Abstract
B7 homolog 4 protein (B7-H4), a member of the B7 family, is a immunomodulatory membrane protein. The aim of the study was to evaluate the expression of this protein in the decidua and placental tissues in case of placental abruption (PA) compared to cases of retained placental tissue (RPT) and controls. Tissue samples were obtained from 47 patients with PA, 60 patients with RPT, and 41 healthy controls. The samples were stained for B7-H4 expression, analyzed by an expert pathologist, and a semi-quantitative scale was applied. A statistical analysis revealed that the expression of B7-H4 was significantly higher in the decidua in PA samples compared to samples from patients with RPT (p-value < 0.001) and healthy controls (p-value < 0.001). The expression of B7-H4 in the placental chorionic villus was significantly higher in PA samples in relation to samples from healthy controls (p-value < 0.001) but not in relation to RPT samples (p-value = 0.0853). This finding suggests that B7-H4 might play an important role in mechanisms restoring reproductive tract homeostasis. Further research is necessary in regard to the role of B7-H4 in PA.
Keywords:
B7-H4; pregnancy; decidua; placental abruption; immunology; cytotoxicity; maternal–fetal interface 1. Introduction
The state of pregnancy is a unique balance between the activation and inhibition of the immune system in the female reproductive tract, which supports the existence and development of the fetus inside the mother’s body [1]. Remarkably, during pregnancy, the immune system is challenged to permit fetal growth in the uterus, while continuing to eliminate attacking pathogens [2].
One of the most important regulators of this balance is the decidua. The human decidua is a highly specialized tissue with many unique regulatory properties. The decidua provides complex immunological protection as well as wide nutritional support to the newly developing life [3]. The decidual cells coexist with a full range of immune cells that consist of a complex, branched system with diverse connections [1,4,5,6]. They include decidual natural killer cells (dNK) [1,7], natural killer T (NKT) cells [8], regulatory T cells (Tregs) [9,10,11], monocytes, macrophages [4,7], lymphocytes, and dendritic cells (DCs) [12]. They also influence the infiltration and activity of one another as well as other cells [13,14]. The coexistence of the decidual and immune cells is feasible due to the development of resistance to the immune-mediated apoptosis of the endometrial cells [3,15,16].
Placental abruption (PA) is defined as a complete or partial separation of the placenta from the uterine wall during pregnancy, with the fetus still being present in the uterine cavity. It is a serious perinatal complication and one of the leading causes of second- and third-trimester bleeding [17,18,19]. Placental abruption occurs in about 1% of cases [20], with the mortality rate of about 10% [21]. Numerous pathophysiological notions are linked to PA, including reduced uteroplacental blood flow [22,23], decidual vasculopathy [24], endothelial cell dysfunction, lack of adequate trophoblastic cell invasion and impaired angiogenesis [25,26,27], thrombosis [28], bacterial infection [29,30,31], chronic inflammation [32,33,34,35,36], hemorrhage [12,35,37,38,39,40,41,42], or genetic predisposition [26,43,44,45]. Notably, the disruption of the uterine cavity may increase the risk of PA [46]. One of the most interesting contemporary perspectives concerning the pathophysiology of PA is the thought of PA as an immunological process taking place locally in the decidua. The significance of the decidual immunomodulatory activity was confirmed in several studies [12,47,48]. Membrane proteins expressed by the decidual cells modulate the maternal immune system activity and participate in the commencement of labor. Available data suggest that PA is a result of the accumulation of cytotoxic immune cells (i.e., neutrophils, macrophages) [35] accompanied by the insufficiency of decidual suppressive activity [12].
B7 homolog 4 protein–B7-H4 (AKA B7S1 or V-set domain containing T-cell activation inhibitor–VCTN1) is a costimulatory transmembrane molecule and a member of the regulatory membrane molecules B7 family [49,50,51,52,53]. The B7 family plays an essential role in maintaining tolerance to the fetus [50]. It is one of the most characterized and widely distributed signaling molecule superfamily, exerting both stimulatory and inhibitory effects via stimulatory and inhibitory receptors, respectively, on T cells [54,55]. B7-H4 was initially described in 2003 [56,57,58], has only an inhibitory receptor and, thus, is responsible for the negative regulation of T-cell-mediated immune responses [49,50,52,53,59,60,61,62]. Notably, B7-H4 only binds to activated T-cells and subsequently inhibits T-cell proliferation by cell cycle arrest apart from inhibition of the production of proinflammatory cytokines [56,57,58,63,64,65]. Conversely, it inhibits neutrophil infiltration and suppresses Th1 immune response [50].
B7-H4 presents with two functional isoforms—the soluble form (sB7-H4) and the membrane-bound form. The source and functional mechanisms of the soluble form of B7-H4 remains obscure [51,53,66,67,68,69,70,71,72,73]. However, there is growing evidence that sB7-H4 acts as a T-cell-negative regulatory molecule, similar to cell-associated B7-H4 [51,52,74,75,76,77]. The inhibitory B7-H4 receptor is undetermined [51], but some recent studies have shown that it binds to the soluble semaphorin (Sema) family member Sema3a protein [78]. The expression of B7-H4 is strictly controlled in peripheral tissues at the transcriptional level, B7-H4 mRNA being widely expressed, while the presence of the B7-H4 protein is mostly limited to the reproductive tract tissues and selected cancers [49,56,57,58,61,62,67,79]. It is mainly present on the antigen presenting cell (APC)–macrophages and DCs [51,80]. The expression of B7-H4 on the APCs varies in different pregnancy pathologies [52,53,81,82].
Importantly, B7-H4 is involved in immunological changes associated with the spontaneous onset of labor as well as with several adverse perinatal outcomes. Its expression is higher during labor than during pregnancy, but it does not change during the course of labor [80]. There is growing evidence that B7-H4 is responsible for the modulation of the immune response during labor and restoring the homeostasis of the reproductive tract after labor [51]. Higher sB7-H4 or B7-H4 expression was described in cases of preterm premature rupture of membranes (PPROM) [52]–the rupture of the amniotic sac before the onset of labor, occurring before 37 weeks of pregnancy [83]. It was also described in case of chorioamnionitis [52], hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome [82], or preeclampsia (PE) [53,81,82,84]. PE is a disorder of pregnancy characterized by high blood pressure and concomitant signs of damage to other organs, with the liver and kidneys being the most commonly affected [85]. All these pregnancy complications are associated with exaggerated immune system activity, with the pathological Th1 cell predominance [86]. According to available data, the disruption of the immunological processes starts long before the visible outcomes (e.g., PE, PPROM or PA), as the increased amount of sB7-H4 was found in such cases as far back as in the first trimester of pregnancy [52,53]. It supports the concept of the induction failure of appropriate maternal immune tolerance and an exaggerated systemic maternal inflammatory response occurring in such cases [87,88,89,90,91]. The costimulatory molecule B7-H4 seems to answer this inflammatory process on the maternal–fetal interface from the very beginning, much before its visible manifestations.
The aim of this study was to explore B7-H4 expression in patients with PA and its significance to the process of placental detachment.
2. Materials and Methods
The study was performed retrospectively at the II Department of Obstetrics and Gynecology, Centre of Postgraduate Medical Education—a tertiary perinatal care center based in Warsaw, Poland. The local Ethics Committee approved the study (approval number 129/PB/2020).
2.1. Patients
We included cases from January 2017 to December 2019, for which the tissue material was available in the archives of the pathology department. We divided the identified cases into three groups: samples from patients diagnosed with PA, samples from patients diagnosed with retained placental tissue (RPT), and samples collected for other reasons (fetal growth restriction, threatening fetal asphyxia, breech presentation, lack of progress in labor, previous cesarean section or without a specified cause). The necessary clinical characteristics of the patients were extracted from the available hospital database. Patients with incomplete medical history were excluded from the study.
We included a total of 148 patients; 47 of them were patients with PA, 60 of them were patients with RPT, and 41 were healthy controls. PA diagnosis was based on the clinical symptoms including rapidly developing uterine tenderness, abdominal pain, severe vaginal bleeding/hemorrhage, and/or fetal distress. In all cases, the diagnosis of PA was first confirmed by the presence of a retroplacental clot and then was retrospectively confirmed in the tissue examined by a pathologist. All cases of diagnosed placental abruption were taken into consideration, despite the fact of the presence of obvious external bleeding [40], the percentage of separated placenta [92], the site of abruption [93], or the grade of the placental abruption [94,95]. The RPT diagnosis was based on the incomplete placental tissue after placental delivery and the presence of RPT inside the uterine cavity following the third stage of labor. It was also retrospectively confirmed by a pathologist in the examined tissues. We excluded patients diagnosed with atony or subatony, as in such cases bleeding could also be caused by a reason different than RPT.
All the participants were of Polish ethnicity. The characteristics of the studied groups are presented in Table 1.

Table 1.
Group characteristics.
2.2. Tissue Samples and Immunohistochemistry
The selected tissue samples were retrieved from the Department of Pathology, Bielański Hospital, Warsaw, Poland. The paraffin-embedded placental chorionic villous and decidual tissue samples were evaluated by an expert pathologist who subsequently selected material sufficient for further analysis. The chosen samples were cut using the microtome into 3 μm slices and sent for immunohistochemical processing.
Immunohistochemical analysis was performed manually in the Department of Pathology, Bielański Hospital, with the Ultravision LPValue Detection System (Thermo Scientific Lab Vision Corporation, Fremont, CA, USA). The visualization of reaction products was performed with 3,3′-diaminobenzidine (DAB+) chromogen (DAKO, Carpinteria, CA, USA) used for 10 min at room temperature, receiving a gold–brown color of the final product. In the next step, the sections were counterstained with Meyer’s hematoxylin and mounted in glycergel. According to the recommendations of the producer, the specificity of the B7-H4 antibody was tested with a specimen of ductal breast cancer, constituting a positive control for B7-H4. The results obtained from the control were in accordance with the producer’s specification.
Afterwards, the slides were washed in tris-buffered saline (TBS) plus 0.025% Triton X-100 and blocked for 2 h at room temperature in 10% normal serum with 1% bovine serum albumin (BSA) in TBS. The slides were drained and incubated with the primary antibody, rabbit polyclonal B7-H4 (ABCAM; Cambridge Biomedical Campus, Cambridge, UK, Catalog No. EPR20236), in 1:100 dilution in TBS with 1% BSA. The incubation with the primary monoclonal antibody took place in a humidified chamber overnight at 4° Celsius. Then, the slides were rinsed twice for 5 min with TBS plus 0.025% Triton and submitted to 0.3% H2O2 in TBS for 15 min. Subsequently, the application of the secondary enzyme-conjugated antibody diluted in TBS with 1% BSA took place. The incubation lasted for 1 h at room temperature. The slides were developed with chromogen for 10 min at room temperature and then rinsed in running tap water and, subsequently, counterstained with hematoxylin.
The decidua is the endometrial tissue of the uterine cavity remodeled during the pregnancy under the influence of hormones. The tissue consists of monomorphic stromal cells closely adjacent to each other, expressing certain features of the epithelial tissue. All of the examined placental samples were collected from patients in the third trimester of pregnancy. The villi of the placenta in the last trimester of pregnancy are composed of connective stromal tissue surrounded by a layer of syncytiotrophoblast with thin-walled blood vessels located on the circumference of the villi under the syncytiotrophoblast. Sinus-type vessels are present in the stroma near the syncytiotrophoblast. All the obtained samples were evaluated by an expert pathologist and qualified as typical and appropriate for further analysis.
A semi-quantitative scale based on the previously published studies [12,82,96] was then applied to the obtained samples (Table 2, Figure 1). The usage of a semi-quantitative scale was necessary because of a significant difference in the staining patterns of cells. Therefore, calculating only the percentage of stained cells would lead to the loss of a significant part of information. The analysis was carried out by an expert pathologist. The number and staining pattern of B7-H4-positive placental chorionic villous and decidual basalis cells per one HPF (high power field–objective magnification ×40, Nikon Eclipse 50i Microscope; Nikon Corporation, Tokyo, Japan) was estimated. The calculation was made through the entire slides (at least 10 HPFs per sample). On the basis of this, the average percentage and staining pattern of B7-H4 positive cells was estimated and the semi-quantitative scale was applied. The criteria of the semi-quantitative scale are presented in Table 2. In case of the lack of reactivity in the whole sample or some reactivity present in <1% of cells the sample was qualified as “Stage 0”. In case of any staining pattern (low or high) present in only 1–20% of cells the sample was qualified as “Stage 1”. In case of a low staining pattern present in 21–50% of cells the sample was qualified as “Stage 2”. In case of a low staining pattern present in >50% of cells or high staining pattern present in >20% of cells the sample was qualified as “Stage 3”. Subsequently, the authors compared B7-H4 expression in PA samples with that in RPT samples and healthy control samples (Figure 2 and Figure 3).

Table 2.
Semi-quantitative analysis of B7-H4 expression in the examined tissues.
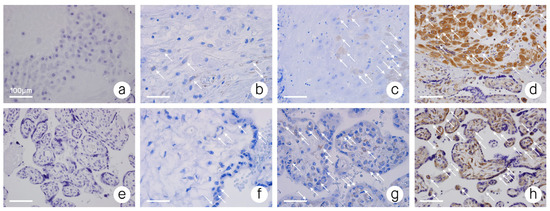
Figure 1.
B7-H4 immunoreactivity; (a) decidua–stage 0–no B7-H4 immunoreactivity; (b) decidua–stage 1–low B7-H4 immunoreactivity; (c) decidua–stage 2–moderate B7-H4 immunoreactivity; (d) decidua–stage 3–high B7-H4 immunoreactivity; (e) placental chorionic villus–stage 0–no B7-H4 immunoreactivity; (f) placental chorionic villus–stage 1–low B7-H4 immunoreactivity; (g) placental chorionic villus–stage 2–moderate B7-H4 immunoreactivity; (h) placental chorionic villus–stage 3–high B7-H4 immunoreactivity. Objective magnification ×40. ↖—Points some of the cells expressing B7-H4.
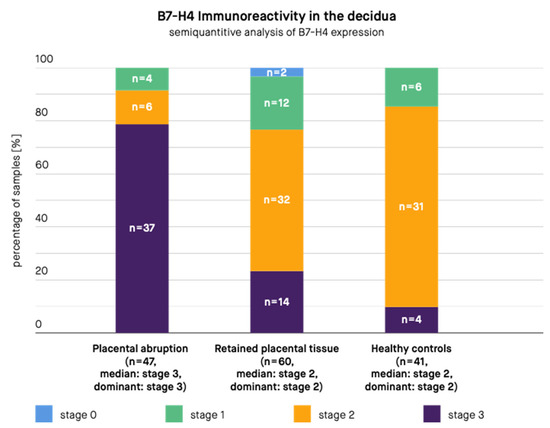
Figure 2.
B7-H4 immunoreactivity in the decidua in samples from patients with placental abruption, retained placental tissue, and healthy controls. n—Number of samples.
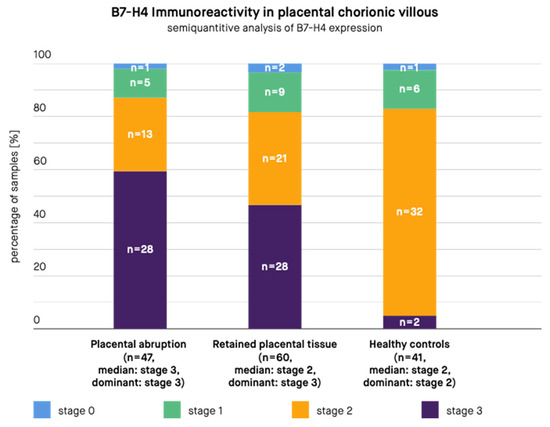
Figure 3.
B7-H4 immunoreactivity in the placental chorionic villus in samples from patients with placental abruption, retained placental tissue, and healthy controls. n—Number of samples.
2.3. Statistical Analysis
The normality of variable and group characteristics distributions were tested with the Shapiro–Wilk test. Regarding the group characteristics data, only the age was normally distributed. The ANOVA test and Kruskal–Wallis test were applied to analyze the differences between group characteristics. B7-H4 expression levels were found to be non-normally distributed. Statistical differences between groups were estimated using standard non-parametric tests (the Dunn’s test with Benjamini–Hochberg adjustment). Significance was accepted at p < 0.05. The R version 4.1.0 software was used for statistical analysis.
3. Results
A total of 148 women were enrolled in this study. The groups did not differ in terms of age, parity, and BMI, but inevitably differed in terms of gestational age and newborn weight and length. The control group had the highest rate of live births (Table 1). The presence of B7-H4 was confirmed in all decidual tissue samples from patients with PA (100%) and healthy controls (100%) and most of the samples from patients with RPT (97%). The presence of B7-H4 in placental chorionic villus was also confirmed in the majority of samples from patients with PA (98%), RPT (97%), and from healthy controls (98%).
The results are presented in Figure 2 and Figure 3. According to the immunohistochemical images, B7-H4 expression was present in the stroma and syncytiotrophoblast of the placental chorionic villus, both in the cytoplasm and at the cell membrane. B7-H4 expression was also noted both in the cytoplasm and at the cell membrane of the decidua. The expression of the B7-H4 molecule in the decidua was significantly higher in case of PA compared to RPT (median in PA group: stage 3, dominant in PA group: stage 3, median in RPT group: stage 2, dominant in RPT group: stage 2, p-value < 0.001) and in case of PA compared to healthy controls (median in PA group: stage 3, dominant in PA group: stage 3, median in healthy controls group: stage 2, dominant in healthy controls group: stage 2, p-value < 0.001). The difference between the control group and RPT group was not significant (median in RPT group: stage 2, dominant in RPT group: stage 2, median in healthy controls group: stage 2, dominant in healthy controls group: stage 2, p-value = 0.3055). The expression of the B7-H4 molecule in the placental chorionic villus did not significantly differ in case of PA compared to RPT (median in PA group: stage 3, dominant in PA group: stage 3, median in RPT group: stage 2, dominant in RPT group: stage 3, p-value = 0.0853), but the difference remained significant in case of PA compared to healthy controls (median in PA group: stage 3, dominant in PA group: stage 3, median in healthy controls group: stage 2, dominant in healthy controls group: stage 2, p-value < 0.001) and in case of RPT compared to the control group (median in RPT group: stage 2, dominant in RPT group: stage 3, median in healthy controls group: stage 2, dominant in healthy controls group: stage 2, p-value = 0.0012).
4. Discussion
According to available data, labor might be considered to be the result of the cessation of maternal immune tolerance toward the fetus. The augmented decidual cytotoxic activity is one of the most important components of the processing cascade leading to the expulsion of the fetus [97,98].
At the molecular level, the process of the development of PA takes place locally on the maternal–fetal interface and is not reflected in the peripheral blood [99,100,101,102,103,104]. When the immunological processes in the decidual microenvironment take place in a proper order, placental detachment occurs after cervical ripening and uterine contractions resulting in the expulsion of the fetus [105]. The disruption of the molecular processes on the maternal–fetal interface may lead to the improper order of these events and PA [1,12,106,107].
Not only during labor, PA is also accompanied by an increase in the cytotoxic activity of lymphocytes [12,108,109,110]. It results from the alteration of the maternal immune tolerance toward fetal antigens [12,48,110,111,112] and the breakthrough of the pregnancy-specific Th2 domination [108,113]. During PA, the levels of immunomodulating membrane proteins on the maternal–fetal interface are reduced and immune cell infiltration is more intense than that during physiological labor [1,12,35,114].
B7-H4 immunosuppressive functions seem to be similar to Tregs. Tregs also stimulate B7-H4 expression on macrophages [57], e.g., via the stimulation of APC production of interleukin 10 (IL-10) [115] and via macrophage sensitization to the action of interleukin 6 (IL-6) [116]. Moreover, IL-6 and IL-10 stimulate the expression of B7-H4 on macrophages [62,116,117]. It is possible that the fetus actively participates in this process by producing IL-6 [80,111]. B7-H4 inhibits interleukin 2 (IL-2) production through interfering with the activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and protein kinase B (PKB, Akt) [118]. Being costimulatory molecules, the members of the B7 family determine the ultimate immune response by the transduction of the second signal [119] (Figure 4). As the antigen is recognized by the T-cell and merges the T-cell receptor (TCR) to the peptide-major histocompatibility complex (MHC), a co-signaling molecule is needed to transduce the second signal, thus controlling the effect of the stimulation and leading to activation or anergy [120,121,122].
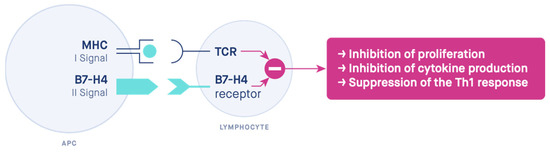
Figure 4.
The two-signal hypothesis. The mechanism of action of the costimulatory molecule B7-H4. MHC—major histocompatibility complex, TCR—T-cell receptor, APC—antigen-presenting cell.
To the best of our knowledge, this is the first study that analyzed B7-H4 immunoreactivity levels in the placental chorionic villus and decidual tissue samples from patients who were diagnosed with PA. Interestingly, B7-H4 expression in the decidual tissues turned out to be significantly higher in PA samples in relation to RPT samples and in healthy controls. The difference was also significant in the placental chorionic villus when comparing PA samples to healthy controls. This effect seemed to be contradictory to formerly investigated molecules responsible for the inhibition of maternal immune response, such as the receptor-binding cancer antigen expressed on SiSo cells (RCAS1) [1] or Tregs [80]. It compelled us to consider the phenomenon from a different perspective. As the augmentation of the immune response must be controlled and finally stopped, mechanisms functioning in the opposite direction have to start simultaneously. The activation of the maternal immune system is accompanied by opposite reactions leading to the restriction of the same activation. It enables the reproductive tract to return to the initial balance. In advanced labor, the total number and activity of immune cells in the decidua decreases [123], which supports the hypothesis of the self-limiting character of the process. B7-H4, as a T-cell activation inhibitor, seemed to be a part of this negative feedback loop.
An important limitation of the presented study is its retrospective character and the unavoidable fact that we could use only the available data and tissue material. Ideally, we would like to obtain samples from healthy controls without any comorbidities, nor pregnancy or labor complications, to avoid the bias of this issues. However, tissue samples are not routinely collected in such cases. It would also be beneficial to have a control group at a more precisely matching pregnancy age, as the difference may exert some influence on the immune response in the decidua. Nonetheless, according to many previous data, the changes of the immune response in the decidua seem mainly related to pregnancy complications or the state of labor. Thus, we assumed the level of bias acceptable [1,12,97,98,99,100,101,102,103,104,105,108,110]. Moreover, regarding the availability of detailed patient data in hospital databases, retrospective studies frequently reveal significant scarcity. Nonetheless, the obtained results are encouraging in terms of continuing the studies in a prospective manner, to enable more detailed and reliable analyses.
The analyzed samples confirmed the involvement of the immunomodulatory molecule B7-H4 in the processes occurring on the maternal–fetal interface. B7-H4 seems to justify the prematurely increased decidual cytotoxic activity present in PA and is responsible for restoring reproductive tract balance. A focused look at the molecular basis of this clinically important pregnancy complication validates, with greater reliability, the significance of this investigated molecule in the placental detachment process. Considering the fact that PA results in perinatal deaths and numerous serious complications for both the mother and the child, it is critical to put effort to thoroughly understand the underlying mechanisms. Any progress that leads to the reduction of mortality and morbidity through the improvement of PA management is valuable.
5. Conclusions
Our study revealed the abnormal expression of B7-H4 in cases of PA in women of Polish ethnicity. According to this finding and previous data, a significant role of this molecule might be indicated in maintaining maternal immune system balance, which is disrupted in case of PA. Our results also indirectly confirm increased cytotoxic activity on the maternal–fetal interface in case of PA, which starts at the early beginning, far before the actual detachment of the placenta. More clinical and molecular studies are still necessary in this matter. The molecular mechanisms underlying PA pathogenesis are an interesting target for further research to better understand and, thus, develop the appropriate management of this serious perinatal complication.
Author Contributions
Conceptualization, M.B. and M.C.; methodology, M.B., M.M.D.-W., E.P., and J.F.; software, M.B.; validation, M.B., M.C. and M.A.; formal analysis, M.B.; investigation, M.B., M.M.D.-W., E.P. and J.F.; resources, M.B. and M.C.; data curation, M.B., M.M.D.-W.; writing—original draft preparation, M.B., M.M.D.-W. and M.C.; writing—review and editing, M.B., M.A., C.W., A.K.; visualization, M.B.; supervision, M.C.; project administration, M.B. and M.C.; funding acquisition, M.B. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded from by the Center of Postgraduate Medical Education, Warsaw, Poland. Grant number 501-1-022-26-22.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (approval number 129/PB/2020).
Informed Consent Statement
Not applicable—a retrospective study.
Data Availability Statement
Data available on request.
Acknowledgments
Special thanks go to Maria Kulecka for statistical calculation consultations and support. Special thanks also go to Krystyna Galazka for the consultations concerning the immunohistochemical analysis. Special thanks go to Łukasz Wicherek for the inspiration and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wicherek, L.; Klimek, M.; Dutsch-Wicherek, M.; Kolodziejski, L.; Skotniczny, K. The molecular changes during placental detachment. Eur. J. Obs. Gynecol. Reprod. Biol. 2006, 125, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Whettlock, E.M.; Male, V. Immune responses in the human female reproductive tract. Immunology 2020, 160, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wicherek, L. The role of the endometrium in the regulation of immune cell activity. Front. Biosci. 2008, 13, 1018–1035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morris, H.; Edwards, J.; Tiltman, A.; Emms, M. Endometrial lymphoid tissue: An immunohistological study. J. Clin. Pathol. 1985, 38, 644–652. [Google Scholar] [CrossRef]
- Wilczynski, J.R.; Tchórzewski, H.; Banasik, M.; Głowacka, E.; Wieczorek, A.; Lewkowicz, P.; Malinowski, A.; Szpakowski, M.; Wilczyński, J. Lymphocyte subset distribution and cytokine secretion in third trimester decidua in normal pregnancy and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 109, 8–15. [Google Scholar] [CrossRef]
- Galazka, K.; Pitynski, K.; Skret-Magierlo, J.; Mach, P.; Knafel, A.; Sikora, J.; Niemiec, T.; Dobrogowski, J.; Basta, A.; Wicherek, L. The Increase in Metallothionein and Ectopic Decidual Immunoreactivity with Respect to the Progression of Labor at Term and the Lack of Analogical Changes in Placental Abruption. Am. J. Reprod. Immunol. 2008, 60, 204–213. [Google Scholar] [CrossRef]
- King, A.; Burrows, T.; Loke, Y.W. Human uterine natural killer cells. Nat. Immunol. 1996, 15, 41–52. [Google Scholar]
- Watanabe, H.; Miyaji, C.; Kawachi, Y.; Iiai, T.; Ohtsuka, K.; Iwanage, T.; Takahashi-Iwanaga, H.; Abo, T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J. Immunol. 1995, 155, 2972–2983. [Google Scholar]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Saito, S.; Sasaki, Y.; Sakai, M. CD4(+)CD25high regulatory T cells in human pregnancy. J. Reprod. Immunol. 2005, 65, 111–120. [Google Scholar] [CrossRef]
- Jacek, R.W.; Wilczynski, J.R.; Kalinka, J.; Radwan, M. The role of T-regulatory cells in pregnancy and cancer. Front. Biosci. 2008, 13, 2275–2289. [Google Scholar] [CrossRef]
- Wicherek, L.; Galazka, K.; Lazar, A. RCAS1 Decidual Immunoreactivity During Placental Abruption: Immune Cell Presence and Activity. Am. J. Reprod. Immunol. 2007, 58, 46–55. [Google Scholar] [CrossRef]
- Ledee-Bataille, N.; Bonnet-Chea, K.; Hosny, G.; Dubanchet, S.; Frydman, R.; Chaouat, G. Role of the endometrial tripod interleukin-18, -15, and -12 in inadequate uterine receptivity in pa-tients with a history of repeated in vitro fertilization-embryo transfer failure. Fertil. Steril. 2005, 83, 598–605. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Stoikos, C.; Stafford-Bell, M.; Clark, L.; Paiva, P.; Kovacs, G.; Salamonsen, L.A. Interleukin-11, IL-11 receptoralpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J. Reprod. Immunol. 2006, 69, 53–64. [Google Scholar] [CrossRef]
- Dmowski, W.P.; Ding, J.; Shen, J.; Rana, N.; Fernandez, B.; Braun, D. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum. Reprod. 2001, 16, 1802–1808. [Google Scholar] [CrossRef]
- Beliard, A.; Noel, A.; Foidart, J.-M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef]
- Harlev, A.; Levy, A.; Zaulan, Y.; Koifman, A.; Mazor, M.; Wiznitzer, A.; Faizayev, E.; Sheiner, E. Idiopathic bleeding during the second half of pregnancy as a risk factor for adverse perinatal outcome. J. Matern. Fetal. Neonatal Med. 2008, 21, 331–335. [Google Scholar] [CrossRef]
- Koifman, A.; Levy, A.; Zaulan, Y.; Harlev, A.; Mazor, M.; Wiznitzer, A.; Sheiner, E. The clinical significance of bleeding during the second trimester of pregnancy. Arch. Gynecol. Obstet. 2008, 278, 47–51. [Google Scholar] [CrossRef]
- Cunningham, J.W. Prompt evaluation and treatment of third-trimester bleeding. J. Am. Acad. Physician Assist. 2021, 34, 26–31. [Google Scholar] [CrossRef]
- Ananth, C.V.; Wilcox, A.J. Placental Abruption and Perinatal Mortality in the United States. Am. J. Epidemiol. 2001, 153, 332–337. [Google Scholar] [CrossRef]
- Tikkanen, M. Placental abruption: Epidemiology, risk factors and consequences. Acta Obstet. Gynecol. Scand. 2011, 90, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Smulian, J.C.; Vintzileos, A.M. Ischemic placental disease: Maternal versus fetal clinical presentations by gestational age. J. Matern. Fetal. Neonatal Med. 2010, 23, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V. Ischemic placental disease: A unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin. Perinatol. 2014, 38, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Peltier, M.R.; Chavez, M.R.; Kirby, R.S.; Getahun, D.; Vintzileos, A.M. Recurrence of Ischemic Placental Disease. Obstet. Gynecol. 2007, 110, 128–133. [Google Scholar] [CrossRef]
- Dommisse, J.; Tiltman, A.J. Placental bed biopsies in placental abruption. BJOG Int. J. Obstet. Gynaecol. 1992, 99, 651–654. [Google Scholar] [CrossRef]
- Signore, C.; Mills, J.L.; Qian, C.; Yu, K.; Lam, C.; Epstein, F.H.; Karumanchi, S.A.; Levine, R.J. Circulating Angiogenic Factors and Placental Abruption. Obstet. Gynecol. 2006, 108, 338–344. [Google Scholar] [CrossRef]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front. Immunol. 2018, 9, 1659. [Google Scholar] [CrossRef]
- Salafia, C.M.; López-Zeno, J.A.; Sherer, D.M.; Whittington, S.S.; Minior, V.K.; Vintzileos, A.M. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. Am. J. Obstet. Gynecol. 1995, 173, 1065–1070. [Google Scholar] [CrossRef]
- Nakatsuka, M.; Asagiri, K.; Kimura, Y.; Kamada, Y.; Tada, K.; Kudo, T. Generation of peroxynitrite and apoptosis in placenta of patients with chorioamnionitis: Possible impli-cations in placental abruption. Hum. Reprod. 1999, 14, 1101–1106. [Google Scholar] [CrossRef]
- Ananth, C.V.; Oyelese, Y.; Srinivas, N.; Yeo, L.; Vintzileos, A.M. Preterm premature rupture of membranes, intrauterine infection, and oligohydramnios: Risk factors for placental abruption. Obstet. Gynecol. 2004, 104, 71–77. [Google Scholar] [CrossRef]
- Avagliano, L.; Falleni, M.; Marconi, A.M.; Bulfoni, C.; Prada, A.; Barbera, A.F.; Doi, P.; Bulfamante, G.P. An imbalance of COX level is not related to placental abruption. J. Clin. Pathol. 2011, 64, 605–609. [Google Scholar] [CrossRef]
- Darby, M.J.; Caritis, S.; Shen-Schwarz, S. Placental abruption in the preterm gestation: An association with chorioamnionitis. Obstet. Gynecol. 1989, 74, 88–92. [Google Scholar]
- Rana, A.; Sawhney, H.; Gopalan, S.; Panigrahi, D.; Nijhawan, R. Abruptio placentae and chorioamnionitis-microbiological and histologic correlation. Acta. Obstet. Gynecol. Scand. 1999, 78, 363–366. [Google Scholar]
- Balkundi, D.R.; Hanna, N.; Hileb, M.; Dougherty, J.; Sharma, S. Labor-Associated Changes in Fas Ligand Expression and Function in Human Placenta. Pediatr. Res. 2000, 47, 301–308. [Google Scholar] [CrossRef][Green Version]
- Ananth, C.V.; Oyelese, Y.; Prasad, V.; Getahun, D.; Smulian, J.C. Evidence of placental abruption as a chronic process: Associations with vaginal bleeding early in pregnancy and placental lesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 15–21. [Google Scholar] [CrossRef]
- Nath, C.A.; Ananth, C.V.; Smulian, J.C.; Shen-Schwarz, S.; Kaminsky, L.; New Jersey–Placental Abruption Study Investigators. Histologic evidence of inflammation and risk of placental abruption. Am. J. Obstet. Gynecol. 2007, 197, 319.e1-6. [Google Scholar] [CrossRef]
- Harger, J.H.; Hsing, A.W.; Tuomala, R.E.; Gibbs, R.S.; Mead, P.B.; Eschenbach, D.A.; Knox, G.E.; Polk, B.F. Risk factors for preterm premature rupture of fetal membranes: A multicenter case-control study. Am. J. Obstet. Gynecol. 1990, 163, 130–137. [Google Scholar] [CrossRef]
- Williams, M.A.; Mittendorf, R.; Lieberman, E.; Monson, R.R. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet. Gynecol. 1991, 78, 14–18. [Google Scholar]
- Lockwood, C.J.; Toti, P.; Arcuri, F.; Paidas, M.; Buchwalder, L.; Krikun, G.; Schatz, F. Mechanisms of Abruption-Induced Premature Rupture of the Fetal Membranes: Thrombin-Enhanced Interleukin-8 Expression in Term Decidua. Am. J. Pathol. 2005, 167, 1443–1449. [Google Scholar] [CrossRef]
- Oyelese, Y.; Ananth, C.V. Placental abruption. Obstet. Gynecol. 2006, 108, 1005–1016. [Google Scholar] [CrossRef]
- Huang, S.-T.J.; Schatz, F.; Salafia, C.; Stocco, C.; Lockwood, C.J.; Krikun, G. Thrombin activation of endometrial endothelial cells: A possible role in intrauterine growth restriction. Thromb. Haemost. 2007, 97, 245–253. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Zdoukopoulos, N.; Zintzaras, E. Genetic risk factors for placental abruption: A HuGE review and meta-analysis. Epidemiology 2008, 19, 309–323. [Google Scholar] [CrossRef]
- Workalemahu, T.; Enquobahrie, D.A.; Gelaye, B.; Sanchez, S.E.; Garcia, P.J.; Tekola-Ayele, F.; Hajat, A.; Thornton, T.A.; Ananth, C.V.; Williams, M.A. Genetic variations and risk of placental abruption: A genome-wide association study and meta-analysis of genome-wide association studies. Placenta 2018, 66, 8–16. [Google Scholar] [CrossRef]
- Workalemahu, T.; Enquobahrie, D.A.; Gelaye, B.; Thornton, T.A.; Tekola-Ayele, F.; Sanchez, S.E.; Garcia, P.J.; Palomino, H.G.; Hajat, A.; Romero, R.; et al. Abruptio placentae risk and genetic variations in mitochondrial biogenesis and oxidative phosphory-lation: Replication of a candidate gene association study. Am. J. Obstet. Gynecol. 2018, 219, 617.e1–617.e17. [Google Scholar] [CrossRef]
- Hemminki, E.; Merilainen, J. Long-term effects of cesarean sections: Ectopic pregnancies and placental problems. Am. J. Obstet. Gynecol. 1996, 174, 1569–1574. [Google Scholar] [CrossRef]
- Skręt-Magierło, J.E.; Wicherek, L.; Basta, P.; Galazka, K.; Sikora, J.; Wilk, M.; Fudali, L.; Skret, A. RCAS1 Decidual Immunoreactivity during Cesarean Section in Scar Deciduosis: Immune Cell Presence and Activity. Gynecol. Obstet. Investig. 2007, 65, 187–194. [Google Scholar] [CrossRef]
- Wicherek, L.; Basta, P.; Galazka, K.; Mak, P.; Dancewicz, L.; Kalinka, J. ORIGINAL ARTICLE: RCAS1 Decidual Immunoreactivity and RCAS1 Serum Level During Cesarean Section with Respect to the Progression of Labor. Am. J. Reprod. Immunol. 2008, 59, 152–158. [Google Scholar] [CrossRef]
- Choi, I.-H.; Zhu, G.; Sica, G.L.; Strome, S.E.; Cheville, J.C.; Lau, J.S.; Zhu, Y.; Flies, D.B.; Tamada, K.; Chen, L. Genomic Organization and Expression Analysis of B7-H4, an Immune Inhibitory Molecule of the B7 Family. J. Immunol. 2003, 171, 4650–4654. [Google Scholar] [CrossRef]
- Petroff, M.G.; Perchellet, A. B7 Family Molecules as Regulators of the Maternal Immune System in Pregnancy. Am. J. Reprod. Immunol. 2010, 63, 506–519. [Google Scholar] [CrossRef]
- Mach, P.; Gellhaus, A.; Wicherek, L.; Schmidt, B.; Kimmig, R.; Kasimir-Bauer, S.; Köninger, A. Changes in the Blood Serum Levels of the Costimulatory Soluble B7-H4 Molecule in Pregnant Women During the Peripartal Phase. Am. J. Reprod. Immunol. 2015, 74, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Mach, P.; Köninger, A.; Wicherek, L.; Kimmig, R.; Kasimir-Bauer, S.; Birdir, C.; Schmidt, B.; Gellhaus, A. Serum concentrations of soluble B7-H4 in early pregnancy are elevated in women with preterm premature rupture of fetal membranes. Am. J. Reprod. Immunol. 2016, 76, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Mach, P.; Nolte-Boenigk, L.; Droste, L.; Fox, L.; Frank, M.; Schmidt, B.; Herse, F.; Verlohren, S.; Wicherek, L.; Iannaccone, A.; et al. Soluble B7-H4 blood serum levels are elevated in women at high risk for preeclampsia in the first trimester, as well as in patients with confirmed preeclampsia. Am. J. Reprod. Immunol. 2018, 80, e12988. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal–Fetal Immunity. Front. Immunol. 2020, 11, 458. [Google Scholar] [CrossRef]
- Prasad, D.V.; Richards, S.; Mai, X.M.; Dong, C. B7S1, a Novel B7 Family Member that Negatively Regulates T Cell Activation. Immunity 2003, 18, 863–873. [Google Scholar] [CrossRef]
- Sica, G.L.; Choi, I.-H.; Zhu, G.; Tamada, K.; Wang, S.-D.; Tamura, H.; Chapoval, A.I.; Flies, D.B.; Bajorath, J.; Chen, L. B7-H4, a Molecule of the B7 Family, Negatively Regulates T Cell Immunity. Immunity 2003, 18, 849–861. [Google Scholar] [CrossRef]
- Zang, X.; Loke, P.; Kim, J.; Murphy, K.; Waitz, R.; Allison, J.P. B7x: A widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 10388–10392. [Google Scholar] [CrossRef]
- Miyatake, T.; Tringler, B.; Liu, W.; Liu, S.H.; Papkoff, J.; Enomoto, T.; Torkkoa, K.C.; Dehna, D.L.; Swishera, A.; Shroyer, K.R.; et al. B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely cor-related with tumor T-cell infiltration. Gynecol. Oncol. 2007, 106, 119–127. [Google Scholar] [CrossRef]
- Park, G.B.; Song, H.; Kim, Y.-S.; Sung, M.; Ryu, J.W.; Lee, H.-K.; Cho, D.H.; Kim, D.; Lee, W.J.; Hur, D.Y.; et al. Cell cycle arrest induced by engagement of B7-H4 on Epstein-Barr virus-positive B-cell lymphoma cell lines. Immunology 2009, 128, 360–368. [Google Scholar] [CrossRef]
- MacGregor, H.L.; Ohashi, P.S. Molecular Pathways: Evaluating the Potential for B7-H4 as an Immunoregulatory Target. Clin. Cancer Res. 2017, 23, 2934–2941. [Google Scholar] [CrossRef]
- Kaur, G.; Janakiram, M. B7x-from bench to bedside. ESMO Open 2019, 4, e000554. [Google Scholar] [CrossRef]
- Suh, W.-K.; Wang, S.; Duncan, G.S.; Miyazaki, Y.; Cates, T.; Walker, T.; Gajewska, B.U.; Deenick, E.; Dawicki, W.; Okada, H.; et al. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol. Cell. Biol. 2006, 26, 6403–6411. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Ahangar, N.K.; Hemmat, N.; Khalaj-Kondori, M.; Shadbad, M.A.; Sabaie, H.; Mokhtarzadeh, A.; Alizadeh, N.; Derakhshani, A.; Baghbanzadeh, A.; Dolatkhah, K.; et al. The Regulatory Cross-Talk between microRNAs and Novel Members of the B7 Family in Human Diseases: A Scoping Review. Int. J. Mol. Sci. 2021, 22, 2652. [Google Scholar] [CrossRef]
- Petroff, M.G.; Chen, L.; Phillips, T.A.; Azzola, D.; Sedlmayr, P.; Hunt, J.S. B7 Family Molecules Are Favorably Positioned at the Human Maternal-Fetal Interface1. Biol. Reprod. 2003, 68, 1496–1504. [Google Scholar] [CrossRef]
- Tringler, B.; Zhuo, S.; Pilkington, G.; Torkko, K.C.; Singh, M.; Lucia, M.S.; Heinz, D.E.; Papkoff, J.; Shroyer, K.R. B7-H4 Is Highly Expressed in Ductal and Lobular Breast Cancer. Clin. Cancer Res. 2005, 11, 1842–1848. [Google Scholar] [CrossRef]
- Christiaens, I.; Zaragoza, D.B.; Guilbert, L.; Robertson, S.; Mitchell, B.F.; Olson, D.M. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 2008, 79, 50–57. [Google Scholar] [CrossRef]
- Repnik, U.; Tilburgs, T.; Roelen, D.; van der Mast, B.; Kanhai, H.; Scherjon, S.; Claas, F. Comparison of Macrophage Phenotype Between Decidua Basalis and Decidua Parietalis by Flow Cytometry. Placenta 2008, 29, 405–412. [Google Scholar] [CrossRef]
- Azuma, T.; Zhu, G.; Xu, H.; Rietz, A.C.; Drake, C.G.; Matteson, E.L.; Chen, L. Potential Role of Decoy B7-H4 in the Pathogenesis of Rheumatoid Arthritis: A Mouse Model Informed by Clinical Data. PLoS Med. 2009, 6, e1000166. [Google Scholar] [CrossRef]
- Kamimura, Y.; Kobori, H.; Piao, J.; Hashiguchi, M.; Matsumoto, K.; Hirose, S.; Azuma, M. Possible involvement of soluble B7-H4 in T cell-mediated inflammatory immune responses. Biochem. Biophys. Res. Commun. 2009, 389, 349–353. [Google Scholar] [CrossRef]
- Lappas, M. Visfatin regulates the terminal processes of human labour and delivery via activation of the nuclear factor-kappaB pathway. Mol. Cell. Endocrinol. 2012, 348, 128–134. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Lu, D.; Li, G.; Sun, C.; Song, H.; Li, J.; Zhai, T.; Huang, L.; Hou, C.; et al. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene 2013, 32, 5347–5358. [Google Scholar] [CrossRef]
- Simon, I.; Zhuo, S.; Corral, L.; Diamandis, E.P.; Sarno, M.J.; Wolfert, R.L.; Kim, N.W. B7-H4 Is a Novel Membrane-Bound Protein and a Candidate Serum and Tissue Biomarker for Ovarian Cancer. Cancer Res. 2006, 66, 1570–1575. [Google Scholar] [CrossRef]
- Thompson, R.H.; Zang, X.; Lohse, C.M.; Leibovich, B.C.; Slovin, S.F.; Reuter, V.E.; Cheville, J.C.; Blute, M.L.; Russo, P.; Kwon, E.D.; et al. Serum-Soluble B7x Is Elevated in Renal Cell Carcinoma Patients and Is Associated with Advanced Stage. Cancer Res. 2008, 68, 6054–6058. [Google Scholar] [CrossRef]
- Leandersson, P.; Kalapotharakos, G.; Henic, E.; Borgfeldt, H.; Petzold, M.; Høyer-Hansen, G.; Borgfeldt, C. A Biomarker Panel Increases the Diagnostic Performance for Epithelial Ovarian Cancer Type I and II in Young Women. Anticancer Res. 2016, 36, 957–965. [Google Scholar]
- Jiang, X.; Liu, G.; Li, Y.; Pan, Y. Immune checkpoint: The novel target for antitumor therapy. Genes Dis. 2019, 8, 25–37. [Google Scholar] [CrossRef]
- Podojil, J.R.; Chiang, M.Y.; Ifergan, I.; Copeland, R.; Liu, L.N.; Maloveste, S.; Miller, S.D. B7-H4 Modulates Regulatory CD4(+) T Cell Induction and Function via Ligation of a Semaphorin 3a/Plexin A4/Neuropilin-1 Complex. J. Immunol. 2018, 201, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.H.; Chen, L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol. Rev. 2009, 229, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Galazka, K.; Wicherek, L.; Pitynski, K.; Kijowski, J.; Zajac, K.; Bednarek, W.; Dutsch-Wicherek, M.; Rytlewski, K.; Kalinka, J.; Basta, A.; et al. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7-H4 macrophages—A preliminary report. Am. J. Reprod. Immunol. 2009, 61, 136–146. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Kolarz, B.; Chmielewski, T.; Tabarkiewicz, J.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The expression of B7-H1 and B7-H4 co-stimulatory molecules on myeloid and plasmacytoid dendritic cells in pre-eclampsia and normal pregnancy. J. Reprod. Immunol. 2013, 99, 33–38. [Google Scholar] [CrossRef]
- Duan, L.; Reisch, B.; Iannaccone, A.; Hadrovic, E.; Wu, Y.; Vogtmann, R.; Winterhager, E.; Kimmig, R.; Köninger, A.; Mach, P.; et al. Abnormal expression of the costimulatory molecule B7-H4 in placental chorionic villous and decidual basalis tissues of patients with preeclampsia and HELLP syndrome. Am. J. Reprod. Immunol. 2021, 86, e13430. [Google Scholar] [CrossRef]
- Prelabor Rupture of Membranes: ACOG Practice Bulletin, Number 217. Obstet. Gynecol. 2020, 135, e80–e97. [CrossRef]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Chmielewski, T.; Kolarz, B.; Rolinski, J.; Oleszczuk, J. PP069. The expressions of B7-H1 and B7-H4 co-stimulatory molecules on myeloid and lymphoid dendritic cells in pre-eclampsia and normal pregnancy. The expressions of B7-H1 and B7-H4 co-stimulatory moleculeson myeloid and lymphoid dendritic cells in pre-eclampsia and normal pregnancy. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2012, 2, 278–279. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Koga, K.; Aldo, P.B.; Mor, G. Toll-like receptors and pregnancy: Trophoblast as modulators of the immune response. J. Obstet. Gynaecol. Res. 2009, 35, 191–202. [Google Scholar] [CrossRef]
- Sasaki, Y.; Darmochwal-Kolarz, D.; Suzuki, D.; Sakai, M.; Ito, M.; Shima, T.; Shiozaki, A.; Rolinski, J.; Saito, S. Proportion of peripheral blood and decidual CD4+ CD25bright regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 2007, 149, 139–145. [Google Scholar] [CrossRef]
- Steinborn, A.; Haensch, G.M.; Mahnke, K.; Schmitt, E.; Toermer, A.; Meuer, S.; Sohn, C. Distinct subsets of regulatory T cells during pregnancy: Is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin. Immunol. 2008, 129, 401–412. [Google Scholar] [CrossRef]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Groth, B.F.D.S.; Nanan, R. Systemic Increase in the Ratio between Foxp3+and IL-17-Producing CD4+T Cells in Healthy Pregnancy but Not in Preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Sargent, I.L. Immunology of Pre-Eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Nuutila, M.; Hiilesmaa, V.; Paavonen, J.; Ylikorkala, O. Clinical presentation and risk factors of placental abruption. Acta Obstet. Gynecol. Scand. 2006, 85, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, D.; Cyr, D.R.; Mack, L.; Wilson, D.; Shuman, W. Sonographic spectrum of placental abruption. Am. J. Roentgenol. 1987, 148, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Page, E.W.; King, E.B.; Merrill, J.A. Abruptio placentae; dangers of delay in delivery. Obstet. Gynecol. 1954, 3, 385–393. [Google Scholar] [PubMed]
- Hurd, W.W.; Miodovnik, M.; Hertzberg, V.; Lavin, J.P. Selective management of abruptio placentae: A prospective study. Obstet. Gynecol. 1983, 61, 467–473. [Google Scholar] [PubMed]
- Wicherek, L.; Basta, P.; Sikora, J.; Galazka, K.; Rytlewski, K.; Grabiec, M.; Lazar, A.; Kalinka, J. RCAS1 Decidual Immunoreactivity in Severe Pre-Eclampsia: Immune Cell Presence and Activity. Am. J. Reprod. Immunol. 2007, 58, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Bonney, E.A.; Condon, J.; Mesiano, S.; Taylor, R.N. Novel concepts on pregnancy clocks and alarms: Redundancy and synergy in human parturition. Hum. Reprod. Updat. 2016, 22, 535–560. [Google Scholar] [CrossRef]
- Bączkowska, M.; Zgliczyńska, M.; Faryna, J.; Przytuła, E.; Nowakowski, B.; Ciebiera, M. Molecular Changes on Maternal–Fetal Interface in Placental Abruption—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6612. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Stocco, C.; Murk, W.; Kayisli, U.A.; Funai, E.F.; Schatz, F. Human Labor Is Associated with Reduced Decidual Cell Expression of Progesterone, But Not Glucocorticoid, Receptors. J. Clin. Endocrinol. Metab. 2010, 95, 2271–2275. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Kayisli, U.A.; Stocco, C.; Murk, W.; Vatandaslar, E.; Buchwalder, L.F.; Schatz, F. Abruption-Induced Preterm Delivery Is Associated with Thrombin-Mediated Functional Progesterone Withdrawal in Decidual Cells. Am. J. Pathol. 2012, 181, 2138–2148. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Semerci, N.; Basar, M.; Buchwalder, L.F.; Buhimschi, C.S.; Lockwood, C.J. Mechanisms of chorioamnionitis-associated preterm birth: Interleukin-1beta inhibits progesterone receptor expression in decidual cells. J. Pathol. 2015, 237, 423–434. [Google Scholar] [CrossRef]
- Ackerman, W.E., IV; Summerfield, T.L.; Mesiano, S.; Schatz, F.; Lockwood, C.J.; Kniss, D.A. Agonist-Dependent Downregulation of Progesterone Receptors in Human Cervical Stromal Fibro-blasts. Reprod. Sci. 2016, 23, 112–123. [Google Scholar] [CrossRef][Green Version]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565. [Google Scholar] [CrossRef]
- Patel, B.; Peters, G.A.; Skomorovska-Prokvolit, Y.; Yi, L.; Tan, H.; Yousef, A.; Wang, J.; Mesiano, S. Control of Progesterone Receptor-A Transrepressive Activity in Myometrial Cells: Implications for the Control of Human Parturition. Reprod. Sci. 2018, 25, 214–221. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Snegovskikh, V.; Schatz, F.; Foyouzi, N.; Rahman, M.; Buchwalder, L.; Lee, H.J.; Funai, E.F.; Buhimschi, C.S.; Buhimschi, I.A.; et al. Progestin inhibits and thrombin stimulates the plasminogen activator/inhibitor system in term decidual stromal cells: Implications for parturition. Am. J. Obstet. Gynecol. 2007, 196, 382.e1–382.e8. [Google Scholar] [CrossRef]
- Steinborn, A.; Rebmann, V.; Scharf, A.; Sohn, C.; Grosse-Wilde, H. Placental Abruption Is Associated with Decreased Maternal Plasma Levels of Soluble HLA-G. J. Clin. Immunol. 2003, 23, 307–314. [Google Scholar] [CrossRef]
- Steinborn, A.; Seidl, C.; Sayehli, C.; Sohn, C.; Seifried, E.; Kaufmann, M.; Schmitt, E. Anti-fetal immune response mechanisms may be involved in the pathogenesis of placental abruption. Clin. Immunol. 2004, 110, 45–54. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Varga, P.; Pacsa, A. Immunologic factors contributing to the initiation of labor—Lymphocyte reactivity in term labor and threatened preterm delivery. Am. J. Obstet. Gynecol. 1986, 155, 108–112. [Google Scholar] [CrossRef]
- Abadia-Molina, A.C.; Ruiz, C.; Montes, M.; King, A.; Loke, Y.; Olivares, E.G. Immune phenotype and cytotoxic activity of lymphocytes from human term decidua against trophoblast. J. Reprod. Immunol. 1996, 31, 109–123. [Google Scholar] [CrossRef]
- Osman, I.; Young, A.; Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 2003, 9, 41–45. [Google Scholar] [CrossRef]
- Steinborn, A.; Sohn, C.; Sayehli, C.; Baudendistel, A.; Hüwelmeier, D.; Solbach, C.; Schmitt, E.; Kaufmann, M. Spontaneous labour at term is associated with fetal monocyte activation. Clin. Exp. Immunol. 1999, 117, 147–152. [Google Scholar] [CrossRef]
- Sindram-Trujillo, A.P.; Scherjon, S.A.; van Hulst-van Miert, P.P.; Kanhai, H.H.; Roelen, D.L.; Claas, F.H. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J. Reprod. Immunol. 2004, 62, 125–137. [Google Scholar] [CrossRef]
- Lin, H.; Mosmann, T.R.; Guilbert, L.; Tuntipopipat, S.; Wegmann, T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 1993, 151, 4562–4573. [Google Scholar]
- Steinborn, A.; Rebmann, V.; Scharf, A.; Sohn, C.; Grosse-Wilde, H. Soluble HLA-DR levels in the maternal circulation of normal and pathologic pregnancy. Am. J. Obstet. Gynecol. 2003, 188, 473–479. [Google Scholar] [CrossRef]
- Kryczek, I.; Wei, S.; Zou, L.; Zhu, G.; Mottram, P.; Xu, H.; Chen, L.; Zou, W. Cutting Edge: Induction of B7-H4 on APCs through IL-10: Novel Suppressive Mode for Regulatory T Cells. J. Immunol. 2006, 177, 40–44. [Google Scholar] [CrossRef]
- Kryczek, I.; Wei, S.; Zhu, G.; Myers, L.; Mottram, P.; Cheng, P.; Chen, L.; Coukos, G.; Zou, W. Relationship between B7-H4, Regulatory T Cells, and Patient Outcome in Human Ovarian Carcinoma. Cancer Res. 2007, 67, 8900–8905. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Zou, L.; Rodriguez, P.; Zhu, G.; Wei, S.; Mottram, P.; Brumlik, M.; Cheng, P.; Curiel, T.; Myers, L.; et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006, 203, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, J.; Metzger, D.; Ao, Z.; Chen, L.; Ou, D.; Verchere, B.; Mui, A.; Warnock, G.L. B7-H4 Treatment of T Cells Inhibits ERK, JNK, p38, and AKT Activation. PLoS ONE 2012, 7, e28232. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lafferty, K.J.; Cunningham, A.J. A NEW ANALYSIS OF ALLOGENEIC INTERACTIONS. Aust. J. Exp. Biol. Med Sci. 1975, 53, 27–42. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Schwartz, R.H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unre-sponsiveness in vitro and in vivo. J. Exp. Med. 1987, 165, 302–319. [Google Scholar] [CrossRef]
- Holt, M.P.; Punkosdy, G.A.; Glass, D.D.; Shevach, E.M. TCR Signaling and CD28/CTLA-4 Signaling Cooperatively Modulate T Regulatory Cell Homeostasis. J. Immunol. 2017, 198, 1503–1511. [Google Scholar] [CrossRef]
- Wicherek, L.; Galazka, K. The possible correlation between the patient’s immune tolerance level during cesaerean section and the incidence of subsequent emergency peripartum hysterectomy. Clin. Dev. Immunol. 2007, 2007, 63596. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).