Abstract
Hypertension is the leading cause of global disease burden. Hypertension can arise from early life. Animal models are valuable for giving cogent evidence of a causal relationship between various environmental insults in early life and the hypertension of developmental origins in later life. These insults consist of maternal malnutrition, maternal medical conditions, medication use, and exposure to environmental chemicals/toxins. There is a burgeoning body of evidence on maternal insults can shift gut microbiota, resulting in adverse offspring outcomes later in life. Emerging evidence suggests that gut microbiota dysbiosis is involved in hypertension of developmental origins, while gut microbiota-targeted therapy, if applied early, is able to help prevent hypertension in later life. This review discusses the innovative use of animal models in addressing the mechanisms behind hypertension of developmental origins. We will also highlight the application of animal models to elucidate how the gut microbiota connects with other core mechanisms, and the potential of gut microbiota-targeted therapy as a novel preventive strategy to prevent hypertension of developmental origins. These animal models have certainly enhanced our understanding of hypertension of developmental origins, closing the knowledge gap between animal models and future clinical translation.
1. Introduction
Hypertension is the most common chronic disease and yields considerable morbidity and mortality globally [1]. Because of the multifactorial nature of hypertension, the use of various animal models, which evoke hypertension by different mechanisms, is advantageous for unraveling disease pathogenesis and developing novel antihypertensive drugs [2,3,4]. Though we are seeing tremendous progress on experimental hypertension, the prevalence of hypertension is still high and continues to rise worldwide [5].
Epidemiological and animal studies support that hypertension may be programmed in utero [6,7,8,9]. The association between fetal development and increased risk of adult disease has emerged as the concept of developmental origins of health and disease (DOHaD) [10]. A wide spectrum of early-life insults can evoke developmental programming resulting hypertension later in life. These insult stimuli include, but are not limited to, maternal malnutrition (both under- and overnutrition), maternal medical conditions, environmental exposure to toxins/chemicals, lifestyle changes, and medicines taken during pregnancy [7,8,9,11,12,13,14].
Over the past decade, the pathogenesis behind hypertension of developmental origins has not been fully elucidated, but data from animal models have proposed several key mechanisms [14]. Until now, the proposed mechanisms consist of aberrant renin–angiotensin system (RAS), oxidative stress, reduced nephron numbers, gut microbiota dysbiosis, dysregulated nutrient-sensing signals, sex differences, epigenetic regulation, etc. [7,8,9,11,12,13,14]. Among them, the interaction between the gut microbiota and the host implicated in hypertension has received significant interest [15,16,17,18]. Despite gut microbiota dysbiosis being observed in multiple animal models of hypertension [15,16], too little attention has been paid to its role in hypertension of developmental origins.
Although blood pressure (BP) is considered with a multifactorial pattern of inheritance, genome-wide association studies cumulatively could only explain ~3.5% of BP trait variability [19]. Accordingly, it is likely that the influence of environmental and epigenetic factors on the developmental programming of hypertension should receive wider recognition. Notably, maternal insults can impair gut microbiota composition and function, leading to adverse offspring outcomes later in life [20]. Conversely, review elsewhere indicted that early-life gut microbiota-targeted therapies have benefits on the prevention of the developmental programming of adult disease, including hypertension [21]. All this raises the notion that we need to pay more attention to prevent and not just treat hypertension, with a focus on the influence of dysbiotic gut microbiota on hypertension of developmental origins. Accordingly, animal models would likely be very useful in unraveling these actions.
In this review, we describe the role of gut microbiota implicated in animal models used for studying the developmental programming of hypertension. Therefore, we summarize the contributions of animal models linking the gut microbiota to developmental programming of hypertension, which helps in developing valuable strategies to prevent hypertension from happening. We specifically focus on addressing gut microbiota-targeted therapies such as probiotics, prebiotics, and postbiotics as a reprogramming strategy for prevention of hypertension of developmental origins.
In view of the above, a search was performed in the electronic bibliographic database PubMed/MEDLINE. Search terms were as follows: “developmental programming”, “DOHaD”, “animal model”, “pregnancy”, “gestation”, “offspring”, “progeny”, “prenatal”, “perinatal”, “mother”, “maternal”, “reprogramming”, “gut microbiota”, “probiotics”, “prebiotics”, “postbiotics”, “synbiotics”, “blood pressure”, and “hypertension.” Relevant abstracts were identified and reviewed to identify appropriate studies. Suitable published articles in English were included, without restriction of the time of publication.
2. Hypertension of Developmental Origins: Choice of Animal Models
Compared to animal models of essential hypertension established in prior research [2,3], the etiologies of hypertension of developmental origins and underlying pathogenic mechanisms are more complex [14]. Animal models of hypertension of developmental origins can be categorized in different ways (Figure 1).
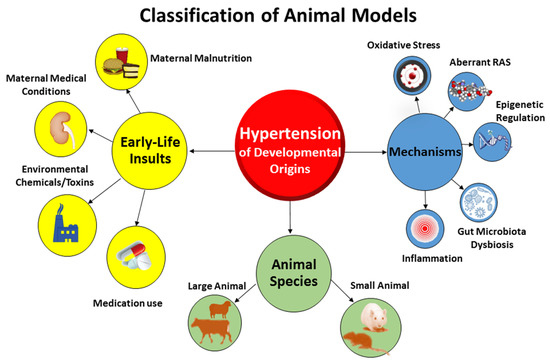
Figure 1.
The classification of major animal models for studying hypertension of developmental origins.
Firstly, these models can be classified according to early-life adverse conditions. Nutritional programming is the most common type of animal model being studied in the field of DOHaD research [22]. Dietary caloric or protein restriction in animals mimics the starvation linked to famine in human cohorts [23,24]. Imbalance of maternal nutrition can have long-term changes in BP, resulting hypertension in adult offspring [25]. Inadequate or excessive intake of a specific nutrient has been established to induce hypertension of developmental origins in animal models, as reviewed elsewhere [11,26]. These models of undernutrition related to hypertension of developmental origins include, but are not limited to, caloric restriction [27], protein restriction [28], and deficiencies in sodium [29], calcium [30], zinc [31], iron [32], methyl donor nutrients (choline; vitamins B2, B6, and B12; folic acid; and methionine) [33], and vitamin D [34]. On the other hand, overnutrition characterized by the consumption of a high-fat [35,36], high-fructose [37,38], or high-protein diet [39] by rodent mothers also leads to early programming of hypertension in the offspring. Additionally, animal models resembling maternal medical conditions have also been evaluated in developmental programming of hypertension. These models include hypertensive disorders of pregnancy [40], preeclampsia [41], diabetes [42], chronic kidney disease (CKD) [43], maternal hypoxia [44], etc. Furthermore, chemical and medication exposures during pregnancy increase the risk of developing hypertension in offspring [13,14]. Prenatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [45], bisphenol A [46], nicotine [47], caffeine [48], cyclosporine [49], gentamicin [50], tenofovir [51], minocycline [52], or glucocorticoids [53] has been reported to induce hypertension of developmental origins in various animal models.
Secondly, animal models can be classified based on molecular mechanisms. In view of different early-life adverse environmental factors producing the same outcome, that is to say hypertension in adult offspring, there might be core mechanisms underlying hypertension of developmental origins. These mechanisms include gut microbiota dysbiosis [21], oxidative stress [12], aberrant RAS [54], reduced nephron numbers [7], dysregulated nutrient-sensing signals [55], sex differences [56,57], epigenetic regulation [58], inflammation [9,14], nitric oxide (NO) deficiency [59], etc. Up to date, various animal models have been developed to test such proposed mechanisms. Because of the multifactorial nature of developmental hypertension, the use of various animal models, each of which induces hypertension by a different mechanism yet with the same end result, is advantageous. This approach would allow for a novel and effective reprogramming intervention targeting a specific molecular pathway to be adopted for preventions and therapies.
Lastly, animal models in DOHaD research can be classified according to species [60]. Diverse large- and small-animal models have been used, each with its own natural advantages and disadvantages [8]. Former reviews demonstrated that cow [61], sheep [62], rat [27], and mice [63] have be used to study hypertension of developmental origins [14]. Considering that rat models are cost-effective and easy to maintain and breed, they became the most common species used in the research field of DOHaD-related hypertension [14]. Although nonhuman primates [64], swine [65], rabbits [66], and guinea pig [67] have been studied for cardiovascular outcomes induced by maternal insult stimuli, none of them have been reported for examining hypertension of developmental origins.
Rats are by far the most often used species in the field of primary hypertension research. Of these, the spontaneously hypertensive rat (SHR) without any doubt is the most popular strain [2]. However, the majority of the rat strains used for studying developmental hypertension are Sprague-Dawley (SD) or Wistar [14]. In view of the genetic background of SHR, offspring develops hypertension spontaneously without programming induced by early-life insults, weakening its application in studying hypertension of developmental origins. Hence, the choices of animal models between essential hypertension and hypertension of developmental origins are quite different. Many more aspects of animal models need to be taken into further consideration, such as the timing of organogenesis [68], life cycle [69], gestation period [70], litter size [71], offspring outcomes, than in human studies [72], and valuable therapeutic interventions need to be evaluated and validated [14].
Together, it is noted that remarkable advances in hypertension of developmental origins have been originated from animal models. However, what is missing in the literature is animal models used for studying hypertension-related complications. Although elevated BP is the core feature of human hypertension, its morbidity and mortality occur with complications in the heart, brain, kidneys, and vessels. The contributions of early-life insults to these hypertension-related complications later in life in an organ-dependent manner have not yet been well-studied in the above-mentioned animal models.
3. Gut Microbiota: Choice of Animal Models
Trillions of bacteria living in the gut—the gut microbiota—coexist with the host in a mutually beneficial relationship [73]. Microbiota refers to all the microorganisms found in the environment, while the term microbiome refers to the collection of genomes from all microorganisms in a given environment. A variety of environmental factors can cause the disturbance of gut microbiota (i.e., dysbiosis), which in turn can influence human health and disease. Although the influence of gut microbiota in hypertension has been extensively reviewed elsewhere [15,16,17,18], less attention was paid to exploring its role in hypertension of developmental origins.
Directly after birth, microbes colonize the neonatal gut immediately [74]. These alterations continue until three years of age and mediate the transition toward an adult-like gut microbiota [75]. During pregnancy and lactation, the mothers share gut microbes and microbial metabolites with their offspring, which highlights the importance of maternal influences in the development of early-life gut microbiota [76]. A diversity of early-life factors governs the establishment of the gut microbiota, such as maternal medical conditions, gestational age, types of delivery, antibiotic exposure, formula feeding, and ecological factors [74,75,76,77].
So far, animal models have been broadly established to investigate human diseases in gut microbiota research [78]. Figure 2 illustrates various approaches to alter the gut microbiota in animal models of disease. Several gut microbiota-targeted therapies have been used to alter gut microbiota compositions and its derived metabolites. These interventions consist of probiotics, prebiotics, synbiotics, postbiotics, etc. [14]. The embryo transfer (ET) method is considered the gold standard for gut microbiota transfer. Additionally, researchers often use other methods to transfer the gut microbiota, such as fecal microbiota transfer (FMT), co-housing (CH), or cross-fostering (CF) [78].
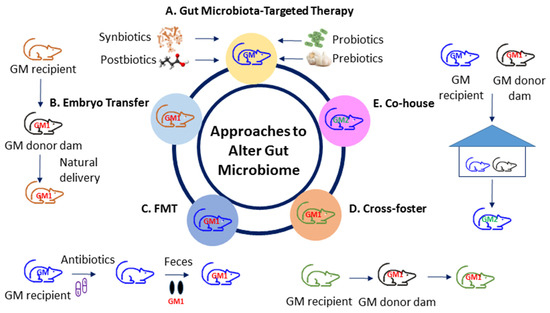
Figure 2.
Different approaches to altering the gut microbiota. (A) Gut microbiota-target therapy; (B) embryo transfer; (C) fecal microbiota transfer; (D) cross-foster; (E) co-house. FMT = fecal microbiota transfer; GM = gut microbiota; GM1 = transferred gut microbiota; GM2 = GM + GM1.
Several gut microbiota-targeted therapies have shown to alter the gut microbiome. Probiotics (i.e., live beneficial microbes) and prebiotics (i.e., substances in foods that promote the growth of healthy microbes) are the most commonly used gut microbiota-targeted modalities in clinical practice [79]. Synbiotics refer to a mixture comprising probiotics and prebiotics that also confers a health benefit [79]. In addition, the use of substances leased or produced through gut microbial metabolism, namely postbiotics, have shown an influence on gut microbiota compositions and metabolites [80].
In the approach to transfer embryos, they are collected from the gut microbiome recipient and surgically transferred to a pseudopregnant donor dam [78]. Accordingly, the recipient pups obtain the vaginal microbiota from the donor dam through vaginal delivery. Nevertheless, this method needs considerable costs and expertise, making it inaccessible for many labs. Using the FMT approach, feces or fecal contents from donors are transferred to recipient animals via gastric gavage. Germ-free mice or antibiotics-treated depleted microbiota animals are commonly used as recipients [81].
Another commonly used method is CH, wherein recipients are co-housed with a donor after weaning [82], leading to the transfer of the donor gut microbiome through coprophagy and grooming [82]. Although the co-housing approach is easy and low-cost, the transfer of the gut microbiota after the critical developmental period results an incomplete transfer as well as a hybridized gut microbiome. When the recipient pups are housed in cages with the donor dam within 24 h after birth, the CF method allows the recipients to obtain most of their gut microbiota from the donor dam [78]. Compared to CH, the CF approach transfers the gut microbiota from an early age during the maternal care process.
All these methods each carry certain advantages and limitations. Researchers should thus be mindful of these method-related differences in the context of the transfer methods used for studying the role of the gut microbiota on hypertension of developmental origins.
4. Gut Microbiota in Hypertension of Developmental Origins
There is mounting evidence to support the pathogenic interconnection between the gut microbiome and hypertension [15,16,17,18]. However, there is paucity of information regarding the influence of the gut microbiota on the developmental programming of hypertension later in life. Therefore, most data obtained from patients with established hypertension and knowledge received from animal models of essential hypertension might be extrapolated to hypertension of developmental origins.
4.1. Gut Microbiota and BP Regulation
A great deal of work on the influence of the gut microbiota and its derived metabolites on BP regulation has been conducted. First, data from several genetic hypertensive rat models (e.g., SHR) indicated that the gut microbiota of hypertensive rats is dysbiotic and significantly different from the microbiota of normotensive control rats [15]. Gut microbiota dysbiosis was also noted for other hypertension models such as animals treated with high salt [83], angiotensin II [84], and deoxycorticosterone acetate-salt [85]. Another line of evidence comes from germ-free animals. The absence of microbiota in germ-free rats resulted with relative hypotension compared with their conventionalized counterparts, suggesting an essential role of gut microbiota in BP regulation [86]. Additionally, germ-free mice that received FMT from a hypertensive human donor developed a gut microbiota similar to that of their donor, as well as elevated BP [87]. There are observations that microbial metabolites are involved in BP homeostasis. Short chain fatty acids (SCFAs) are the main metabolites produced during bacterial fermentation of carbohydrates. SCFAs are generally known to regulate BP via activating their SCFA receptor, including olfactory receptor 78 (Olfr78), G protein-coupled receptors (GPR) GPR41, GPR43, and GRP109A [88]. Another example is trimethylamine-N-oxide (TMAO). TMAO is a small colorless amine oxide produced by gut microbiota metabolism [89]. A high TMAO level correlates with CVD mortality [90]. Fourth, the uses of probiotics [91] or prebiotics [92] have shown benefits on hypertensive patients.
4.2. Animal Models Linking Gut Microbiota Dysbiosis to Hypertension of Developmental Origins
Much work investigating the actions of the gut microbiome has directly studied the hypertension models, yet relatively little data exists on its programming effect related to hypertension of developmental origins. A summary of animal studies indicating the association between dysbiotic gut microbiota and developmental hypertension in adult offspring is provided in Table 1 [40,52,93,94,95,96,97,98,99,100,101,102,103,104,105,106].

Table 1.
Animal models reporting hypertension of developmental origins associated with dysbiotic gut microbiota.
The current review is only restricted to early-life insults starting in the pregnancy and/or lactation period. Table 1 shows that rats are the most common species being used. A variety of early-life insults have been reported to induce developmental hypertension, accompanying alterations of the gut microbiota, including a maternal high-fructose diet [93,94,95], maternal high-fructose diet plus TCDD exposure [96], maternal high-fat/high-cholesterol diet [97], maternal high-fat and/or post-weaning high-fat diet [98,99], gestational hypertension [40,100], maternal CKD [43], maternal dyslipidemia [101], maternal NG-nitro-L-arginine-methyl ester (L-NAME) administration plus postnatal high-fat diet [102], maternal administration of minocycline [52], maternal TMAO and asymmetric dimethylarginine (ADMA) exposure [103], maternal TCDD exposure [104,105], and prenatal androgen exposure [106].
Table 1 lists the timing of hypertension determined from rat models, with age ranging from 12 weeks to four months. As every month of an adult rat corresponds to three human years [55], the observed periods correspond with humans from childhood to early adulthood.
4.3. Gut Microbiota Dysbiosis in Hypertension of Developmental Origins
The study of the gut microbiome in animal models of developmental hypertension mainly focuses on four types of dysbiosis: loss of diversity, decreases in beneficial microbes, shifts in key taxa, and alterations of microbial metabolites. A schematic summarizing the gut microbiota and a possible molecular pathway linked to hypertension of developmental origins is presented in Figure 3.
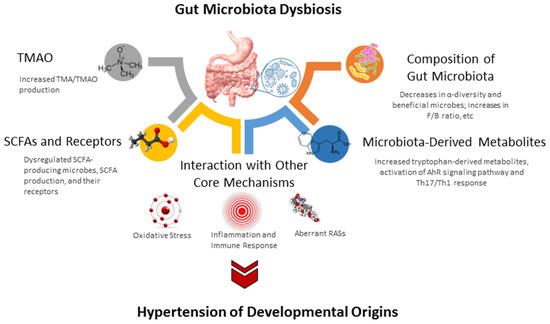
Figure 3.
Overview of the gut microbiota and potential molecular mechanisms related to hypertension of developmental origins. SCFA short chain fatty acid. TMAO = trimethylamine N-oxide; TMA = trimethylamine; SCFA = short chain fatty acid; RAS = renin-angiotensin system; TH17 = T helper 17 cells; TH1 = T helper 1 cells; F/B ratio = Firmicutes to Bacteroidetes ratio; AhR = aryl hydrocarbon receptor.
4.3.1. Alterations in Gut Microbiota Compositions
First, α-diversity is decreased in models of maternal high-fat and high-cholesterol diet [97] and maternal TCDD exposure [104,105]. A similar pattern of gut dysbiosis was reported in several hypertensive animal models [15]. Second, a maternal plus post-weaning high-fat diet programming offspring’s hypertension coincides with an increased Firmicutes to Bacteroidetes (F/B) ratio and a reduction of genera Lactobacillus and Akkermansia [98,99]. This was found to be consistent with hypertension models showing the F/B ratio was increased and served as a microbial marker of hypertension [15]. Likewise, the increase of the F/B ratio is noted in other models of developmental hypertension programmed by a variety of maternal insults, including CKD [43], minocycline administration [52], hypertension [100], L-NAME administration plus high-fat diet [102], and TCDD exposure [104,105]. Both Akkermansia and Lactobacillus are known as one of the beneficial probiotic bacterial strains [107,108]. Decreases of certain beneficial microbes were also found in developmental models of hypertension, like maternal minocycline administration [52], maternal high-fructose diet [94], maternal hypertension [40], maternal dyslipidemia [101], and maternal TCDD exposure [104,105].
4.3.2. SCFAs and Their Receptors
Notably, an association between microbiota-derived metabolites and hypertension has been found in several models of developmental hypertension [43,93,94,106].
SCFAs, the main metabolites produced by the gut microbiota, have one to six carbon atoms (C1–C6), mainly consisting of acetic acid (C2), propionic acid (C3), and butyric acid (C4) [88]. In SHR, hypertension is associated with decreased abundance of acetate- and butyrate-producing bacteria [15]. Similarly, SCFAs and their receptors are involved in hypertension of developmental origins, as reported in several animal models [52,93,106].
In a model of maternal administration of minocycline, minocycline-induced hypertension is associated with a reduction of plasma acetate and butyrate [52]. Another report demonstrated that dam rats receiving a 60% fructose diet caused offspring’s hypertension, coinciding with an increased plasma acetate level and a reduction of renal GPR41 and GPR43 expression [93]. As acetate is a ligand for GPR41 to induce vasodilatation, and Olfr78 exhibits vasoconstrictive action [109], these findings support the notion that SCFAs and their receptors contribute to maternal high-fructose-diet-induced hypertension in adult offspring. Additionally, maternal garlic oil therapy protects against offspring hypertension programmed by a high-fat diet, which is related to increased acetate, butyrate, and propionate, as well as their producing microorganisms [110]. Moreover, maternal SCFA supplementation have been reported to be protective on hypertension of developmental origins [85,94]. These findings support the notion that SCFAs and their receptors might be a crucial mechanism behind developmental programming of hypertension.
4.3.3. TMAO
TMAO is an end-product of microbial carnitine and choline metabolism [89]. TMAO is converted from trimethylamine (TMA) by flavin-containing monooxygenase (FMO). TMAO is able to activate nuclear factor-κB (NF-κB) signaling, enhance leukocyte-endothelial cell adhesion, and induce inflammatory gene expression, all of which are related to the development of hypertension [111].
Maternal exposure to TMAO results in hypertension in adult male offspring [103]. Conversely, microbe-dependent TMA and TMAO formation can be inhibited by 3,3-dimethyl-1-butanol (DMB), a structural analogue of choline [112].
In a maternal high-fructose diet model, maternal DMB therapy showed protection against hypertension in adult rat offspring, which was relevant to the reduction of TMA and TMAO levels [94]. Another study demonstrated that perinatal resveratrol therapy protected adult rat offspring against maternal CKD-induced hypertension, which was associated with a decrease of the TMAO-to-TMA ratio [113]. These observations suggest a pathogenic link between the TMAO metabolic pathway and hypertension of developmental origins.
4.4. Core Mechanisms Linking to Gut Microbiota
Considering that various early-life insults during fetal development produce the same outcome―hypertension in adulthood—there might be some core mechanisms involved in the pathogenesis of hypertension of developmental origins. A number of mechanisms so far have been proposed, such as aberrant RAS, oxidative stress, reduced nephron numbers, dysregulated nutrient-sensing signals, inflammation, sex differences, epigenetic regulation [7,8,9,11,12,13,14]. Among them, some are interconnected to gut microbiota dysbiosis and will be discussed in turn.
4.4.1. Oxidative Stress
During pregnancy, the presence of excessive reactive oxygen species (ROS) under suboptimal in utero conditions may prevail over the defensive antioxidant system and compromise fetal development, leading to oxidative stress damage [114]. A review elsewhere indicated that there are various types of in utero insult stimuli linked to oxidative stress in mediating hypertension of developmental origins [115]. The main mechanisms underlying the actions of oxidative stress-related hypertension of developmental origins include increased ROS-producing enzyme expression [116], increased ROS formation [117], decreased antioxidant capacity [118], impaired NO signaling pathway [27], increased lipid peroxidation [119], increased oxidative DNA damage [43], and increased peroxynitrite production [120].
Data from several animal models listed in Table 1 shows that the connections between gut microbiota dysbiosis and oxidative stress may be involved in the pathogenesis of programmed hypertension, including maternal CKD [43], high-fructose diet [95], and high-fat diet models [110]. Gut microbial communities are able to elicit redox signaling and maintain host–microbiota homeostasis [121]. An imbalance in the redox state can lead to inflammatory responses and gut damage, resulting in gut microbiota dysbiosis. In a maternal CKD model, offspring developed hypertension related to increased oxidative stress and impaired NO signaling [43]. In a subsequent study, perinatal resveratrol therapy protected adult offspring against hypertension programmed by maternal CKD, accompanied with reshaping the gut microbiota and reducing oxidative stress concurrently [113].
Together, oxidative stress may work together with the gut microbiota under hypertension of developmental origins. More attention needs to be paid to evaluate how the gut microbiota interconnects with oxidative stress to elicit organ-specific programming processes behind hypertension, and whether antioxidant therapy in pregnancy may also benefit the gut microbiota to protect adult offspring against hypertension of developmental origins.
4.4.2. Aberrant RAS
The RAS is a major regulatory network that maintains BP, and blockade of the RAS has emerged as a therapeutic option for hypertension [122]. An increasing number of animal models linked to aberrant RAS are now being developed to evaluate hypertension of developmental programming, as reviewed elsewhere [54].
Within the RAS, regulation is achieved through a cascade of proteases generating some bioactive peptides [122]. The classical RAS consists of angiotensin-converting enzyme (ACE), angiotensin (ANG) II, and angiotensin II type 1 receptor (AT1R). Activation of the classical RAS elicits vasoconstriction and inflammation under pathophysiological conditions, consequently resulting in hypertension and its related complications [123].
On the other hand, the nonclassical RAS is composed of ACE2/angiotensin-(1-7) (Ang-(1-7))/Mas receptor/ANG II type 2 receptor (AT2R), by which it can counterbalance the adverse effects of ANG II [124]. A growing body of evidence supports that aberrant RAS plays a key role in developmental hypertension, and RAS-based interventions can be used as a reprogramming strategy to prevent hypertension [54].
Mounting evidence suggests a bidirectional interaction between the gut microbiota and RAS; alterations in RAS shift microbiota composition and metabolic activity, while gut microbiota-derived metabolites can modulate the gut RAS [125]. Through regulation of intestinal amino acid transport, prior research reported that ACE2 plays a key non-catalytic role in gut biology and modulation of the gut microbiota [126].
Adult rat progeny of CKD mothers developed hypertension, coinciding with decreased expression of Mas receptor and AT2R [43]. In another high-fructose diet plus TCDD exposure model, 3,3-dimethyl-1-butanol (DMB) therapy protected against hypertension, coinciding with a reduction of AT1R but an increase of AT2R protein abundance, as well as reshaping the gut microbiota [96]. Other developmental hypertension models such as a perinatal high-fat diet [99], maternal administration of minocycline [52], and maternal TMAO plus ADMA exposure [103] also interfere with aberrant RAS and gut microbiota dysbiosis.
As gut microbiota dysbiosis has been linked to hypertension by modulating the systemic and local RAS [127], these observations endorse the idea that the interaction between the RAS and the gut microbiota implicates the pathogenesis of developmental programming of hypertension, although this remains speculative.
4.4.3. Inflammation and Immune Response
Pregnancy is considered a physiologic systemic inflammatory response; compromised pregnancies and associated complications may be attributed to inflammation [128]. The accumulation of T cells, monocyte/macrophages, and T cell-derived cytokines is involved in the pathogenesis of hypertension [129]. An imbalance of T regulatory cells (Treg) and T helper 17 (TH17) cells has been linked to hypertension [129], which can be restored by postbiotic therapy [130]. In CKD, the interplay between Treg/TH17 balance and inflammation has also been related to hypertension [131]. Treg and TH17 cells can both be regulated by aryl hydrocarbon receptor (AhR) [132].
It is noted that several microbial tryptophan metabolites are uremic toxins as well as AhR ligands. AhR signaling can initiate inflammation through increasing monocyte adhesion, upregulating proinflammatory gene expression, reducing NO bioavailability, and inducing the expression of endothelial adhesion molecules [133]. Several gut microbiota-derived uremic toxins, like indoleacetic acid and indoxyl sulfate, have pro-oxidant, proinflammatory, procoagulant, and proapoptotic effects, all of which are involved in the pathogenesis of hypertension [134].
Using a rat model of maternal CKD-induced hypertension, we observed that maternal tryptophan therapy preventing offspring’s hypertension coincides with restoration of the AhR signaling pathway and several tryptophan-metabolizing microbes [134]. Another study showed that TCDD-induced programming hypertension is related to TH17-induced renal inflammation, the activation of AhR signaling, and alterations of gut microbiota compositions [105]. In contrast, TCDD-induced activation of AhR signaling and TH17 responses can be restored by perinatal supplementation with resveratrol, an AhR antagonist. Additionally, resveratrol is reported to have benefits on offspring hypertension in several developmental hypertension models [45,46,95,102].
Although results from animal models support the role of inflammation and immunity on hypertension of developmental origins, more research is required to gain comprehensive insight into their interconnections with the gut microbiota and develop therapeutic potential of inflammation- or immune-targeted therapies in hypertension of developmental origins and associated organ damage.
5. Reprogramming Strategy: Gut Microbiota-Targeted Therapy
The idea from DOHaD research creates opportunities to reverse the programming process, namely reprogramming, by early intervention aiming to prevent hypertension of developmental origins later in life [135]. Current literature on animal studies for hypertension of developmental origins supports that gut microbiota-targeted therapy can work as a reprogramming strategy to prevent hypertension induced by various early-life insult stimuli.
Animal Models Used for Reprogramming
Here, we show Table 2 that summarizes studies documenting microbiota-targeted reprogramming therapies in animal models of developmental hypertension, restricting those starting before or upon disease onset [93,94,96,99,103,105,110,113,134,136,137]. The therapeutic duration is from pregnancy through lactation, which cover the periods of organogenesis. The literature review states that gut microbiota-targeted strategies used to prevent hypertension include probiotics, prebiotics, postbiotics, and dietary nutrients.

Table 2.
Summary of animal models documenting gut microbiota-targeted therapies for hypertension of developmental origins.
Table 2 illustrates that the most commonly used species are rats. Several models of developmental hypertension have been used to examine gut microbiota-targeted interventions, such as maternal high-fructose diet [93,94], perinatal high-fat diet [99,110,136], perinatal TCDD exposure [105], maternal adenine-induced CKD [113,134,137], maternal TMAO and ADMA exposure [103], and maternal high-fructose intake plus TCDD exposure [96].
Despite probiotics and prebiotics showing benefits in hypertension [91,92], there was very limited evidence in regard to their role on hypertension of developmental origins. Probiotic treatment with Lactobacillus casei in pregnancy and lactation prevents the development of hypertension in adult male rat offspring raised on a maternal high-fructose diet [93] or perinatal high-fat diet model [99].
As a prebiotic, inulin has a protective effect in hypertension of developmental origins [93,99]. A previous study using a maternal high-fructose model demonstrated that perinatal inulin treatment protects against offspring’s hypertension, accompanied by increased abundance of Lactobacillus, the most-known probiotic strain [93]. Another study demonstrated that perinatal supplementing with inulin protects against maternal high-fructose-diet-induced hypertension, accompanied by increases of plasma propionate concentrations [99].
Additionally, resveratrol could be used to protect against adult disease of developmental origins due to its probiotic properties [138]. Studies using a maternal TMAO plus ADMA exposure rat model indicate that adult rat progeny born to dams exposed to uremic toxins develop hypertension [103]. Nonetheless, maternal resveratrol therapy rescues from hypertension programmed by TMAO plus ADMA exposure, accompanied by increased butyrate-producing bacteria and fecal butyrate level.
Another study demonstrated that adult rat progeny born to dams exposed to TCDD have hypertension [105], which is related to activation of AhR signaling, induction of TH17-dependent renal inflammation, and shifts of gut microbiota compositions [105]. Conversely, the induction of TH17- and AhR-mediated inflammation can be counterbalanced by perinatal resveratrol supplementation. The beneficial effects of resveratrol are also relevant to reshaping the gut microbiota by augmenting microbes that can inhibit TH17 responses and reducing the F/B ratio. In a maternal CKD model, adult rat progeny developed renal hypertrophy and hypertension [82]. Perinatal resveratrol therapy protects from hypertension, accompanied by restoration of microbial richness and diversity and an increase in beneficial microbes, Lactobacillus and Bifidobacterium [113]. Nevertheless, the low bioavailability of resveratrol limits its clinical translation [139]. In this regard, we synthesized resveratrol butyrate ester (RBE) from resveratrol and butyrate by esterification to improve the efficacy [140]. We recently found that low-dose RBE (25 mg/L) is as effective as resveratrol (50 mg/L) in protecting against CKD-induced hypertension [141], despite the beneficial effect of RBE in models of developmental hypertension awaiting further evaluation.
Although there are many other prebiotic foods, only garlic oil has shown benefits on protection of perinatal high-fat-diet-induced hypertension in adult progeny [110]. The beneficial effects of garlic oil include increased α-diversity; increased plasma acetate, butyrate, and propionate; and increased beneficial bacteria Lactobacillus and Bifidobacterium.
In addition to probiotics and prebiotics, postbiotics are another gut microbiota-targeted modality. Postbiotics include various components like microbial cell fractions, extracellular vesicles, cell lysates, extracellular polysaccharides, functional proteins, cell wall-derived muropeptides, etc. [80]. Nevertheless, very limited information exists regarding the use of postbiotics in hypertension. As a postbiotic, acetate for perinatal supplementing showed benefits on maternal high-fructose-diet-induced hypertension [94].
Additionally, two studies demonstrated that maternal DMB therapy, an inhibitor of TMA formation, protects from hypertension in adult rat progeny exposed to maternal high-fructose diet with or without TCDD exposure [94,96]. Its beneficial effect was accompanied by affection of the metabolic pathway of TMA-TMAO and reshaping the gut microbiota. Another example of postbiotics use for hypertension of developmental origins is conjugated linoleic acid. One study showed the benefit of conjugated linoleic acid, a gut microbial metabolite derived from dietary polyunsaturated fatty acids, on high-fat-diet-induced hypertension [136].
Moreover, dietary nutrients have also been applied as gut microbiota-targeted therapies for hypertension of developmental origins. Former reviews have sufficiently illustrated the impact of diet on the gut microbiome [142,143]. Prior research demonstrating that specific nutrient intake can be beneficial to protecting from hypertension of developmental origins in various animal models [26,144]. These nutrients include folic acid [145], vitamin E [146], polyunsaturated fatty acids [147], and certain amino acids [27,148,149]. However, very few of them have been studied about their impact in gut microbiota related to developmental hypertension. One recent study showed that L-cysteine therapy protects adult offspring against maternal CKD-induced hypertension, associated with enhancement of beneficial genera Oscillibacter and Butyricicoccus, as well as depletion of indole-producing genera Alistipes and Akkermansia [137]. Another study reported that tryptophan supplementation during gestation prevents maternal CKD-induced offspring hypertension. The protective effect of tryptophan supplementation is related to alterations to several tryptophan-metabolizing microbes and the AHR signaling pathway [134].
Notably, increasing evidence supports the notion that altered gut microbial composition and function is evident in hypertension that can be evoked with diets high in salt [83,150,151,152]. Although maternal high salt consumption has been associated with offspring hypertension [29], much remains to be elucidated about the interplay between gut microbiota with high-sodium diets and the role of low-salt diet as a reprogramming strategy for hypertension of developmental origins.
Together, current evidence from animal models supports that modulation of gut microbiota compositions and its derived metabolites through gut microbiota-targeted therapies, in the long term, may enable the capacity to prevent the development of hypertension in a desired favorable direction.
6. Conclusions and Perspectives
Animal models have made significant contributions to research in hypertension of developmental origins, giving rise to substantial evidence of an interconnection between early-life insult stimuli, gut microbiota dysbiosis, and hypertension in adulthood. Our review provides insights into the importance of animal models not only in investigating underlying mechanisms behind hypertension of developmental origins, but also in developing early-life gut microbiota-targeted therapy to help prevent hypertension in later life. Considering that hypertension is a major hallmark of metabolic syndrome, aforementioned animal models may be potential models to evaluate developmental programming of metabolic syndrome-related disorders, not to mention that the link between gut microbiota and metabolic syndrome have been extensively discussed in many research papers [153,154].
Animal models are generally considered an intermediate step between bench and human trials. While animal studies are a regulatory requirement for validating preliminary experimental data, animals will remain indispensable in research for some time [155]. However, alternative approaches to animal models need to be investigated and adopted. The integration of computer models with modeling in vitro tissues and organs should be considered as alternative protocols to reduce the use of animals in scientific research [156].
To move the field forward, some unsolved aspects toward clinical translation need to be considered. Despite abovementioned early-life insults having been identified in animal models of developmental hypertension, there may be more risk factors in nature that can adversely influence the BP of adult progeny awaiting to be discovered.
Another important aspect is that the implication of the gut microbiota transfer early in life to the development of hypertension is still unknown, although FMT has been extensively studied in microbiome-associated pathologies, including hypertension [78,81,82]. However, currently, little information exists about their potential application in hypertension of developmental origins.
Animal studies suggest that early use of certain prebiotics, probiotics, or postbiotics may prevent the developmental programming of hypertension, while the exact mechanisms have not been entirely elucidated. What is absent in the literature is whether other prebiotic-rich foods or prebiotic-like components, either individually or in combination, in pregnancy and lactation can also change the gut microbiota to protect adult progeny against hypertension in various animal models.
In conclusion, gut microbiota dysbiosis is a meaningfully pathogenetic link for hypertension of developmental origins. Each of the aforementioned animal models was applied to examine a specific hypothesis, and neither can be considered superior in respect of all aspects of research on hypertension of developmental origins. After all this greater understanding of animal models used for DOHaD research and remarkable growth in gut microbiota-targeted therapies, we believe that translating this growing body of evidence into clinical practice is a valuable strategy to reduce the global hypertension-related burden.
Author Contributions
Conceptualization, C.-N.H. and Y.-L.T.; data curation, C.-N.H. and Y.-L.T.; funding acquisition, Y.-L.T.; project administration, C.-N.H. and Y.-L.T.; writing-original draft, C.-N.H. and Y.-L.T.; writing-review and editing, C.-N.H. and Y.-L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chang Gung Memorial Hospital, Kaohsiung, Taiwan, grants CORPG8M0201, CORPG8M0151, and CORPG8M0081, and the Ministry of Science and Technology, Taiwan, grants MOST 110-2314-B-182-020-MY3 (Y.-L.T.) and MOST 110-2314-B-182A-029 (C.-N.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Hypertension. 2022. Available online: https://www.who.int/health-topics/hypertension#tab=tab_1 (accessed on 25 February 2022).
- Pinto, Y.M.; Paul, M.; Ganten, D. Lessons from rat models of hypertension: From Goldblatt to genetic engineering. Cardiovasc. Res. 1998, 39, 77–88. [Google Scholar] [CrossRef]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, e87–e120. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Joe, B. Towards precision medicine for hypertension: A review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol. Rev. 2017, 97, 1469–1528. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; E Hoy, W.; E Ozanne, S.; E Vikse, B. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef]
- Bagby, S.P. Maternal nutrition, low nephron number, and hypertension in later life: Pathways of nutritional programming. J. Nutr. 2007, 137, 1066–1072. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Grigore, D.; Alexander, B.T. Developmental programming of hypertension: Insight from animal models of nutritional manipulation. Hypertension 2008, 52, 44–50. [Google Scholar] [CrossRef]
- Paauw, N.D.; Van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2016, 219, 241–259. [Google Scholar] [CrossRef]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The Double-Edged Sword Effects of Maternal Nutrition in the Developmental Programming of Hypertension. Nutrients 2018, 10, 1917. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Adverse Impact of Environmental Chemicals on Developmental Origins of Kidney Disease and Hypertension. Front. Endocrinol. 2021, 12, 745716. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Animal Models for DOHaD Research: Focus on Hypertension of Developmental Origins. Biomedicines 2021, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Palmu, J.; Lahti, L.; Niiranen, T. Targeting Gut Microbiota to Treat Hypertension: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1248. [Google Scholar] [CrossRef]
- Avery, E.G.; Bartolomaeus, H.; Maifeld, A.; Marko, L.; Wiig, H.; Wilck, N.; Rosshart, S.P.; Forslund, S.K.; Müller, D.N. The Gut Microbiome in Hypertension: Recent Advances and Future Perspectives. Circ. Res. 2021, 128, 934–950. [Google Scholar] [CrossRef]
- Seidel, E.; Scholl, U.I. Genetic mechanisms of human hypertension and their implications for blood pressure physiology. Physiol. Genom. 2017, 49, 630–652. [Google Scholar] [CrossRef]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Cardiovascular Diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients 2021, 13, 2290. [Google Scholar] [CrossRef]
- McMullen, S.; Mostyn, A. Animal models for the study of the developmental origins of health and disease. Proc. Nutr. Soc. 2009, 68, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.D.; Zybert, P.A.; van der Pal-de Bruin, K.; Lumey, L.H. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: Evidence from the Dutch Famine. Eur. J. Epidemiol. 2006, 21, 759–765. [Google Scholar] [CrossRef]
- Langley-Evans, S.C.; Langley-Evans, A.J.; Marchand, M.C. Nutritional programming of blood pressure and renal morphology. Arch. Physiol. Biochem. 2003, 111, 8–16. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, V.; Lim, K.; Pearson, J.T.; Black, M.J. Developmental programming of cardiovascular disease following intra uterine growth restriction: Findings utilising a rat model of maternal protein restriction. Nutrients 2014, 7, 119–152. [Google Scholar] [CrossRef]
- Koleganova, N.; Piecha, G.; Ritz, E.; Becker, L.E.; Müller, A.; Weckbach, M.; Nyengaard, J.R.; Schirmacher, P.; Gross-Weissmann, M.L. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am. J. Physiol. Ren. Physiol. 2011, 301, F344–F354. [Google Scholar] [CrossRef]
- Bergel, E.; Belizán, J.M. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. BJOG 2002, 109, 540–545. [Google Scholar] [CrossRef]
- Tomat, A.; Elesgaray, R.; Zago, V.; Fasoli, H.; Fellet, A.; Balaszczuk, A.M.; Schreier, L.; Costa, M.A.; Arranz, C. Exposure to zinc deficiency in fetal and postnatal life determines nitric oxide system activity and arterial blood pressure levels in adult rats. Br. J. Nutr. 2010, 104, 382–389. [Google Scholar] [CrossRef]
- Gambling, L.; Dunford, S.; Wallace, D.I.; Zuur, G.; Solanky, N.; Srai, K.S.; McArdle, H.J. Iron deficiency during pregnancy affects post-natal blood pressure in the rat. J. Physiol. 2003, 552, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal melatonin therapy attenuates methyl-donor diet-induced programmed hypertension in male adult rat offspring. Nutrients 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Tare, M.; Emmett, S.J.; Coleman, H.A.; Skordilis, C.; Eyles, D.W.; Morley, R.; Parkington, H.C. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J. Physiol. 2011, 589, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Tsai, C.C.; Huang, L.T.; Hsu, C.N. High fat diets sex-specifically affect the renal transcriptome and program obesity, kidney injury, and hypertension in the offspring. Nutrients 2017, 9, 357. [Google Scholar] [CrossRef]
- Seong, H.Y.; Cho, H.M.; Kim, M.; Kim, I. Maternal High-Fructose Intake Induces Multigenerational Activation of the Renin Angiotensin-Aldosterone System. Hypertension 2019, 74, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chan, J.Y.; Hsu, C.N. Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life. Nutrients 2016, 8, 757. [Google Scholar] [CrossRef]
- Thone-Reineke, C.; Kalk, P.; Dorn, M.; Klaus, S.; Simon, K.; Pfab, T.; Godes, M.; Persson, P.; Unger, T.; Hocher, B. High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1025–R1030. [Google Scholar] [CrossRef][Green Version]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants 2020, 9, 856. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Camm, E.J.; Niu, Y.; Richter, H.G.; Blanco, C.E.; Gottschalk, R.; Blake, E.Z.; Horder, K.A.; Thakor, A.S.; Hansell, J.A.; et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE 2012, 7, e31017. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018, 19, 2459. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Tain, Y.L. Maternal exposure to bisphenol A combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Huang, X.; Li, Y.; Dasgupta, C.; Wang, L.; Zhang, L. Antenatal Antioxidant Prevents Nicotine-Mediated Hypertensive Response in Rat Adult Offspring. Biol. Reprod. 2015, 93, 66. [Google Scholar] [CrossRef]
- Serapiao-Moraes, D.F.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Faria, T.S. Maternal caffeine administration leads to adverse effects on adult mice offspring. Eur. J. Nutr. 2013, 52, 1891–1900. [Google Scholar] [CrossRef]
- Slabiak-Blaz, N.; Adamczak, M.; Gut, N.; Grajoszek, A.; Nyengaard, J.R.; Ritz, E.; Wiecek, A. Administration of cyclosporine a in pregnant rats—The effect on blood pressure and on the glomerular number in their offspring. Kidney Blood Press. Res. 2015, 40, 413–423. [Google Scholar] [CrossRef]
- Chahoud, I.; Stahlmann, R.; Merker, H.J.; Neubert, D. Hypertension and nephrotoxic lesions in rats 1 year after prenatal exposure to gentamicin. Arch. Toxicol. 1988, 62, 274–284. [Google Scholar] [CrossRef]
- Gois, P.H.; Canale, D.; Luchi, W.M.; Volpini, R.A.; Veras, M.M.; Costa Nde, S.; Shimizu, M.H.; Seguro, A.C. Tenofovir during pregnancy in rats: A novel pathway for programmed hypertension in the offspring. J. Antimicrob. Chemother. 2015, 70, 1094–1105. [Google Scholar] [CrossRef][Green Version]
- Hsu, C.N.; Chan, J.Y.H.; Wu, K.L.H.; Yu, H.R.; Lee, W.C.; Hou, C.Y.; Tain, Y.L. Altered Gut Microbiota and Its Metabolites in Hypertension of Developmental Origins: Exploring Differences between Fructose and Antibiotics Exposure. Int. J. Mol. Sci. 2021, 22, 2674. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Targeting the Renin-Angiotensin-Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021, 22, 2298. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Interplay between Oxidative Stress and Nutrient Sensing Signaling in the Developmental Origins of Cardiovascular Disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Intapad, S.; Alexander, B.T. Sex differences in the developmental programming of hypertension. Acta Physiol. 2014, 210, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Tomat, A.L.; Salazar, F.J. Mechanisms involved in developmental programming of hypertension and renal diseases. Gender differences. Horm. Mol. Biol. Clin. Investig. 2014, 18, 63–77. [Google Scholar] [CrossRef]
- Scherrer, U.; Rimoldi, S.F.; Sartori, C.; Messerli, F.H.; Rexhaj, E. Fetal programming and epigenetic mechanisms in arterial hypertension. Curr. Opin. Cardiol. 2015, 30, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease. Int. J. Mol. Sci. 2019, 20, 681. [Google Scholar] [CrossRef]
- Dickinson, H.; Moss, T.J.; Gatford, K.L.; Moritz, K.M.; Akison, L.; Fullston, T.; Hryciw, D.H.; Maloney, C.A.; Morris, M.J.; Wooldridge, A.L.; et al. A review of fundamental principles for animal models of DOHaD research: An Australian perspective. J. Dev. Orig. Health Dis. 2016, 7, 449–472. [Google Scholar] [CrossRef]
- Mossa, F.; Carter, F.; Walsh, S.W.; Kenny, D.A.; Smith, G.W.; Ireland, J.L.; Hildebrandt, T.B.; Lonergan, P.; Ireland, J.J.; Evans, A.C. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 2013, 88, 92. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.S.; Gardner, D.S.; Rhind, S.M.; Rae, M.T.; Kyle, C.E.; Brooks, A.N.; Walker, R.M.; Ramsay, M.M.; Keisler, D.H.; Stephenson, T.; et al. Programming of adult cardiovascular function after early maternal undernutrition in sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R12–R20. [Google Scholar] [CrossRef] [PubMed]
- Brain, K.L.; Allison, B.J.; Niu, Y.; Cross, C.M.; Itani, N.; Kane, A.D.; Herrera, E.A.; Skeffington, K.L.; Botting, K.J.; Giussani, D.A. Intervention against hypertension in the next generation programmed by developmental hypoxia. PLoS Biol. 2019, 17, e2006552. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.H.; Li, C.; Li, J.; Huber, H.F.; Nathanielsz, P.W.; Clarke, G.D. Cardiac remodeling in a baboon model of intrauterinegrowth restriction mimics accelerated ageing. J. Physiol. 2017, 595, 1093–1110. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in swine: Implications for animal production and biomedical research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef]
- Chavatte-Palmer, P.; Tarrade, A.; Rousseau-Ralliard, D. Diet before and during Pregnancy and Offspring Health: The Importance of Animal Models and What Can Be Learned from Them. Int. J. Environ. Res. Public Health 2016, 13, 586. [Google Scholar] [CrossRef]
- Morrison, J.L.; Botting, K.J.; Darby, J.R.T.; David, A.L.; Dyson, R.M.; Gatford, K.L.; Gray, C.; Herrera, E.A.; Hirst, J.J.; Kim, B.; et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol. 2018, 596, 5535–5569. [Google Scholar] [CrossRef]
- Hartman, H.A.; Lai, H.L.; Patterson, L.T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007, 310, 379–387. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Barry, J.S.; Anthony, R.V. The pregnant sheep as a model for human pregnancy. Theriogenology 2008, 69, 55–67. [Google Scholar] [CrossRef]
- Chahoud, I.; Paumgartten, F.J.R. Influence of litter size on the postnatal growth of rat pups: Is there a rationale for litter-size standardization in toxicity studies? Environ. Res. 2009, 109, 1021–1027. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Hagaman, J.R.; Smithies, O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 1995, 25, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; De La Cochetiere, M.-F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Zhang, C.; Franklin, C.L.; Ericsson, A.C. Consideration of Gut Microbiome in Murine Models of Diseases. Microorganisms 2021, 9, 1062. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Zółkiewicz, J.; Marzec, A.; Ruszczyn’ski, M.; Feleszko, W. Postbiotics—A step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Personett, A.R.; Turner, G.; Dorfmeyer, R.A.; Franklin, C.L. Variable Colonization after reciprocal fecal microbiota transfer between mice with low and high richness microbiota. Front. Microbiol. 2017, 8, 196. [Google Scholar] [CrossRef]
- Robertson, S.J.; Lemire, P.; Maughan, H.; Goethel, A.; Turpin, W.; Bedrani, L.; Guttman, D.S.; Croitoru, K.; Girardin, S.E.; Philpott, D.J. Comparison of co-housing and littermate methods for microbiota standardization in mouse models. Cell Rep. 2019, 27, 1910–1919.e2. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; de la Visitación, N.; Toral, M.; Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Yang, T.; Izquierdo-García, J.L.; Guerra-Hernández, E.; Ruiz-Cabello, J.; et al. Probiotic Bifidobacterium breve prevents DOCA-salt hypertension. FASEB J. 2020, 34, 13626–13640. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Joe, B.; McCarthy, C.G.; Edwards, J.M.; Cheng, X.; Chakraborty, S.; Yang, T.; Golonka, R.M.; Mell, B.; Yeo, J.Y.; Bearss, N.R.; et al. Microbiota Introduced to Germ-Free Rats Restores Vascular Contractility and Blood Pressure. Hypertension 2020, 76, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Chi, C.; Li, C.; Wu, D.; Buys, N.; Wang, W.; Fan, H.; Sun, J. Effects of Probiotics on Patients with Hypertension: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 34. [Google Scholar] [CrossRef]
- Ried, K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp. Ther. Med. 2020, 19, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Tain, Y.L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients 2018, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on Gut Microbial Metabolite Trimethylamine-N-Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption Through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chan, J.Y.H.; Yu, H.R.; Lee, W.C.; Wu, K.L.H.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on Gut Microbiota-Derived Metabolite Trimethylamine to Protect Adult Male Rat Offspring against Hypertension Programmed by Combined Maternal High-Fructose Intake and Dioxin Exposure. Int. J. Mol. Sci. 2020, 21, 5488. [Google Scholar] [CrossRef]
- Guimarães, K.S.L.; Braga, V.A.; Noronha, S.I.S.R.; Costa, W.K.A.D.; Makki, K.; Cruz, J.C.; Brandão, L.R.; Chianca Junior, D.A.; Meugnier, E.; Leulier, F.; et al. Lactiplantibacillus plantarum WJL administration during pregnancy and lactation improves lipid profile, insulin sensitivity and gut microbiota diversity in dyslipidemic dams and protects male offspring against cardiovascular dysfunction in later life. Food Funct. 2020, 11, 8939–8950. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Lee, C.T.; Chan, J.Y.H.; Tain, Y.L. The Interplay between Maternal and Post-Weaning High-Fat Diet and Gut Microbiota in the Developmental Programming of Hypertension. Nutrients 2019, 11, 1982. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef]
- Li, H.B.; Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Maternal Treatment with Captopril Persistently Alters Gut-Brain Communication and Attenuates Hypertension of Male Offspring. Hypertension 2020, 75, 1315–1324. [Google Scholar] [CrossRef]
- De Oliveira, Y.; Cavalcante, R.G.S.; Cavalcanti Neto, M.P.; Magnani, M.; Braga, V.A.; de Souza, E.L.; de Brito Alves, J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020, 11, 5581–5594. [Google Scholar] [CrossRef]
- Chen, H.E.; Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-N-oxide. J. Nutr. Biochem. 2021, 93, 108630. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Lee, C.T.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal 3,3-Dimethyl-1-Butanol Therapy Protects Adult Male Rat Offspring against Hypertension Programmed by Perinatal TCDD Exposure. Nutrients 2021, 13, 3041. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hung, C.H.; Hou, C.Y.; Chang, C.I.; Tain, Y.L. Perinatal Resveratrol Therapy to Dioxin-Exposed Dams Prevents the Programming of Hypertension in Adult Rat Offspring. Antioxidants 2021, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.B.; Sarsour, N.; Salehi, M.; Schroering, A.; Mell, B.; Joe, B.; Hill, J.W. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 2018, 9, 400–421. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- DiRienzo, D.B. Effect of probiotics on biomarkers of cardiovascular disease: Implications for heart-healthy diets. Nutr. Rev. 2014, 72, 18–29. [Google Scholar] [CrossRef]
- Pluznick, J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol. Ren. Physiol. 2013, 305, F439–F444. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Garlic Oil Supplementation Prevents High-Fat Diet-Induced Hypertension in Adult Rat Offspring: Implications of H2S-Generating Pathway in the Gut and Kidneys. Mol. Nutr. Food Res. 2021, 65, e2001116. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Perinatal Resveratrol Therapy Prevents Hypertension Programmed by Maternal Chronic Kidney Disease in Adult Male Offspring: Implications of the Gut Microbiome and Their Metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Oxidative Stress-Induced Hypertension of Developmental Origins: Preventive Aspects of Antioxidant Therapy. Antioxidants 2022, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, L.; Wu, J.; Xu, T.; Yang, C.; Chen, X.; Lv, J.; Xu, Z. Prenatal high-salt diet impaired vasodilatation with reprogrammed renin-angiotensin system in offspring rats. J. Hypertens. 2018, 36, 2369–2379. [Google Scholar] [CrossRef]
- Svitok, P.; Okuliarova, M.; Varga, I.; Zeman, M. Renal impairment induced by prenatal exposure to angiotensin II in male rat offspring. Exp. Biol. Med. 2019, 244, 923–931. [Google Scholar] [CrossRef]
- Do Nascimento, L.C.P.; Neto, J.P.R.C.; de Andrade Braga, V.; Lagranha, C.J.; de Brito Alves, J.L. Maternal exposure to high-fat and high-cholesterol diet induces arterial hypertension and oxidative stress along the gut-kidney axis in rat offspring. Life Sci. 2020, 261, 118367. [Google Scholar] [CrossRef]
- Chen, L.; Zadi, Z.H.; Zhang, J.; Scharf, S.M.; Pae, E.K. Intermittent hypoxia in utero damages postnatal growth and cardiovascular function in rats. J. Appl. Physiol. 2018, 124, 821–830. [Google Scholar] [CrossRef]
- Piecha, G.; Koleganova, N.; Ritz, E.; Müller, A.; Fedorova, O.V.; Bagrov, A.Y.; Lutz, D.; Schirmacher, P.; Gross-Weissmann, M.L. High salt intake causes adverse fetal programming--vascular effects beyond blood pressure. Nephrol. Dial. Transplant. 2012, 27, 3464–3476. [Google Scholar] [CrossRef]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Koper, M.; Ufnal, M. Gut microbiota and renin-angiotensin system: A complex interplay at local and systemic levels. Am. J. Physiol. Liver Physiol. 2021, 321, G355–G366. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Andrade, J.M.; de Farias Lelis, D.; Mafra, V.; Cota, J. The angiotensin converting enzyme 2 (ACE2), gut microbiota, and cardiovascular health. Protein Pept. Lett. 2017, 24, 827–832. [Google Scholar] [CrossRef]
- Richards, E.M.; Pepine, C.J.; Raizada, M.K.; Kim, S. The gut, its microbiome, and hypertension. Curr. Hypertens. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F.; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef]
- McMaster, W.G.; Kirabo, A.; Madhur, M.S.; Harrison, D.G. Inflammation, immunity, and hypertensive end-organ damage. Circ. Res. 2015, 116, 1022–1033. [Google Scholar] [CrossRef]
- Ren, J.; Crowley, S.D. Role of T-cell activation in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1345–H1353. [Google Scholar] [CrossRef]
- Zhang, J.; Hua, G.; Zhang, X.; Tong, R.; Du, X.; Li, Z. Regulatory T cells/T-helper cell 17 functional imbalance in uraemic patients on maintenance haemodialysis: A pivotal link between microinflammation and adverse cardiovascular events. Nephrology 2010, 15, 33–41. [Google Scholar] [CrossRef]
- Stevens, E.A.; Mezrich, J.D.; Bradfield, C.A. The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology 2009, 127, 299–311. [Google Scholar] [CrossRef]
- Sallée, M.; Dou, L.; Cerini, C.; Poitevin, S.; Brunet, P.; Burtey, S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins 2014, 6, 934–949. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Tiao, M.M.; Tain, Y.L. Maternal Tryptophan Supplementation Protects Adult Rat Offspring against Hypertension Programmed by Maternal Chronic Kidney Disease: Implication of Tryptophan-Metabolizing Microbiome and Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 4552. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2016, 17, 23. [Google Scholar] [CrossRef]
- Gray, C.; Vickers, M.H.; Segovia, S.A.; Zhang, X.D.; Reynolds, C.M. A maternal high fat diet programmes endothelial function and cardiovascular status in adult male offspring independent of body weight, which is reversed by maternal conjugated linoleic acid (CLA) supplementation. PLoS ONE 2015, 10, e0115994. [Google Scholar]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 28, 483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Tain, Y.L. Preventive Aspects of Early Resveratrol Supplementation in Cardiovascular and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021, 22, 4210. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chang, S.K.C.; Liao, J.X.; Chen, Y.W.; Huang, H.T.; Li, Y.L.; Hou, C.Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants 2021, 10, 420. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang, C.I.; Tain, Y.L. Resveratrol Butyrate Ester Protects Adenine-Treated Rats against Hypertension and Kidney Disease by Regulating the Gut-Kidney Axis. Antioxidants 2021, 11, 83. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Nutraceuticals as modulators of gut microbiota: Role in therapy. Br. J. Pharmacol. 2020, 177, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Amino Acids and Developmental Origins of Hypertension. Nutrients 2020, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Torrens, C.; Brawley, L.; Anthony, F.W.; Dance, C.S.; Dunn, R.; Jackson, A.A.; Poston, L.; Hanson, M.A. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension 2006, 47, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; D’Armiento, F.P.; Witztum, J.L.; de Nigris, F.; Casanada, F.; Condorelli, M.; Silvestre, M.; Napoli, C. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ. Res. 2001, 89, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Gregório, B.M.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Maternal fish oil supplementation benefits programmed offspring from rat dams fed low-protein diet. Am. J. Obstet. Gynecol. 2008, 199, e1–e7. [Google Scholar] [CrossRef]
- Fujii, T.; Yura, S.; Tatsumi, K.; Kondoh, E.; Mogami, H.; Fujita, K.; Kakui, K.; Aoe, S.; Itoh, H.; Sagawa, N.; et al. Branched-chain amino acid supplemented diet during maternal food restriction prevents developmental hypertension in adult rat offspring. J. Dev. Orig. Health Dis. 2011, 2, 176–183. [Google Scholar] [CrossRef]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar] [PubMed]
- Smiljanec, K.; Lennon, S.L. Sodium, hypertension, and the gut: Does the gut microbiota go salty? Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1173–H1182. [Google Scholar] [CrossRef]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Figueiredo, P.; Martins, J.P.; Santos, H.A. Requirements for Animal Experiments: Problems and Challenges. Small 2021, 17, e2004182. [Google Scholar] [CrossRef] [PubMed]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).