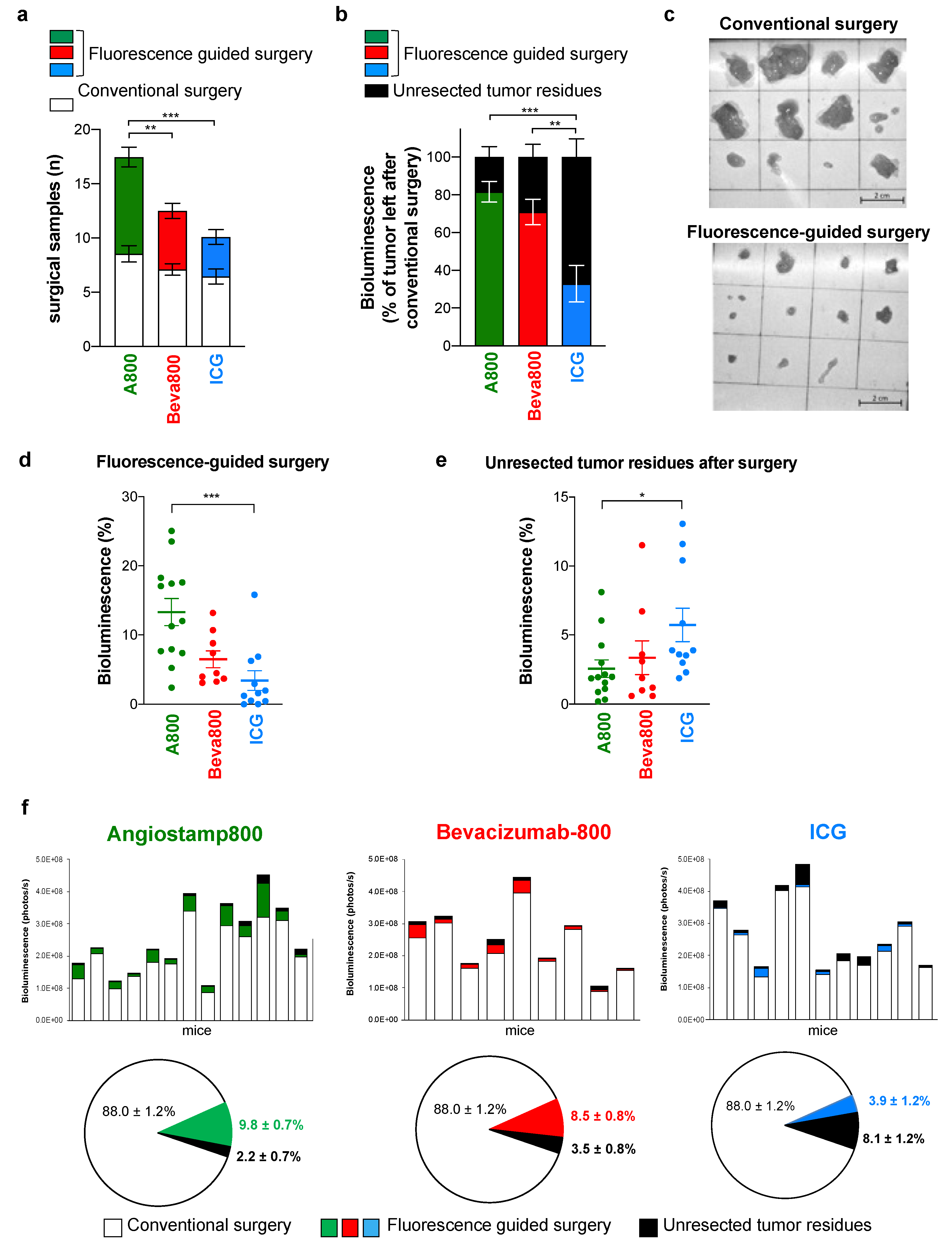
2.5. Ex Vivo Surgical Specimen Examination by Sequential Bioluminescence and Fluorescence Imaging
2.6. Statistical Analyses
3. Results
3.1. Bevacizumab-IRDye 800CW Circulated Longer in the Bloodstream than Angiostamp800 and ICG
3.2. Angiostamp800, Bevacizumab-IRDye 800CW, and ICG Accumulated in Primary Ovarian Tumors
3.3. Angiostamp800 and Bevacizumab-IRDye 800CW Improved Fluorescence-Guided Surgery of Peritoneal Carcinomatosis More than ICG
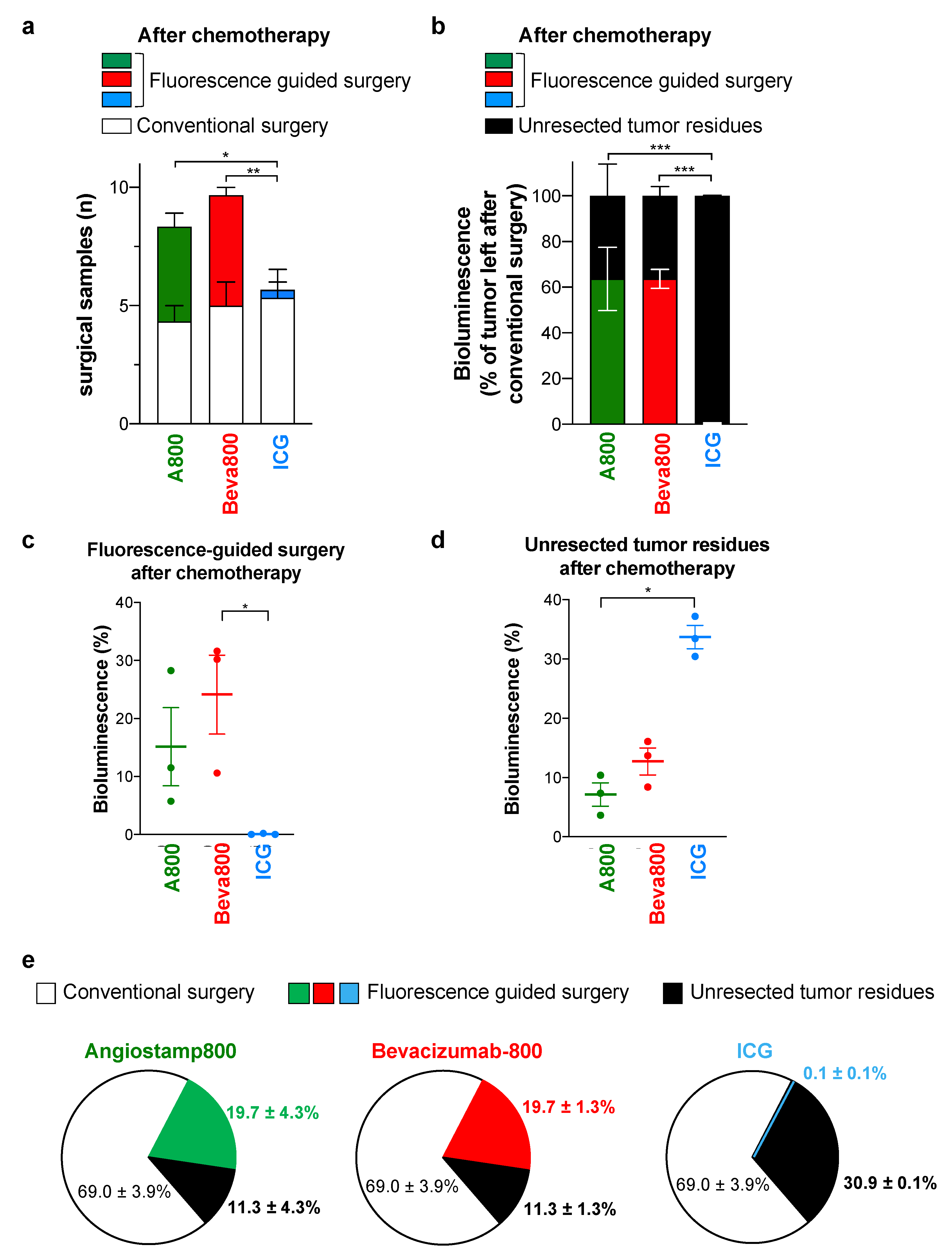
3.4. In Contrast to ICG, Angiostamp800 and Bevacizumab-IRDye 800CW Further Improved Surgery for Peritoneal Carcinomatosis after Neoadjuvant Chemotherapy
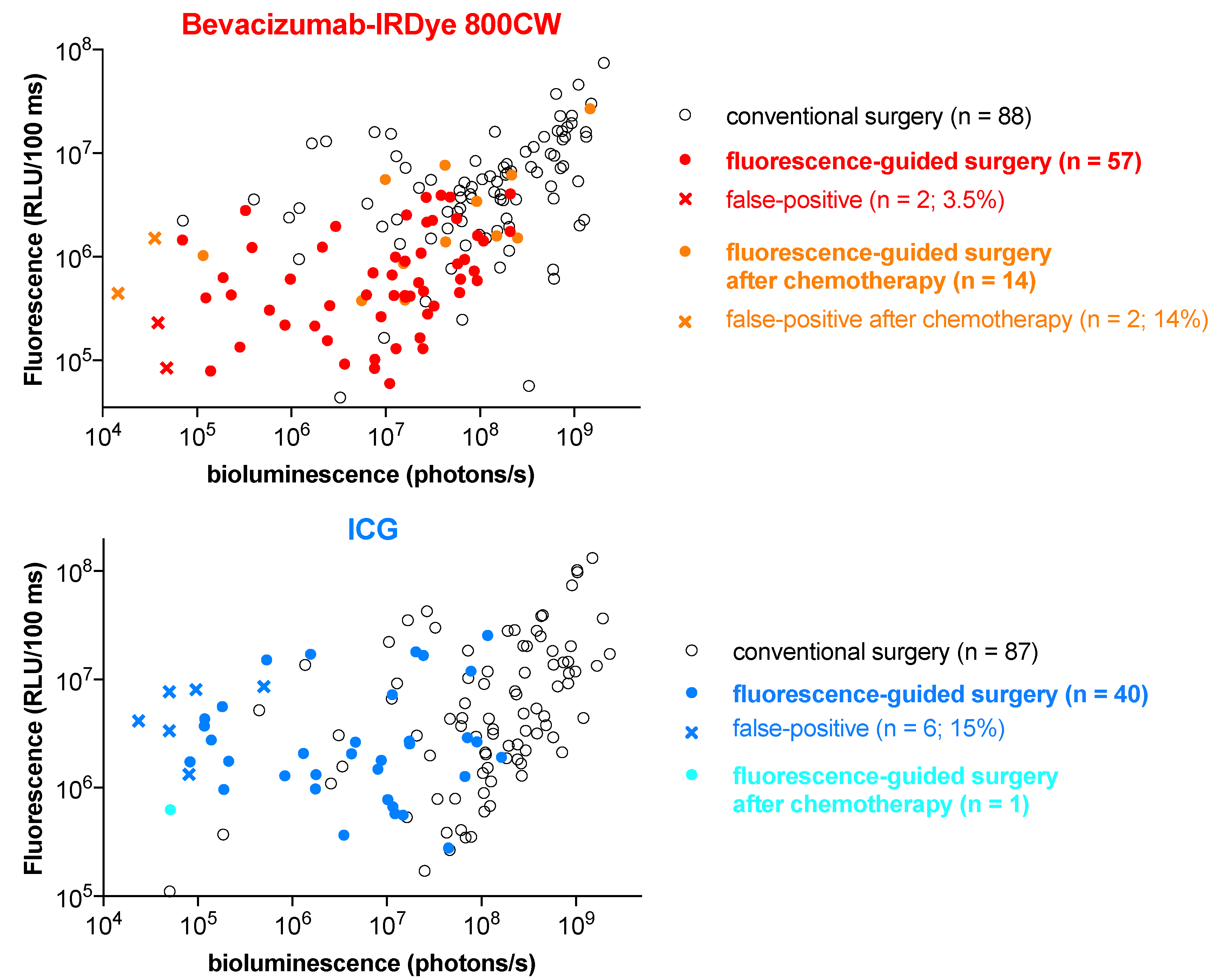
3.5. Angiostamp800 and Bevacizumab-IRDye 800CW Showed Higher Specificity and Lower False-Positive Rate than ICG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Azais, H.; Estevez, J.P.; Foucher, P.; Kerbage, Y.; Mordon, S.; Collinet, P. Dealing with microscopic peritoneal metastases of epithelial ovarian cancer. A surgical challenge. Surg. Oncol. 2017, 26, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Xu, S.; Bulin, A.-L.; Hurbin, A.; Elleaume, H.; Coll, J.-L.; Broekgaarden, M. Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives. Cancers 2020, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and developments in fluorescence-guided cancer surgery. Nat. Rev. Clin. Oncol. 2021, 19, 9–22. [Google Scholar] [CrossRef]
- Van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiocchi, G.L.; Diana, M.; Boni, L. indocyanine green-based uorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J. Gastroenterol. 2018, 24, 2921–2930. [Google Scholar] [CrossRef]
- Liberale, G.; Bourgeois, P.; Larsimont, D.; Moreau, M.; Donckier, V.; Ishizawa, T. Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: A systematic review. Eur. J. Surg. Oncol. 2017, 43, 1656–1667. [Google Scholar] [CrossRef]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Peters, A.A.W.; de Kroon, C.D.; Trimbos, J.B.M.Z.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef]
- Veys, I.; Pop, F.C.; Vankerckhove, S.; Barbieux, R.; Chintinne, M.; Moreau, M.; Nogaret, J.M.; Larsimont, D.; Donckier, V.; Bourgeois, P.; et al. ICG-fluorescence imaging for detection of peritoneal metastases and residual tumoral scars in locally advanced ovarian cancer: A pilot study. J. Surg. Oncol. 2018, 117, 228–235. [Google Scholar] [CrossRef]
- Lamberts, L.E.; Koch, M.; de Jong, J.S.; Adams, A.L.L.; Glatz, J.; Kranendonk, M.E.G.; Terwisscha van Scheltinga, A.G.T.; Jansen, L.; de Vries, J.; Lub-de Hooge, M.N.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.; de Jong, J.S.; Glatz, J.; Symvoulidis, P.; Lamberts, L.E.; Adams, A.L.; Kranendonk, M.E.; Terwisscha van Scheltinga, A.G.; Aichler, M.; Jansen, L.; et al. Threshold Analysis and Biodistribution of Fluorescently Labeled Bevacizumab in Human Breast Cancer. Cancer Res. 2017, 77, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagengast, W.B.; Hartmans, E.; Garcia-Allende, P.B.; Peters, F.T.M.; Linssen, M.D.; Koch, M.; Koller, M.; Tjalma, J.J.J.; Karrenbeld, A.; Jorritsma-Smit, A.; et al. Near-infrared fluorescence molecular endoscopy detects dysplastic oesophageal lesions using topical and systemic tracer of vascular endothelial growth factor A. Gut 2019, 68, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmans, E.; Tjalma, J.J.J.; Linssen, M.D.; Allende, P.B.G.; Koller, M.; Jorritsma-Smit, A.; Nery, M.; Elias, S.G.; Karrenbeld, A.; de Vries, E.G.E.; et al. Potential Red-Flag Identification of Colorectal Adenomas with Wide-Field Fluorescence Molecular Endoscopy. Theranostics 2018, 8, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: A single-centre feasibility study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Garanger, E.; Boturyn, D.; Jin, Z.; Dumy, P.; Favrot, M.C.; Coll, J.L. New multifunctional molecular conjugate vector for targeting, imaging, and therapy of tumors. Mol. Ther. 2005, 12, 1168–1175. [Google Scholar] [CrossRef]
- Wenk, C.H.; Ponce, F.; Guillermet, S.; Tenaud, C.; Boturyn, D.; Dumy, P.; Watrelot-Virieux, D.; Carozzo, C.; Josserand, V.; Coll, J.L. Near-infrared optical guided surgery of highly infiltrative fibrosarcomas in cats using an anti-alphavss3 integrin molecular probe. Cancer Lett. 2013, 334, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Dutour, A.; Josserand, V.; Jury, D.; Guillermet, S.; Decouvelaere, A.V.; Chotel, F.; Pointecouteau, T.; Rizo, P.; Coll, J.L.; Blay, J.Y. Targeted imaging of alpha(v)beta(3) expressing sarcoma tumor cells in vivo in pre-operative setting using near infrared: A potential tool to reduce incomplete surgical resection. Bone 2014, 62, 71–78. [Google Scholar] [CrossRef]
- Atallah, I.; Milet, C.; Henry, M.; Josserand, V.; Reyt, E.; Coll, J.L.; Hurbin, A.; Righini, C.A. Near-infrared fluorescence imaging-guided surgery improves recurrence-free survival rate in novel orthotopic animal model of head and neck squamous cell carcinoma. Head Neck 2016, 38 (Suppl. S1), E246–E255. [Google Scholar] [CrossRef] [Green Version]
- Bellanger, A.; Donini, C.F.; Vendrell, J.A.; Lavaud, J.; Machuca-Gayet, I.; Ruel, M.; Vollaire, J.; Grisard, E.; Győrffy, B.; Bièche, I.; et al. The critical role of the ZNF217 oncogene in promoting breast cancer metastasis to the bone. J. Pathol. 2017, 242, 73–89. [Google Scholar] [CrossRef]
- Lavaud, J.; Henry, M.; Gayet, P.; Fertin, A.; Vollaire, J.; Usson, Y.; Coll, J.L.; Josserand, V. Noninvasive monitoring of liver metastasis development via combined multispectral photoacoustic imaging and fluorescence diffuse optical tomography. Int. J. Biol. Sci. 2020, 16, 1616–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josserand, V.; Keramidas, M.; Lavaud, J.; Righini, C.; Vollaire, J.; Bellard, E.; Rols, M.P.; Teissie, J.; Coll, J.L.; Golzio, M. Electrochemotherapy guided by intraoperative fluorescence imaging for the treatment of inoperable peritoneal micro-metastases. J. Control. Release 2016, 233, 81–87. [Google Scholar] [CrossRef]
- Keramidas, M.; Josserand, V.; Righini, C.A.; Wenk, C.; Faure, C.; Coll, J.L. Intraoperative near-infrared image-guided surgery for peritoneal carcinomatosis in a preclinical experimental model. Br. J. Surg. 2010, 97, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Mery, E.; Jouve, E.; Guillermet, S.; Bourgognon, M.; Castells, M.; Golzio, M.; Rizo, P.; Delord, J.P.; Querleu, D.; Couderc, B. Intraoperative fluorescence imaging of peritoneal dissemination of ovarian carcinomas. A preclinical study. Gynecol. Oncol. 2011, 122, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Michy, T.; Massias, T.; Bernard, C.; Vanwonterghem, L.; Henry, M.; Guidetti, M.; Royal, G.; Coll, J.L.; Texier, I.; Josserand, V.; et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers 2019, 11, 1760. [Google Scholar] [CrossRef] [Green Version]
- Tomkowiak, M.; Ghittoni, R.; Teixeira, M.; Blanquier, B.; Szecsi, J.; Negre, D.; Aubert, D.; Coupet, C.A.; Brunner, M.; Verhoeyen, E.; et al. Generation of transgenic mice expressing EGFP protein fused to NP68 MHC class I epitope using lentivirus vectors. Genesis 2013, 51, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Murillo, A.; Lozano, M.L.; Alvarez, L.; Jacome, A.; Almarza, E.; Navarro, S.; Segovia, J.C.; Hanenberg, H.; Guenechea, G.; Bueren, J.A.; et al. Development of lentiviral vectors with optimized transcriptional activity for the gene therapy of patients with Fanconi anemia. Hum. Gene Ther. 2010, 21, 623–630. [Google Scholar] [CrossRef]
- Gilson, P.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Vollaire, J.; Baulin, V.; Werner, M.; Orlowska, A.; Josserand, V.; Mahuteau-Betzer, F.; et al. The pyrrolopyrimidine colchicine-binding site agent PP-13 reduces the metastatic dissemination of invasive cancer cells in vitro and in vivo. Biochem. Pharmacol. 2019, 160, 1–13. [Google Scholar] [CrossRef]
- Bouclier, C.; Simon, M.; Laconde, G.; Pellerano, M.; Diot, S.; Lantuejoul, S.; Busser, B.; Vanwonterghem, L.; Vollaire, J.; Josserand, V.; et al. Stapled peptide targeting the CDK4/Cyclin D interface combined with Abemaciclib inhibits KRAS mutant lung cancer growth. Theranostics 2020, 10, 2008–2028. [Google Scholar] [CrossRef]
- Jeannot, V.; Gauche, C.; Mazzaferro, S.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Didier, C.; Vollaire, J.; Josserand, V.; Coll, J.L.; et al. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Control. Release 2018, 275, 117–128. [Google Scholar] [CrossRef]
- Porcu, E.P.; Salis, A.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol. Adv. 2016, 34, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Day, K.E.; Beck, L.N.; Heath, C.H.; Huang, C.C.; Zinn, K.R.; Rosenthal, E.L. Identification of the optimal therapeutic antibody for fluorescent imaging of cutaneous squamous cell carcinoma. Cancer Biol. Ther. 2013, 14, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Scheltinga, A.G.T.T.; van Dam, G.M.; Nagengast, W.B.; Ntziachristos, V.; Hollema, H.; Herek, J.L.; Schroder, C.P.; Kosterink, J.G.; Lub-de Hoog, M.N.; de Vries, E.G. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J. Nucl. Med. 2011, 52, 1778–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.; Xie, Y.; Bae, S.; et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153. [Google Scholar] [CrossRef]
- Garcia de Jalon, E.; Kleinmanns, K.; Fosse, V.; Davidson, B.; Bjorge, L.; Haug, B.E.; McCormack, E. Comparison of Five Near-Infrared Fluorescent Folate Conjugates in an Ovarian Cancer Model. Mol. Imaging Biol. 2021, 1–21. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Morimoto, Y.; Takahata, R.; Nomura, S.; Yoshida, K.; Horiguchi, H.; Hiraki, S.; Ono, S.; Miyazaki, H.; Saito, D.; et al. Photodynamic therapy using nanoparticle loaded with indocyanine green for experimental peritoneal dissemination of gastric cancer. Cancer Sci. 2014, 105, 1626–1630. [Google Scholar] [CrossRef]
- He, P.; Huang, T.; Fang, C.; Su, S.; Tian, J.; Xia, X.; Li, B. Identification of extrahepatic metastasis of hepatocellular carcinoma using indocyanine green fluorescence imaging. Photodiagn. Photodyn. Ther. 2019, 25, 417–420. [Google Scholar] [CrossRef]
- Wu, F.; Tamhane, M.; Morris, M.E. Pharmacokinetics, Lymph Node Uptake, and Mechanistic PK Model of Near-Infrared Dye-Labeled Bevacizumab After IV and SC Administration in Mice. AAPS J. 2012, 14, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Nagengast, W.B.; de Vries, E.G.; Hospers, G.A.; Mulder, N.H.; de Jong, J.R.; Hollema, H.; Brouwers, A.H.; van Dongen, G.A.; Perk, L.R.; Lub-de Hooge, M.N. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J. Nucl. Med. 2007, 48, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Rizov, M.; Andreeva, P.; Dimova, I. Molecular regulation and role of angiogenesis in reproduction. Taiwan. J. Obstet. Gynecol. 2017, 56, 127–132. [Google Scholar] [CrossRef]
- Atallah, I.; Milet, C.; Quatre, R.; Henry, M.; Reyt, E.; Coll, J.L.; Hurbin, A.; Righini, C.A. Role of near-infrared fluorescence imaging in the resection of metastatic lymph nodes in an optimized orthotopic animal model of HNSCC. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, K.; Fujiwara, Y.; Yano, M.; Motoori, M.; Sugimura, K.; Takahashi, H.; Ohue, M.; Sakon, M. Usefulness of diagnostic laparoscopy with 5-aminolevulinic acid (ALA)-mediated photodynamic diagnosis for the detection of peritoneal micrometastasis in advanced gastric cancer after chemotherapy. Surg. Today 2016, 46, 1427–1434. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Josserand, V.; Bernard, C.; Michy, T.; Guidetti, M.; Vollaire, J.; Coll, J.-L.; Hurbin, A. Tumor-Specific Imaging with Angiostamp800 or Bevacizumab-IRDye 800CW Improves Fluorescence-Guided Surgery over Indocyanine Green in Peritoneal Carcinomatosis. Biomedicines 2022, 10, 1059. https://doi.org/10.3390/biomedicines10051059
Josserand V, Bernard C, Michy T, Guidetti M, Vollaire J, Coll J-L, Hurbin A. Tumor-Specific Imaging with Angiostamp800 or Bevacizumab-IRDye 800CW Improves Fluorescence-Guided Surgery over Indocyanine Green in Peritoneal Carcinomatosis. Biomedicines. 2022; 10(5):1059. https://doi.org/10.3390/biomedicines10051059
Chicago/Turabian StyleJosserand, Véronique, Claire Bernard, Thierry Michy, Mélanie Guidetti, Julien Vollaire, Jean-Luc Coll, and Amandine Hurbin. 2022. "Tumor-Specific Imaging with Angiostamp800 or Bevacizumab-IRDye 800CW Improves Fluorescence-Guided Surgery over Indocyanine Green in Peritoneal Carcinomatosis" Biomedicines 10, no. 5: 1059. https://doi.org/10.3390/biomedicines10051059
APA StyleJosserand, V., Bernard, C., Michy, T., Guidetti, M., Vollaire, J., Coll, J.-L., & Hurbin, A. (2022). Tumor-Specific Imaging with Angiostamp800 or Bevacizumab-IRDye 800CW Improves Fluorescence-Guided Surgery over Indocyanine Green in Peritoneal Carcinomatosis. Biomedicines, 10(5), 1059. https://doi.org/10.3390/biomedicines10051059






