Abstract
Wounds are considered a clinically critical issue, and effective treatment will decrease complications, prevent chronic wound formation, and allow rapid healing. The development of products based on naturally occurring materials is an efficient approach to wound healing. Natural polysaccharides can mimic the extracellular matrix and promote cell growth, thus making them attractive for wound healing. In this context, the aim of this work was to produce a gel based on chicha gum, chitosan, and Mauritia flexuosa oil (CGCHO) for wound treatment. TG and DTG analyzed the thermal behavior of the materials, and SEM investigated the surface roughness. The percentages of total phenolic compounds, flavonoids, and antioxidants were determined, presenting a value of 81.811 ± 7.257 µmol gallic acid/g Mauritia flexuosa oil, 57.915 ± 0.305 µmol quercetin/g Mauritia flexuosa oil, and 0.379 mg/mL, respectively. The anti-inflammatory was determined, presenting a value of 10.35 ± 1.46% chicha gum, 16.86 ± 1.00% Mauritia flexuosa oil, 10.17 ± 1.05% CGCHO, and 15.53 ± 0.65% chitosan, respectively. The materials were tested against Gram-negative (Klebsiella pneumoniae) and Gram-positive (Staphylococcus aureus) bacteria and a fungus (Candida albicans). The CGCHO formulation showed better antimicrobial activity against Gram-positive bacteria. In addition, an in vivo wound healing study was also performed. After 21 days of treatment, the epidermal re-epithelialization process was observed. CGCHO showed good thermal stability and roughness that can help in cell growth and promote the tissue healing process. In addition to the good results observed for the antimicrobial, antioxidant, anti-inflammatory activities and providing wound healing, they provided the necessary support for the healing process, thus representing a new approach to the wound healing process.
1. Introduction
Wound healing is a dynamic and complex physiological process that occurs following a natural response to injury, in which some irregularities may occur. Research has been developed to accelerate the process of wound healing [1]. The healing process involves four phases: coagulation, inflammation, migration-proliferation, and remodeling. These phases occur in sequence; however, different stages can coincide in other areas of the injury site during the repair process. [2,3,4,5]. The hemostasis stage occurs immediately after the injury. The inflammation phase, characterized by signs of heat, redness, swelling, and pain, is fully established within 24 h, but it may last longer if there is an infection, trauma, or other problem. With their degradative enzymes and reactive oxygen species (ROS), phagocytes decompose and remove cell fragments at tissue damage sites [6]. The extracellular matrix (ECM) provides the structure for repair and is one of the main components of wound healing, along with stromal cells (fibroblasts, myofibroblasts). The healing stage can be promoted by pharmaceutical formulations containing antioxidant, antimicrobial, hemostatic, or anti-inflammatory compounds [7,8].
Many studies have been carried out to develop an ideal material to facilitate healing and provide the physiological environment for the wound [5]. Natural polymers can act as an accelerating agent in the wound healing process, as an anti-inflammatory agent, and as safe and biocompatible scaffolds for skin tissue regeneration. Polymers such as chitosan, gelatin, alginate, and hyaluronic acid have been used in wound healing [9,10]. These materials can adhere to the injured tissue through their surface molecules by means of chemical bonds, electrostatic bonds, and diffusive phenomena. The pattern of chemical bonds involved in these materials can also have an influence, as diffusion is believed to occur through the movement and penetration of polymer chains between the macromolecules of the cell membrane [11]. Materials such as polysaccharides have aroused great interest in the pharmacological sector. Some interesting properties can be found in some polysaccharides, especially biocompatible, biodegradable, adhesive, non-toxic, antimicrobial, antioxidant, and anti-inflammatory activities. Additionally, they have been used as materials with potential uses in the wound healing process [5,12,13,14,15,16].
Chitosan (CH), a polysaccharide obtained from crustacean shells, has a bioactive potential that partially meets the requirements for fast curing [17,18]. The structure of CH is similar to the ECM of the skin, composed of hyaluronic acid (HA), a polysaccharide that has N-acetyl-D-glucosamine groups in its structure [19,20]. CH hydrogels improved mucoadhesion properties and accelerated the healing rate by successfully reconstructing an epidermis [21,22]. CH promotes the growth of wound healing-related cells, such as the growth of fibroblasts, epithelial cells, keratinocytes, and macrophages to produce active factors that contribute to wound healing. In addition, the molecular structure of chitosan is similar to the stroma of mucopolysaccharide matrix cells [23,24,25]. Another biopolymer with interesting biological properties for treating skin lesions is chicha gum (CG), a polysaccharide from the exudate of the Sterculia striata tree. CG is an anionic polysaccharide, with numerous acid groups in its structure, with excellent swelling capacity, adhesion, anti-inflammatory, and antibacterial activity, important features for the wound healing process [26,27,28]. Due to its properties, GC has the potential to be explored for asset delivery systems. GC hydrogels have been developed and have shown firmness, consistency, and cohesiveness. The mucoadhesion of the CG hydrogel was superior to that of chitosan, being a promising polymer for mucoadhesive formulations [29,30].
Mauritia flexuosa oil is a natural activity extracted from the fruit of the Mauritia flexuosa L. palm, a common plant in the Amazon region of Brazil. This product is available in large- and small-scale production, acquisition, and processing [31]. Due to compounds such as fatty acids and carotenoids, this natural oil can help to heal wounds and acts as a healing accelerator [32]. Carotenoids, especially β-carotene, are antioxidant agents [24]. BO has fatty acids in its composition, such as oleic acid, which acts in the construction of the cell membrane and is one of the constituents of the epidermis [33,34,35,36,37]. Hexadecanoic and octadecanoic acids are other constituents of Mauritia flexuosa oil and tend to increase antimicrobial and antioxidant activity [38,39]. The antimicrobial activity occurs because fatty acids act as detergents in the amphipathic structure of a bacterial cell membrane, increasing cell permeability and impairing essential processes [40]. Mauritia flexuosa oil has the potential for use in the pharmaceutical and cosmetic industries. Oil emulsions were considered potential vehicles to transport antioxidant precursors. Thus, new products with Mauritia flexuosa oil can prevent pathologies associated with oxidative damage [41,42]
The properties offered by CH, CG and Mauritia flexuosa oil are attractive for wound treatment. Thus, this work aimed to synthesize a gel formulation of CH associated with CG and Mauritia flexuosa oil (CGCHO) for wound healing.
2. Materials and Methods
2.1. Materials
The reagents sodium hydroxide, sodium chloride, ethanol, methanol, acetone, acetic acid, hyaluronidase enzyme (350 UI), potassium salt of the human umbilical cord, hyaluronic acid, Trolox, quercetin, and 2,2-diphenyl-1-picryl-hydrazine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Chitosan with a medium degree of deacetylation of 95% was supplied by Primex. Mauritia flexuosa oil was purchased at local markets in the city of Bom Jesus, State of Piaui, Brazil. Brain heart infusion and Mueller–Hinton agar were obtained fro, HIMEDIA (Mumbai, Maharashtra India). Sodium sulfate was provided by FLUKA (Steinheim, Renânia do Norte-Vestefália, Germany), and gallic acid was obtained from Panreac. Folin, sodium carbonate, and aluminum chloride were obtained from MERCK (Hohenbrunn, Baviera, Germany).
2.2. Purification of Chicha Gum
Crude CG samples were collected from bark-free nodules of chicha tree (Sterculia striata) planted at EMBRAPA-Meio Norte, located in Teresina, Piauí, Brazil. The material was deposited and registered by the Graziela Barroso Herbarium and received the following registration number, TEPB:30418. CG (Mw = 6.3 × 106 g/mol) was isolated and purified according to the method proposed by Braz et al. [26]. The exudate (1.0 g) was dissolved in distilled water with stirring (100.0 mL) at room temperature for 24 h. Subsequently, 1 g of NaCl was added to the solution, and the pH was adjusted to 7. This polysaccharide was precipitated in ethanol P.A (99.5%). Later, the precipitate was washed with ethanol, followed by acetone, dried at 40 °C for 24 h, and ground until a powder was formed.
2.3. Preparation of Chicha Gum/Chitosan/Mauritia flexuosa Oil Gel (CGCHO) and Chitosan-Based Gel (CHB)
The CGCHO gel was fabricated using 1.0 g of CG, 1.5 g of CH, and 0.5 mL of Mauritia flexuosa oil in 25.0 mL of acetic acid. The gel was homogenized using a mechanical stirrer for 10 min. The oil-free gel was also obtained with the same concentrations of polysaccharides. The CHG gel was prepared by adding 0.75 g in 25.0 mL of acetic acid (2% v/v); the solution was stirred to form a homogeneous gel.
2.4. Characterization of Polysaccharides and Gels
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
FTIR spectra of the polysaccharides and gels were obtained using a Varian 660-IR spectrophotometer. The range was 600 to 4000 cm−1, combining 16 scans, with a resolution of 4 cm−1.
2.4.2. Thermal Gravimetric Analysis (TGA)
TGA analysis was performed using TA Instruments SDT Q600 V20.9 Build 20, model DSC-TGA Standard (New Castle, DE, USA). Approximately 10.0 mg of each dried sample was placed in an aluminum crucible and heated from 25 to 600 °C at 10 °C min−1 in a nitrogen atmosphere.
2.4.3. Scanning Electron Microscopy (SEM)
A freeze-dried gel sample was put on double-sided carbon tape and sputter-coated with silver. SEM images of samples were observed using an FEI, model Quanta FEG 250 (FEI Company, Eindhoven, Holanda). For the lyophilization process, the sample was frozen in an ultrafreezer (−80 °C), and it was not necessary to use cryoprotectant.
2.5. Characterization of Mauritia flexuosa Oil
2.5.1. Antioxidant Activity of Mauritia flexuosa Oil-DPPH Radical Scavenging Assay
Mauritia flexuosa oil’s antioxidant activity was determined by the DPPH• [43]. The analysis used 20.0 μL of oil mixed with 180.0 μL of DPPH• solution (150 μmol/L) in methanol. The concentrations of the oil were 30.0 to 150.0 μL/mL. The mixture was incubated at room temperature for 40 min in the dark. The absorbance was measured at 517 nm. Trolox was used as a control. The experiments were performed in triplicate. The inhibition percent was calculated from the control using Equation (1):
where:
% DPPH• = activity of DPPH•
Abs sample = Absorbance at the sample
Abs control = Absorbance at the control
The concentrations of the Mauritia flexuosa oil were determined when 50% inhibition (IC50) was observed.
2.5.2. Total Phenolics and Flavonoids of Mauritia flexuosa Oil
The total phenolic content (TPC) of the Mauritia flexuosa oil was determined using the Folin-Ciocalteu method [44,45]. Briefly, 20.0 μL of a dilute solution of oil in ethanol (10.0 μL/mL and 2.5 μL/mL) was mixed with 100.0 μL of Folin-Ciocalteu reagent. The solution was stirred for 1 min and kept at rest for 4 min. After, 75.0 μL of Na2CO3 (10% w/v) was added and stirred for 1 min. After incubation in the dark for 2 h, at room temperature, the absorbance of the solution and the blank were determined by UV-vis at 750 nm (VWR UV-3100 PC Spectrophotometer). The total phenolic content was calculated from a calibration curve, using gallic acid as standard. The result was expressed as milligrams of gallic acid equivalents per gram of Mauritia flexuosa oil.
To determine the total content of flavonoids in Mauritia flexuosa oil, the aluminum chloride method was used [45,46]. Briefly, 20.0 μL of a diluted solution of oil in ethanol (10.0 μL/mL and 2.5 μL/mL) was mixed with 210.0 μL of methanol (80%) and 20.0 μL of 2% AlCl3 was added. The solution was mixed and kept in the dark at room temperature for 30 min, and the absorbance of the mixture was monitored by UV-Vis at 427 nm (VWR UV-3100 PC Spectrophotometer). The total flavonoid content was expressed as milligrams of quercetin equivalents per gram of Mauritia flexuosa oil.
2.6. Anti-Inflammatory Activity of Polysaccharides and Gels
The anti-inflammatory activity was indirectly evaluated from the inhibitory effect of samples on reactions catalyzed by hyaluronidase [32,47]. The analysis was performed for samples of CG, Mauritia flexuosa oil, CGCHO and, CHG. Briefly, 50.0 μL of each sample was used in 50.0 μL (350 UI) of hyaluronidase enzyme (Type IV-S) incubated at 37 °C for 20 min. Then, calcium chloride (1.2 mL of 2.5 × 10−3 mol/L) was added to activate the enzyme, and the mixture was incubated for a further 20 min at 37 °C. After this time, 0.5 mL of hyaluronic acid sodium salt (1.0 × 10−1 mol/L) was added to each sample and incubated for another 40 min at 37 °C. Then, 0.1 mL of potassium tetraborate was added at a concentration of 8.0 × 10−1 mol/L. Then, the solutions were boiled in a water bath for 3 min. The solutions were cooled to 10 °C with the addition of 3.0 mL of p-dimethylaminabenzaldehyde and incubated for 20 min at 37 °C. Finally, absorbance was measured by UV-Vis at 585 nm. All tests were performed in triplicate.
2.7. Antimicrobial Tests
Minimum Inhibitory Concentration (MIC)
The experiments were conducted against three different microorganisms: Staphylococcus aureus (ATCC 43300), Klebsiella pneumoniae (ATCC 13883), and the fungus Candida albicans (ATCC 10231).
The minimum inhibitory concentration (MIC) was determined in a 96-well microplate, and the experiments were performed according to the CLSI [48]. In brief, isolated colonies were suspended in sterile saline with 0.85% to reach 0.5 on the MacFarland scale. The broths used were Muller–Hinton broth for bacteria and yeasts peptone dextrose broth for the fungus. CG, CGCHO, and CHG were tested at a concentration of 0.156 to 40.0 mg/mL. Isolated Mauritia flexuosa oil was also tested at a concentration of 2.5 mg/mL to 320.0 mg/mL. To all wells in the plates were added the isolated colonies previously prepared (106 colony forming units (CFU)/mL) and incubated at 37 °C for 24 h and at 25 °C for 48 h for the bacteria and fungus, respectively. Then, the antimicrobial activity was determined by the addition of 20.0 μL of 2, 3, 5-triphenyl-2H-tetrazoliumchloride (TTC, 5 mg/mL) solution. The antibacterial and antifungal controls used were gentamicin and amphotericin, respectively. All tests were performed in triplicate.
2.8. Healing Assays
2.8.1. Excision Procedure, Wound Treatment, and Skin Lesion Assessment in Rats
The wound excision procedure was described by Ferreira et al. (2020) [32]. The animals were anesthetized with xylazine hydrochloride (0.04 mL/100 g) and ketamine hydrochloride (10% 0.08 mL/100 g). Then, skin tissue approximately 0.6 cm in diameter was removed to form a wound with exposure of the dorsal fascia muscle. The animals were treated with topical application of 10.0 mg of each sample of CHB, Mauritia flexuosa oil and CGCHO.
Skin lesion evaluation in animals was performed, observing the repair of the lesion and the presence or absence of edema, exudate, and crust. Lesions were measured on the 1st, 3rd, 7th, 14th and 21st days of treatment. After the test, the animals were euthanized with sodium pentobarbital (10–15 mg/100 g). A skin fragment from the back of three animals from each group was dissected on the 3rd, 7th, 14th and 21st days of treatment. The injured skin samples were fixed in 10% buffered formalin for 24 h and taken to a battery with an increasing sequence of alcohol and xylene. Then, they were subjected to paraffin baths at 60 °C and embedded in wax blocks. After this, 5 µm-thick sections were made using a Leica microtome (Buffalo Grove, IL, USA), stained with hematoxylin-eosin (H.E.). The slides were evaluated by light microscopy and the images were digitally photographed.
2.8.2. Statistical Analysis
Data were analyzed using Graph Pad Prism Version 6.0 software, and the results of healing on the 3rd, 7th, 14th and 21st days of treatment were expressed by the significance of the difference between the mean using variance analysis (ANOVA) with p ≤ 0.05.
3. Results and Discussion
3.1. Characterizations
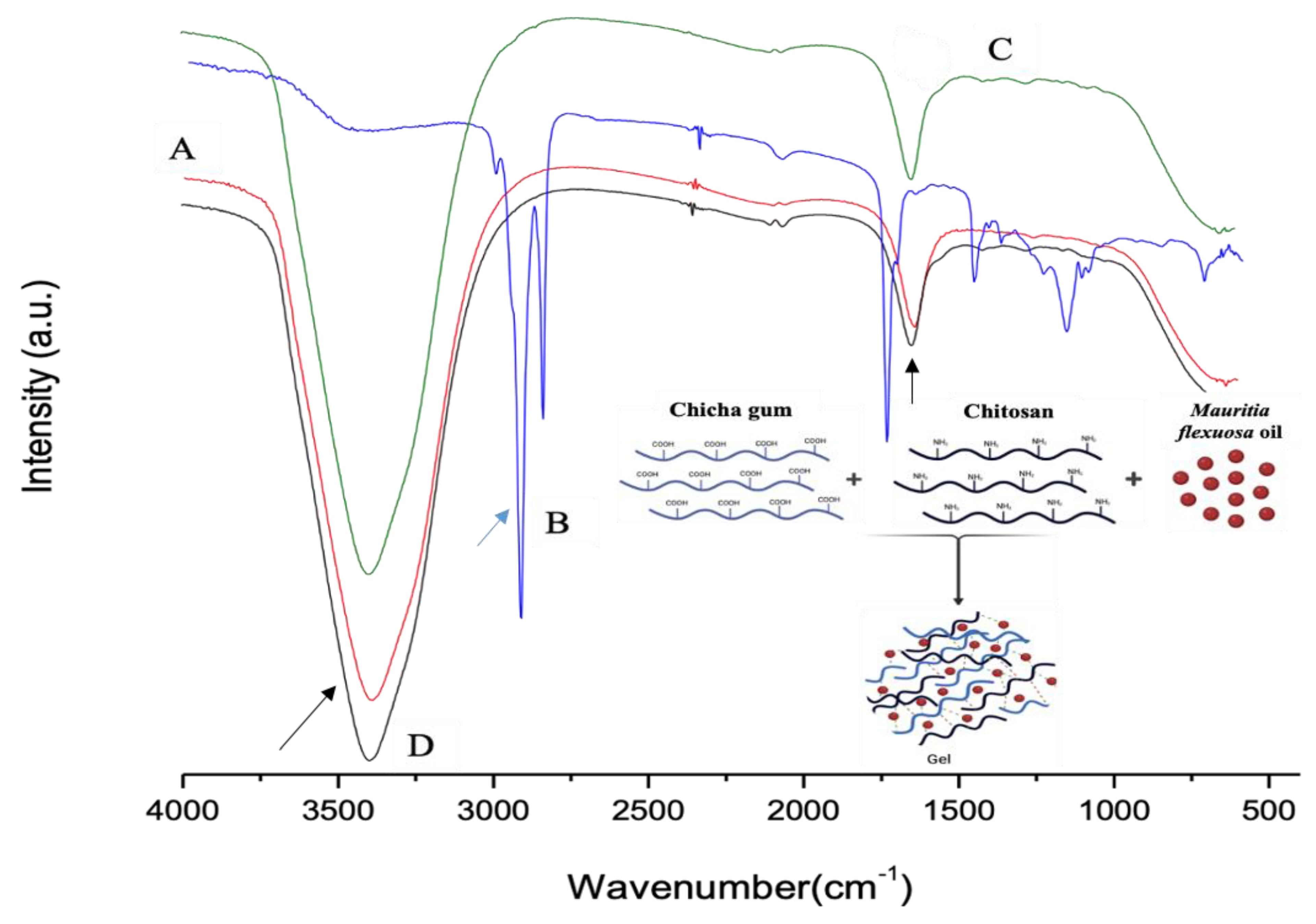
Figure 1A–D show the FTIR spectra of CG, Mauritia flexuosa oil, CGCHO and CHG, respectively. Figure 1A shows bands in the region of 3500 cm−1 related to the elongation of the galactopyranose and glucopyranose ring alcohols, and the band at 1643 cm−1 indicates the presence of carboxylic acid groups (black arrow) [26,28]. In Figure 1B, the region above 3000 cm−1 is related to the hydroxyl groups of the acid groups present in the BO structure. The bands in the region of 2900 cm−1 are related to the CH bonds of the carbons (blue arrow) [32]. In the regions of 1700–1500 cm−1, the presence of carbonyls was observed, referring to the fatty acids in the BO structure [49]. In Figure 1C,D, the band observed at 3100 cm−1 is related to OH stretching with large broadening caused by gel formation [32,50]. Close to 1650 cm−1, bands of C=O were observed (black arrow). Thus, in Figure 1D, the combination of the main characteristic bands of the polymers and BO is observed.
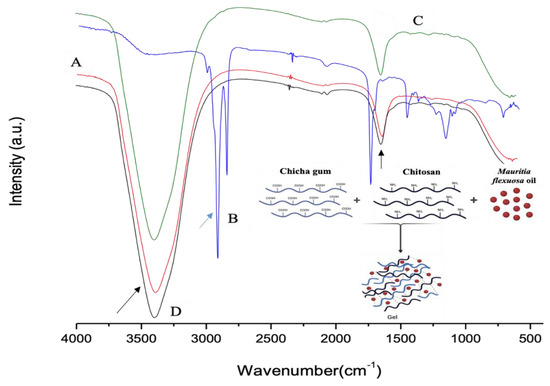
Figure 1.
FTIR spectra for (A) chicha gum (CG), (B) Mauritia flexuosa oil, (C) chicha gum/chitosan/Mauritia flexuosa oil gel (CGCHO), and (D) chitosan-based gel (CHB).
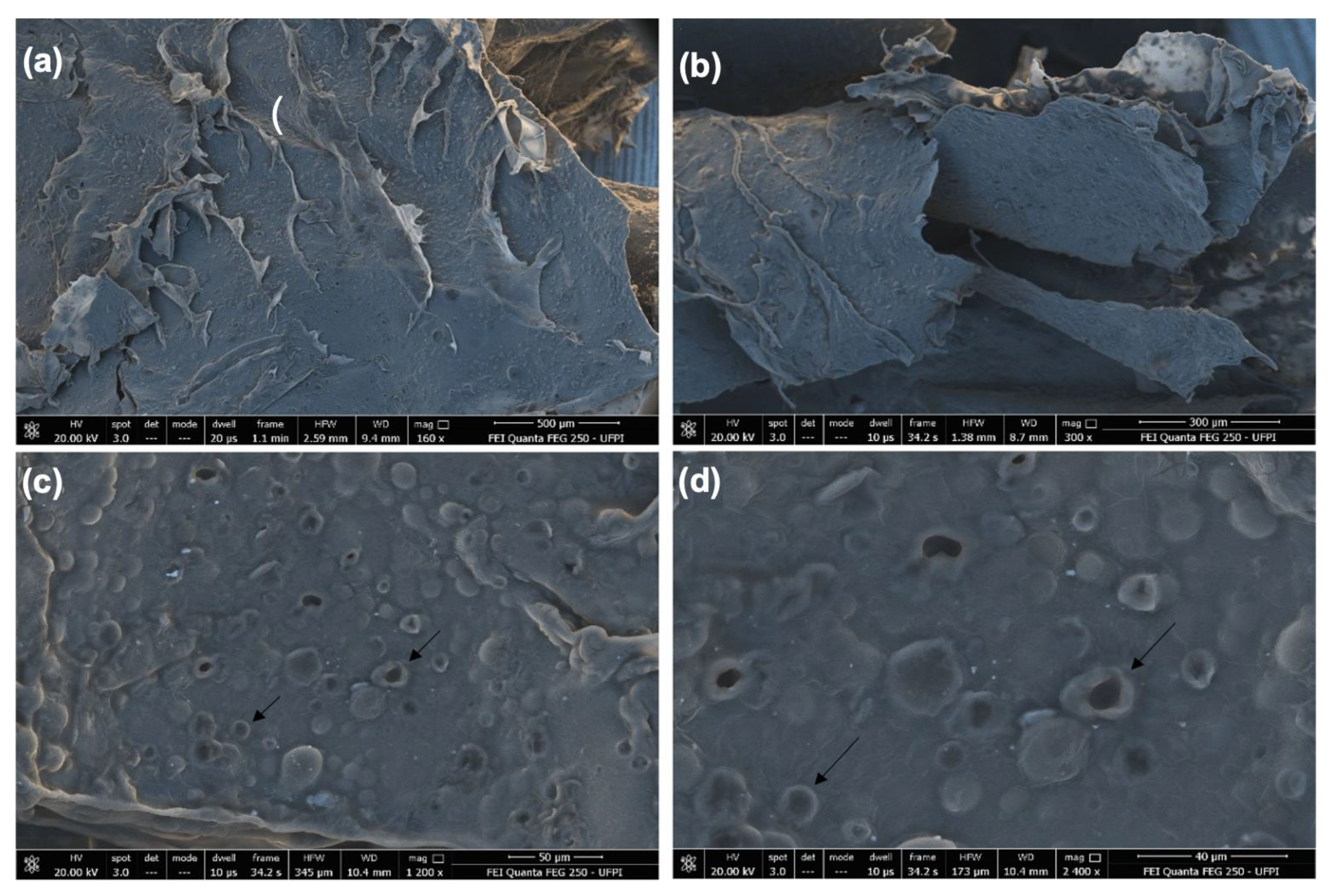
In Figure 2, SEM images of the CGCHO are shown at different magnifications. An irregular and porous surface with surface roughness was observed (black arrow). The presence of roughness can facilitate cell growth and favor the tissue healing process, due to its similarity to the extracellular matrix of the skin [51]. The porosity of CGCHO can be favorable to the transfer of nutrients and oxygen to the wound cells. This process can contribute to vascularization, better adhesion of the gel to the skin and cell proliferation [32,52]. In Figure 2c,d, it can be seen that Mauritia flexuosa oil is dispersed throughout the gel, suggesting uniform incorporation.
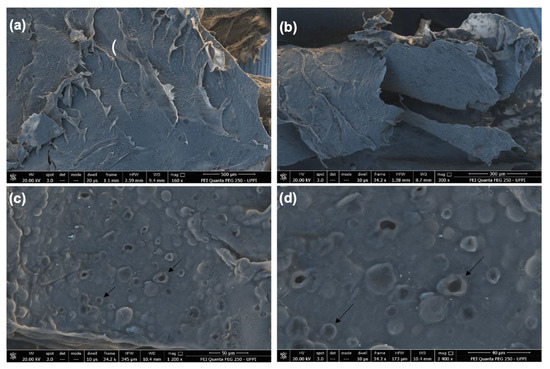
Figure 2.
Scanning electron microscope (SEM) images of chicha gum/chitosan/Mauritia flexuosa oil gel (CGCHO) at the magnitudes of (a) 500 µm, (b) 300 µm, (c) 50 µm and (d) 40 µm.
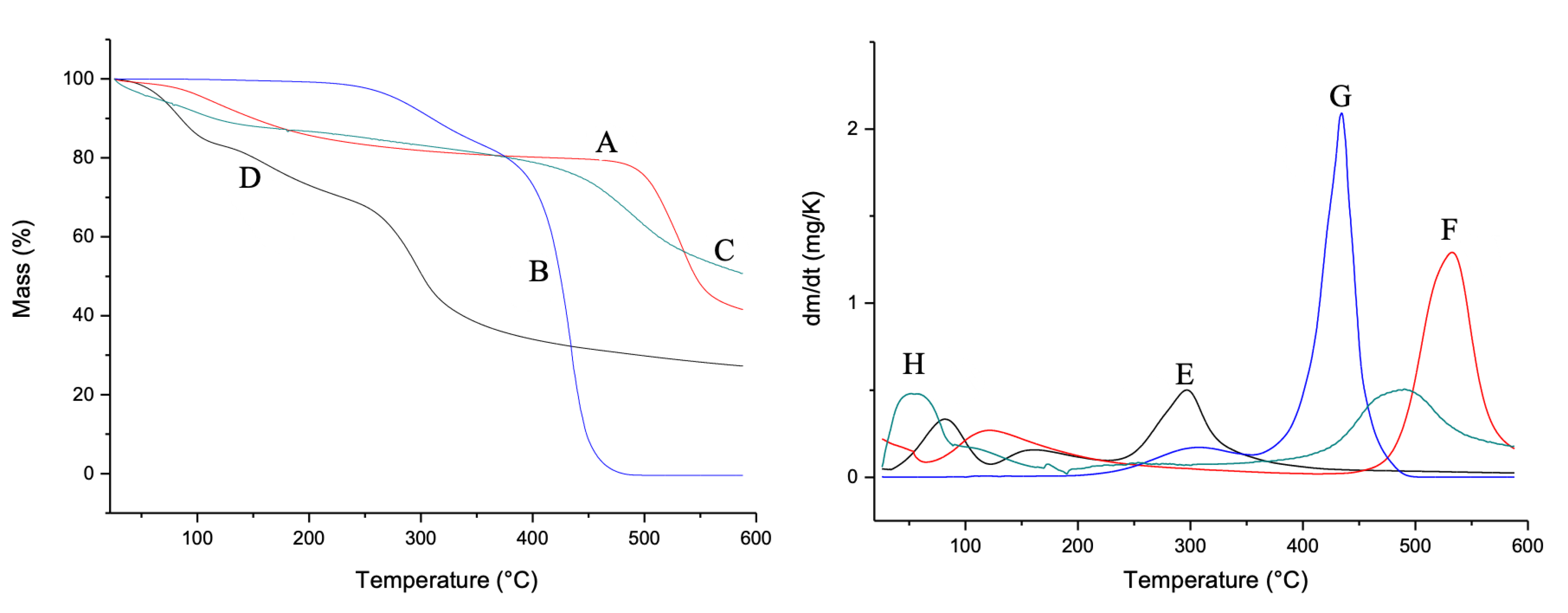
In Figure 3, the TG and DTG curves are shown. For the CG (Figure 3A,E), two mass loss events were observed. The first event is related to water loss and the second event is related to the degradation of the main polysaccharide polymer chain, according to Braz et al. [26]. Mauritia flexuosa oil presented two events in the temperature range between 250 and 350 °C. The first event is related to the condensation of surface groups. The second refers to the total thermal decomposition (Figure 3B,F). For CGCHO, two decomposition events were observed. The first event that occurred at 100 °C is related to the loss of water from the polymers. The second event occurred between 400 and 600 °C and is related to Mauritia flexuosa oil (Figure 3C,G). CGCHO showed greater thermal stability, indicating a good interaction between biopolymers and Mauritia flexuosa oil; thus, the use of this material allows it to be used in wound healing without suffering significant mass loss up to 50 °C. CHB showed three decomposition events. The first event occurred in the range of 50 °C and was related to the loss of water and other volatile components. The second event occurred between 100 and 200 °C and was related to the condensation of the hydroxyl and amine groups. The last event indicated decomposition of the polysaccharide structure (Figure 3D,H) [49].
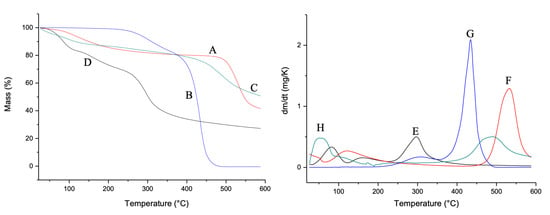
Figure 3.
Thermogravimetric curves (TG) of (A) chicha gum (CG), (B) Mauritia flexuosa oil, (C) chicha gum/chitosan/Mauritia flexuosa oil gel (CGCHO), and (D) chitosan-based gel (CHB). Derivative thermogravimetric curves (DTG) of CG (E), Mauritia flexuosa oil (F), CGCHO (G), and CHG (H).
3.2. Antioxidant Activity
The IC50 value was determined for Mauritia flexuosa oil and is related to the amount of compound required to lower the initial DPPH• radical concentration by 50%. The Mauritia flexuosa oil had a value of 0.379 mg/mL, indicating good activity. One of the factors responsible for the antioxidant activity in Mauritia flexuosa oil is the presence of phenolic compounds and flavonoids, in addition to other components such as carotenoids. High oxidative stability can neutralize free radicals naturally produced by neutrophils at the site of inflammation and contribute to rapid healing [32,44,45]. One of the main factors that aggravate pathological conditions is oxidative stress, being responsible for damage and molecular alterations [32,43].
3.3. Total Phenolics and Flavonoids
The content of phenolic compounds found for Mauritia flexuosa oil was 81.811 ± 7.257 µmol gallic acid/g Mauritia flexuosa oil. Phenolic compounds act as hydrogen donors, being effective antioxidants and powerful free radical scavengers capable of reducing oxidative damage [53,54,55,56,57]. It is noteworthy that the biological properties of these natural antioxidants present in Mauritia flexuosa oil can contribute to rapid healing.
The content of flavonoid found was 57.915 ± 0.305 µmol quercetin/g Mauritia flexuosa oil. Flavonoids act in the inflammatory process by mediating macrophages and the production of pro-inflammatory molecules. These results demonstrate that Mauritia flexuosa oil can be an enhancer in the healing process.
3.4. Anti-Inflammatory Activity
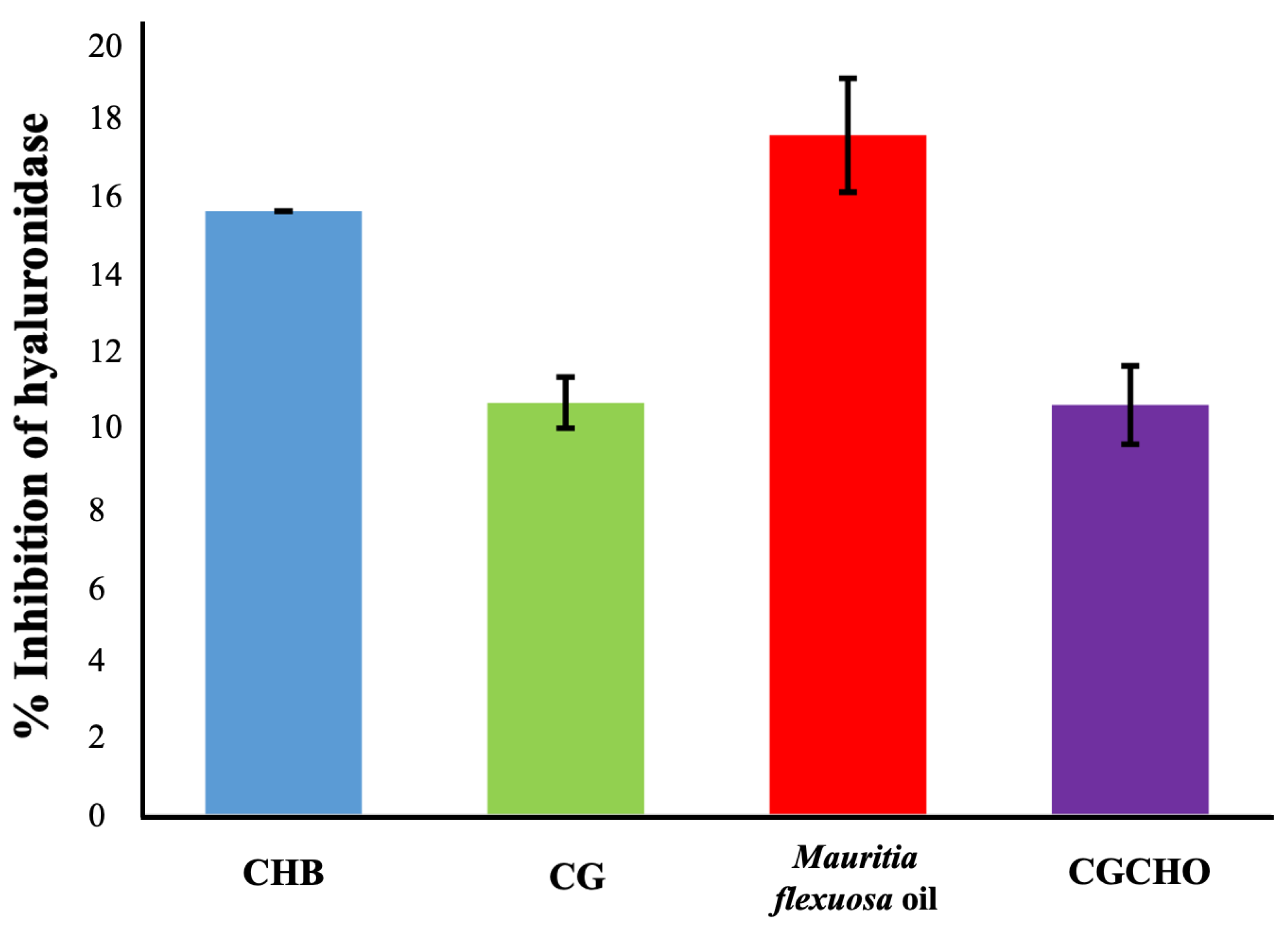
For anti-inflammatory activity, the materials showed enzyme inhibition percentages of 10.35 ± 1.46% for CG, 16.86 ± 1.00% for Mauritia flexuosa oil, 10.17 ± 1.05% for CGCHO and 15.53 ± 0.65% CH, as shown in Figure 4. It was observed that the CG was responsible for the decreased anti-inflammatory activity of the CGCHO gel; this result corroborates Ferreira et al. [32]. The inflammatory process is complex, induced by different pathways, and occurs mainly in the first three days after tissue injury. Cell proliferation, mainly of macrophages and neutrophils, at the wound site has a pro-inflammatory and anti-inflammatory action [47]. The materials used to produce the gel, such as Mauritia flexuosa oil, have antioxidant activity that inhibits nitric oxide and reduces the inflammatory process [47,58]. CH is responsible for stimulating cell proliferation, which justifies its anti-inflammatory action [59,60]. Chang et al. [61] reported that CH inhibited the production of pro-inflammatory cytokines (TNF-α, IL-6). CG has biological properties similar to CH, and so a similar anti-inflammatory activity is expected [26]. Thus, the use of natural polymers to produce CGCHO gel has good anti-inflammatory activities and has the potential to help in the wound healing process.
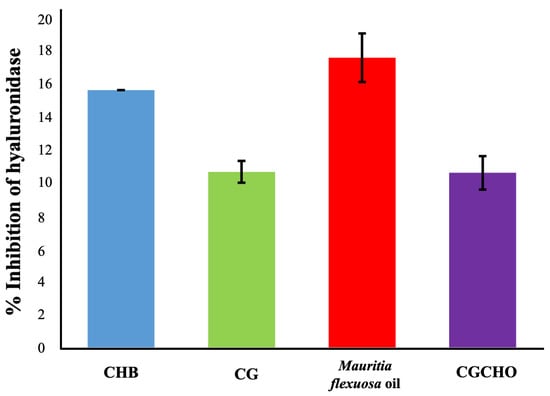
Figure 4.
Inhibition of hyaluronidase activity by chicha gum (CG), Mauritia flexuosa oil, chicha gum/chitosan/Mauritia flexuosa oil gel (CGCHO), and chitosan-based gel (CHB).
3.5. Antibacterial Tests
Table 1 presents the results of the antimicrobial tests. CGCHO gel showed better MIC results for S. aureus when compared to CHG. Mauritia flexuosa oil and CG did not show antibacterial activity at the concentrations tested. The activity observed for the CGCHO gel may be related to the presence of CH [28,32]. The cationic nature of CH interacts with the negatively charged microbial cell membrane, which leads to extravasation of intracellular components, causing bacterial cell death [62,63]. S. aureus is a pathogenic bacterium widely known to cause infections based on biofilm formation in its hosts. Thus, the use of a gel that has antibacterial activity can promote the healing process.

Table 1.
Minimum inhibitory concentration (mic) for CG, Mauritia flexuosa oil, CGCHO and CHB against S. aureus, K. pneumoniae and C. albicans.
The CGCHO gel showed a superior inhibitory effect than that observed for CHG for Klebsiella pneumoniae. K. pneumoniae is associated with a variety of infections, including wounds and disseminated infections. Babassu oil fibers with PLA were produced to aid in the healing process, and in the antimicrobial assay, only inhibition was observed for Gram-negative bacteria [64]. Mauritia flexuosa oil and CG showed no antibacterial action against this strain at the concentrations tested. Between the two strains tested, it was possible to observe a better result for S. aureus. This result may be related to the structural differences in the cell wall of both bacteria. Gram-positive bacteria have only one cell membrane, whereas Gram-negative bacteria have a cell membrane and an additional outer layer membrane that is impermeable to most molecules.
The antifungal activity tested against C. albicans was observed for CGCHO and CHG gel. C. albicans is an opportunistic human pathogen involved in various infections and causes disease in immunocompetent and immunocompromised patients, accounting for a high mortality rate in the United States [65,66,67]. Antimicrobial action may help to reduce wound contamination by bacterial and fungal strains. Thus, CGCHO gel can be a viable alternative for the wound healing process.
3.6. Healing Activity
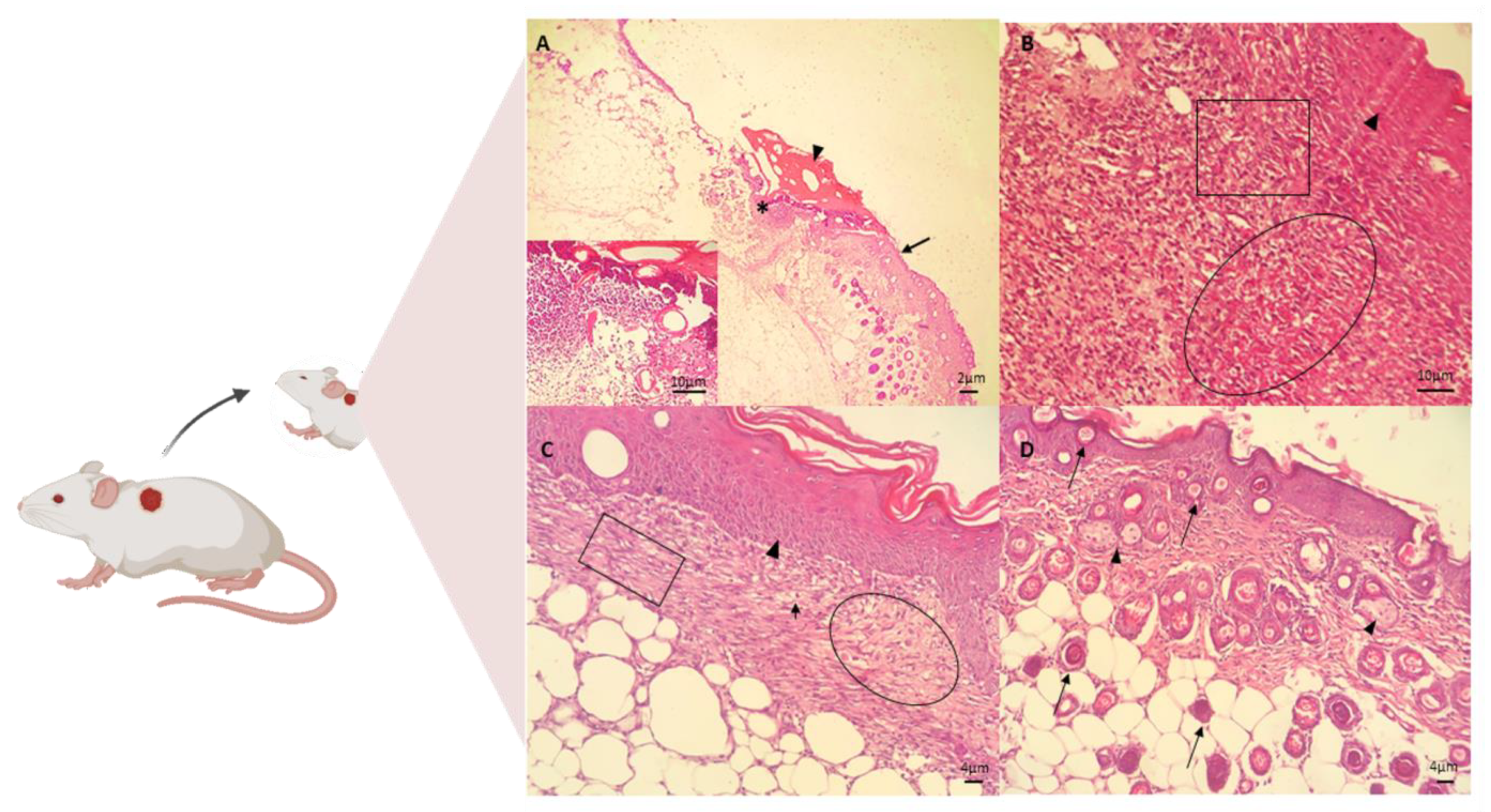
The in vivo assay for the treatment of skin wounds in mice was performed using the CGCHO. In Figure 5, the histopathological evaluations performed on days 3, 7, 14 and 21 of treatment are shown. Signs of the inflammatory phase were identified on the third day of treatment by histopathological analysis, such as the presence of ulcers, necrosis, and inflammatory response [68]. Inflammatory infiltrate was identified, with the presence of cellular debris and mononuclear cell infiltrate. In the deep dermis, mononuclear infiltrate, hemorrhage, fibrin network and cellular debris were observed in the wound (Figure 5A). On the seventh day of the experiment, the presence of fibrin, mononuclear cells, the formation of granulation tissue in the dermis and fibroblasts producing fundamental amorphous substances was observed. An angiogenesis process and some mast cells were observed. In the deeper dermis, young collagen fibers without individualization were observed. The re-epithelialization process was completed after 7 days of treatment with CGCHO, with a thicker epidermis close to the lesion area (Figure 5B). CH gel with nerolidol was produced and in the in vivo healing assays on day 7, the beginning of reepithelialization was also observed [3].
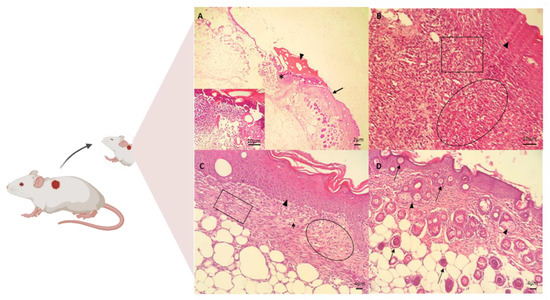
Figure 5.
Photomicrographs of skin wounds on the (A) 3rd, (B) 7th, (C) 14th, and (D) 21st day of treatment for the group treated with chicha gum/chitosan/Mauritia flexuosa oil gel (CGCHO). H.E. stain. (Scale bar: 2 µm, 4 µm, and 10 µm). (A) Skin ulcer (*) with the epidermis on the right edge (arrows), fibrin clot (arrowheads), and inflammatory infiltrate of polymorphonuclear cells in the dermis (detail). (B) Continuing reepithelialization of the epidermis (arrowhead), presence of newly formed vessels in granulation tissue in the dermis (circle), and macrophages and new fibroblasts (square). (C) Epidermal reepithelialization (arrowheads) and extensive granulation tissue formation (circle), with neoformed vessels (arrows), and collagen fibers(square). (D) Presence of hairs follicle (bulbus-arrows) and sebaceous glands (arrowheads).
On the 14th day, the formation of capillaries and granulation tissue with dense collagen fibers and the presence of congested mononuclear cells were observed (Figure 5C). On the last day of treatment (day 21), the complete process of epidermal re-epithelialization was observed, with hair bulbs in the dermis, contractile fibroblasts, and denser and thicker collagen fibers (Figure 5D).
The healing process is a complex physiological reaction, being a dynamic procedure that requires the participation of different tissues and cell lines [69]. Events such as re-epithelialization and tissue repair play important roles in wound healing [70]. The healing properties observed in this study may be related to the characteristics of the CGCHO gel. CH has a structure similar to the glycosaminoglycans present in the extracellular matrix (ECM), having the ability to stimulate the re-epithelialization process [5,17,71]. CG is an anionic polysaccharide with swelling power and can facilitate wound repair [36,68]. Mauritia flexuosa oil is rich in fatty acids, has an immunomodulatory character, acting as a chemotactic agent and an angiogenesis promoter, and may interfere with the inflammatory process [23,24,25,26,27,28,29,30,31,32]. It has already been observed that extra virgin olive oil, Moringa oleifera oil, and Melaleuca alternifolia oil have demonstrated promising efficacy in the treatment, healing, and closure of wounds through their anti-inflammatory, antioxidant, antimicrobial properties and by stimulating the angiogenic activities necessary for wound healing [72,73,74]. The combination of biomaterials has a common interest because they act on molecular and cellular events that aim to repair the defect in tissue integrity [75,76]. Thus, the gel produced can provide moisture for re-epithelialization, and contains antibacterial agents, phenolic compounds and flavonoids that are antioxidant agents and act to control the production of pro-inflammatory molecules. In addition, it has adequate roughness to allow gas exchange, which can be useful in the healing process. However, since these are natural products, there is a need for further toxicological tests on the components of the CGCHO gel to demonstrate the efficacy and tolerance for topical treatment, as well as to identify synergy or antagonism between the components.
4. Conclusions
It was possible to develop a gel with chicha gum, chitosan and Mauritia flexuosa oil for the treatment of wounds. The gel showed interesting properties, such as roughness, antimicrobial activity against Staphylococcus aureus and Klebsiella pneumoniae and good anti-inflammatory activity. All of these properties probably favored the re-epithelialization process, maintaining a moist environment and stimulating the angiogenic activities necessary for wound healing, as observed in the in vivo study. However, further studies of toxicity, biodegradation profile and release profile of Mauritia flexuosa oil are needed for CGCHO gel. However, CGCHO gel may be a promising candidate for the treatment of wounds.
Author Contributions
Investigation, writing—original draft, visualization: M.O.G.F.; formal analysis, writing—original draft: M.S.R.; writing—review and editing: A.C.d.J.O.; funding acquisition, validation, supervision, project administration: A.B.R., J.A.O., L.M.E. and E.C.S.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Swiss strain mice (Mus musculus) were obtained from the Central Bioterium of the Federal University of Piaui, Brazil. The animals used were 2 to 4 months old and had a body mass around 25–30 g. The animals were kept under temperature conditions up to 26 ± 1 °C, under a 12 h light/12 h dark cycle, and with access to food and water. The animals were randomly divided, and topical treatment was with the CG, Mauritia flexuosa oil, CGCHO and, CHG. The procedures were guided by the ethical principles established by the National Council for the Control of Animal Experimentation and the UFPI Animal Experimentation Ethics Committee (207/16, 16 September 2016), in accordance with the provisions of Law 11,794, of 8 October 2008.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors acknowledge the Coordination for the Improvement of Higher Education Personnel, the Brazilian Ministry of Education, financial support for the scholarship. To National Council for Scientific and Technological Development (CNPq), and Piaui State Research Support Foundation (FAPEPI). The authors are thankful to CNPq for financial support (Postdoctoral fellow 164646/2020-5), and NORTE-01-0247-FEDER-113540-“Pharmapitox-Desenvolvimento de um coletor inovador e protocolo para purificação da apitoxina para uso nas indústrias farmacêutica e cosmética”.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In Vitro, Ex Vivo evaluation and In Vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; d’Ayala, G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.G.; Leite, L.L.R.; de Lima, I.S.; Barreto, H.M.; Nunes, L.; Ribeiro, A.B.; Osajima, J.A.; Filho, E.S. Chitosan Hydrogel in combination with Nerolidol for healing wounds. Carbohydr. Polym. 2016, 152, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of bioactive compounds-loaded chitosan films by using a QbD approach–A novel and potential wound dressing material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from nature: Biopolymers and biocomposites as smart wound care materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-inflammatory and anti-oxidant properties of laccase-synthesized phenolic-O-carboxymethyl chitosan hydrogels. New Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Moholkar, D.N.; Sadalage, P.S.; Peixoto, D.; Paiva-Santos, A.C.; Pawar, K.D. Recent advances in biopolymer-based formulations for wound healing applications. Eur. Polym. J. 2021, 160, 110784. [Google Scholar] [CrossRef]
- Shukla, A.; Choudhury, S.; Chaudhary, G.; Singh, V.; Prabhu, S.N.; Pandey, S.; Garg, S.K. Chitosan and gelatin biopolymer supplemented with mesenchymal stem cells (Velgraft®) enhanced wound healing in goats (Capra hircus): Involvement of VEGF, TGF and CD31. J. Tissue Viability 2021, 30, 59–66. [Google Scholar] [CrossRef]
- Hazrati, R.; Davaran, S.; Omidi, Y. Bioactive functional scaffolds for stem cells delivery in wound healing and skin regeneration. React. Funct. Polym. 2022, 174, 105233. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Z.; Wu, D.; Gan, W.; Zhu, S.; Li, W.; Tian, J.; Li, L.; Zhou, C.; Lu, L. Antibacterial poly (ethylene glycol) diacrylate/chitosan hydrogels enhance mechanical adhesiveness and promote skin regeneration. Carbohydr. Polym. 2019, 225, 115110. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Yang, M.; Zhang, H.; Zhu, L. Fabrication of a novel blended membrane with chitosan and silk microfibers for wound healing: Characterization, in vitro and in vivo studies. J. Mater. Chem. B 2015, 3, 3634–3642. [Google Scholar] [CrossRef] [PubMed]
- Seon, G.M.; Lee, M.H.; Kwon, B.-J.; Kim, M.S.; Koo, M.-A.; Seomun, Y.; Kim, J.-T.; Kim, T.H.; Park, J.-C. Recombinant batroxobin-coated nonwoven chitosan as hemostatic dressing for initial hemorrhage control. Int. J. Biol. Macromol. 2018, 113, 757–763. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Ferreira, M.O.G.; de Lima, I.S.; Morais, A.S.; Silva, S.O.; de Carvalho, R.B.F.; Ribeiro, A.B.; Osajima, J.A.; Filho, E.C.S. Chitosan associated with chlorhexidine in gel form: Synthesis, characterization and healing wounds applications. J. Drug Deliv. Sci. Technol. 2019, 49, 375–382. [Google Scholar] [CrossRef]
- Tamer, T.M.; Valachová, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; Eldin, M.S.M.; Šoltés, L. Chitosan/hyaluronan/edaravone membranes for anti-inflammatory wound dressing: In vitro and in vivo evaluation studies. Mater. Sci. Eng. C 2018, 90, 227–235. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B 2016, 4, 4801–4812. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Luo, L.; Ding, Q.; Tang, S. Bio-orthogonally crosslinked catechol–chitosan hydrogel for effective hemostasis and wound healing. Carbohydr. Polym. 2022, 281, 119039. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Elasaly, M.K.; Omar, S.A.; El-Nabarawi, M.A.; Shoueir, K.R. Wound healing activities of polyurethane modified chitosan nanofibers loaded with different concentrations of linezolid in an experimental model of diabetes. J. Drug Deliv. Sci. Technol. 2022, 67, 102982. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Sukumaran, H.G.; Dara, P.K.; Ganesan, B.; Ashraf, M.; Anandan, R.; Mathew, S.; Nagarajarao, R.C. Nano-encapsulation of curcumin in fish collagen grafted succinyl chitosan hydrogel accelerates wound healing process in experimental rats. Food Hydrocoll. Health 2022, 2, 100061. [Google Scholar] [CrossRef]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, L.; Li, H.; Xia, L.; Hu, H.; Wang, S.; Liu, C.; Chi, J.; Yang, Y.; Song, F.; et al. Preparation, biocompatibility, and wound healing effects of O-carboxymethyl chitosan nonwoven fabrics in partial-thickness burn model. Carbohydr. Polym. 2022, 280, 119032. [Google Scholar] [CrossRef] [PubMed]
- Braz, E.M.A.; Silva, S.C.C.C.; Brito, C.A.R.S.; Carvalho, F.A.A.; Alves, M.M.M.; Barreto, H.M.; Silva, D.A.; Magalhães, R.; Oliveira, A.L.; Silva-Filho, E.C. Modified chicha gum by acetylation for antimicrobial and antiparasitic applications: Characterization and biological properties. Int. J. Biol. Macromol. 2020, 160, 1177–1188. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.C.; Jung, W.K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef]
- Silva, S.C.C.C.; Braz, E.M.D.A.; Carvalho, F.A.D.A.; Brito, C.A.R.D.S.; Brito, L.M.; Barreto, H.M.; Filho, E.C.D.S.; da Silva, D.A. Antibacterial and cytotoxic properties from esterified Sterculia gum. Int. J. Biol. Macromol. 2020, 164, 606–615. [Google Scholar] [CrossRef]
- Da Silva, J.S.F.; de Jesus Oliveira, A.C.; Soares, M.F.D.L.R.; Soares-Sobrinho, J.L. Recent advances of Sterculia gums uses in drug delivery systems. Int. J. Biol. Macromol. 2021, 193, 481–490. [Google Scholar] [CrossRef]
- Ferreira, S.R.D.S.; Mesquita, M.V.N.; de Sá, L.L.F.; Nogueira, N.C.; Rizzo, M.D.S.; Silva-Filho, E.C.; da Costa, M.P.; Ribeiro, A.B. Sustainable natural gums for industrial application: Physiochemical and texturometric evaluation. J. Drug Deliv. Sci. Technol. 2019, 54, 101306. [Google Scholar] [CrossRef]
- Costa, A.N.; de Sá, R.A.; Bezerra, R.D.; Souza, J.L.; Lima, F.D.C. Constituents of buriti oil (Mauritia flexuosa L.) like inhibitors of the SARS-Coronavirus main peptidase: An investigation by docking and molecular dynamics. J. Biomol. Struct. Dyn. 2020, 39, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- FFerreira, M.O.G.; Lima, I.S.; Ribeiro, A.B.; Lobo, A.O.; Rizzo, M.S.; Osajima, J.A.; Estevinho, L.M.; Silva-Filho, E.C. Biocompatible Gels of Chitosan–Buriti Oil for Potential Wound Healing Applications. Materials 2020, 13, 1977. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, E.; Curi, R. Ácidos graxos e cicatrização: Uma revisão. Rev. Bras. Farm. 2007, 88, 53–58. [Google Scholar]
- Aquino, J.D.S.; Pessoa, D.C.; Araújo, K.D.L.G.; Epaminondas, P.S.; Schuler, A.R.P.; Souza, A.G.D.; Stamford, T.L.M. Refining of buriti oil (Mauritia flexuosa) originated from the Brazilian Cerrado: Physicochemical, thermal-oxidative and nutritional implications. J. Braz. Chem. Soc. 2012, 23, 212–219. [Google Scholar] [CrossRef]
- Agregán, R.; Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Carballo, J.; Franco, D. Assessment of the antioxidant activity of Bifurcaria bifurcata aqueous extract on canola oil. Effect of extract concentration on the oxidation stability and volatile compound generation during oil storage. Food Res. Int. 2017, 99, 1095–1102. [Google Scholar] [CrossRef]
- Gonçalves, V.; Galego, L.; Almeida, V.; Costa, M.; Monteiro, I.; Matos, F. Antioxidant activity of the essential oils of Thymbra capitata, Origanum vulgare, Thymus mastichina, and Calamintha baetica. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Plants as Food and Medicine: The Utilization, Seoul, Korea, 13 August 2006; Volume 765, pp. 325–334. [Google Scholar]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Für Nat. C 2008, 63, 331–336. [Google Scholar] [CrossRef]
- Lima, L.A.R.D.S.; Johann, S.; Cisalpino, P.S.; Pimenta, L.P.S.; Boaventura, M.A.D. In Vitro antifungal activity of fatty acid methyl esters of the seeds of Annona cornifolia A. St.-Hil.(Annonaceae) against pathogenic fungus Paracoccidioides brasiliensis. Rev. Soc. Bras. Med. Trop. 2011, 44, 777–780. [Google Scholar] [CrossRef]
- Nobre, C.B.; Sousa, E.O.; Camilo, C.J.; Machado, J.F.; Silva, J.M.; Jaime Filho, R.; Coutinho, H.D.M.; Costa, J.G. Antioxidative effect and phytochemical profile of natural products from the fruits of “babaçu”(Orbignia speciose) and “buriti”(Mauritia flexuosa). Food Chem. Toxicol. 2018, 121, 423–429. [Google Scholar] [CrossRef]
- Cruz, M.B.; da Silva Oliveira, W.; Araújo, R.L.; França, A.C.H.; Pertuzatti, P.B. Buriti (Mauritia Flexuosa L.) pulp oil as an immunomodulator against enteropathogenic Escherichia coli. Ind. Crops Prod. 2020, 149, 112330. [Google Scholar] [CrossRef]
- Zanatta, C.F.; Mitjans, M.; Urgatondo, V.; Rocha-Filho, P.A.; Vinardell, M.P. Photoprotective potential of emulsions formulated with Buriti oil (Mauritia flexuosa) against UV irradiation on keratinocytes and fibroblasts cell lines. Food Chem. Toxicol. 2010, 48, 70–75. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, P.D.S.; de Araujo Boleti, A.P.; do Carmo Vieira, M.; Carollo, C.A.; da Silva, D.B.; Estevinho, L.M.; Dos Santos, E.L.; de Picoli Souza, K. Microbiological quality, chemical profile as well as antioxidant and antidiabetic activities of Schinus terebinthifolius Raddi. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 220, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef] [PubMed]
- Koolen, H.H.; da Silva, F.M.; Gozzo, F.C.; de Souza, A.Q.; de Souza, A.D. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L.) by UPLC–ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef]
- Medeiros, M.C.; Aquino, J.S.; Soares, J.; Figueiroa, E.B.; Mesquita, H.M.; Pessoa, D.C.; Stamford, T.M. Buriti oil (Mauritia flexuosa L.) negatively impacts somatic growth and reflex maturation and increases retinol deposition in young rats. Int. J. Dev. Neurosci. 2015, 46, 7–13. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Approved Standard M07–A10; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Pereira, L.A.; da Silva Reis, L.; Batista, F.A.; Mendes, A.N.; Osajima, J.A.; Silva-Filho, E.C. Biological properties of chitosan derivatives associated with the ceftazidime drug. Carbohydr. Polym. 2019, 222, 115002. [Google Scholar] [CrossRef]
- Silva, R.F.J.; Pighinelli, L. Application of chitosan and buriti oil (Mauritia Flexuosa L.) in skin wound healing. J. Appl. Biotechnol. Bioeng. 2017, 3, 00056. [Google Scholar]
- Pereira, G.G.; Santos-Oliveira, R.; Albernaz, M.S.; Canema, D.; Weismüller, G.; Barros, E.B.; Magalhães, L.; Lima-Ribeiro, M.H.M.; Pohlmann, A.; Guterres, S.S. Microparticles of Aloe vera/vitamin E/chitosan: Microscopic, a nuclear imaging and an in vivo test analysis for burn treatment. Eur. J. Pharm. Biopharm. 2014, 86, 292–300. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2018, 112, 1300–1309. [Google Scholar] [CrossRef]
- Ferreira, B.S.; De Almeida, C.G.; Faza, L.P.; De Almeida, A.; Diniz, C.G.; Silva, V.L.D.; Le Hyaric, M. Comparative properties of amazonian oils obtained by different extraction methods. Molecules 2011, 16, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, S.; Ncube, B.; Finnie, J.F.; McGaw, L.J.; Njoya, E.M.; Coopoosamy, R.M.; Van Staden, J. Antioxidant, anti-inflammatory and wound healing properties of medicinal plant extracts used to treat wounds and dermatological disorders. S. Afr. J. Bot. 2019, 126, 232–240. [Google Scholar] [CrossRef]
- dos Santos, U.P.; Campos, J.F.; Torquato, H.F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; Souza, K.D.P.; Dos Santos, E.L. Antioxidant, antimicrobial and cytotoxic properties as well as the phenolic content of the extract from Hancornia speciosa Gomes. PLoS ONE 2016, 11, e0167531. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.G.; Arruda, H.S.; Araujo, N.M.P.; Braga, L.E.D.O.; Banzato, T.P.; Pereira, G.A.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Eberlin, M.N.; de Carvalho, J.E.; et al. Antioxidant, antiproliferative and healing properties of araticum (Annona crassiflora Mart.) peel and seed. Food Res. Int. 2020, 133, 109168. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef]
- Ojeda-Martínez, M.L.; Yáñez-Sánchez, I.; Velásquez-Ordoñez, C.; Martínez-Palomar, M.M.; Álvarez-Rodríguez, A.; A Garcia-Sánchez, M.; Rojas-González, F.; Gálvez-Gastélum, F.J. Skin wound healing with chitosan thin films containing supported silver nanospheres. J. Bioact. Compat. Polym. 2015, 30, 617–632. [Google Scholar] [CrossRef]
- Bai, M.Y.; Chou, T.C.; Tsai, J.C.; Yu, W.C. The effect of active ingredient-containing chitosan/polycaprolactone nonwoven mat on wound healing: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 2324–2333. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 225, 115238. [Google Scholar] [CrossRef]
- Sasikala, L.; Rathinamoorthy, R.; Dhurai, B. Optimization of process conditions for chitosan-manuka honey film as wound contact layer for wound dressings. Wound Med. 2018, 23, 11–21. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Barbosa, W.S.; Rangel, W.S.; Valle, I.M.M.; Matos, A.P.D.S.; Melgaço, F.G.; Dias, M.L.; Júnior, E.R.; da Silva, L.C.P.; de Abreu, L.C.L.; et al. Polymeric membrane based on polyactic acid and babassu oil for wound healing. Mater. Today Commun. 2021, 26, 102173. [Google Scholar] [CrossRef]
- Pascoal, A.; Estevinho, L.M.; Martins, I.M.; Choupina, A.B. Novel sources and functions of microbial lipases and their role in the infection mechanisms. Physiol. Mol. Plant Pathol. 2018, 104, 119–126. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Importance of Candida–bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011, 19, 557–563. [Google Scholar] [CrossRef]
- Hughes, C.; Ferguson, J. Phenotypic chlorhexidine and triclosan susceptibility in clinical Staphylococcus aureus isolates in Australia. Pathology 2017, 49, 633–637. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Mi, B.; Yan, C.; Chen, L.; Liu, G.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Liu, X.; You, L.; Tarafder, S.; Zou, L.; Fang, Z.; Chen, J.; Lee, C.H.; Zhang, Q. Curcumin-releasing chitosan/aloe membrane for skin regeneration. Chem. Eng. J. 2019, 359, 1111–1119. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; de Luna-Bertos, E.; Ramos-Torrecillas, J.; Illescas-Montesa, R.; Costela-Ruiz, V.J.; García-Martínez, O. Potential Effects of Phenolic Compounds That Can Be Found in Olive Oil on Wound Healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef]
- Halcón, L.; Milkus, K. Staphylococcus aureus and wounds: A review of tea tree oil as a promising antimicrobial. Am. J. Infect. Control. 2004, 32, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.C.S.S.B.; de Paula, T.; Gonçalves, J.P.; da Silva Soley, B.; Cretella, A.B.M.; Otuki, M.F.; Cabrini, D.A. The oil from Moringa oleifera seeds accelerates chronic skin wound healing. Phytomed. Plus 2021, 1, 100099. [Google Scholar] [CrossRef]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The effects of lavender essential oil on wound healing: A review of the current evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).