Gelsolin as a Potential Biomarker for Endoscopic Activity and Mucosal Healing in Ulcerative Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Sample Collection
2.2. Measurement of Serum GSN Level
2.3. Mass Spectrometry
2.4. Data Analysis
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
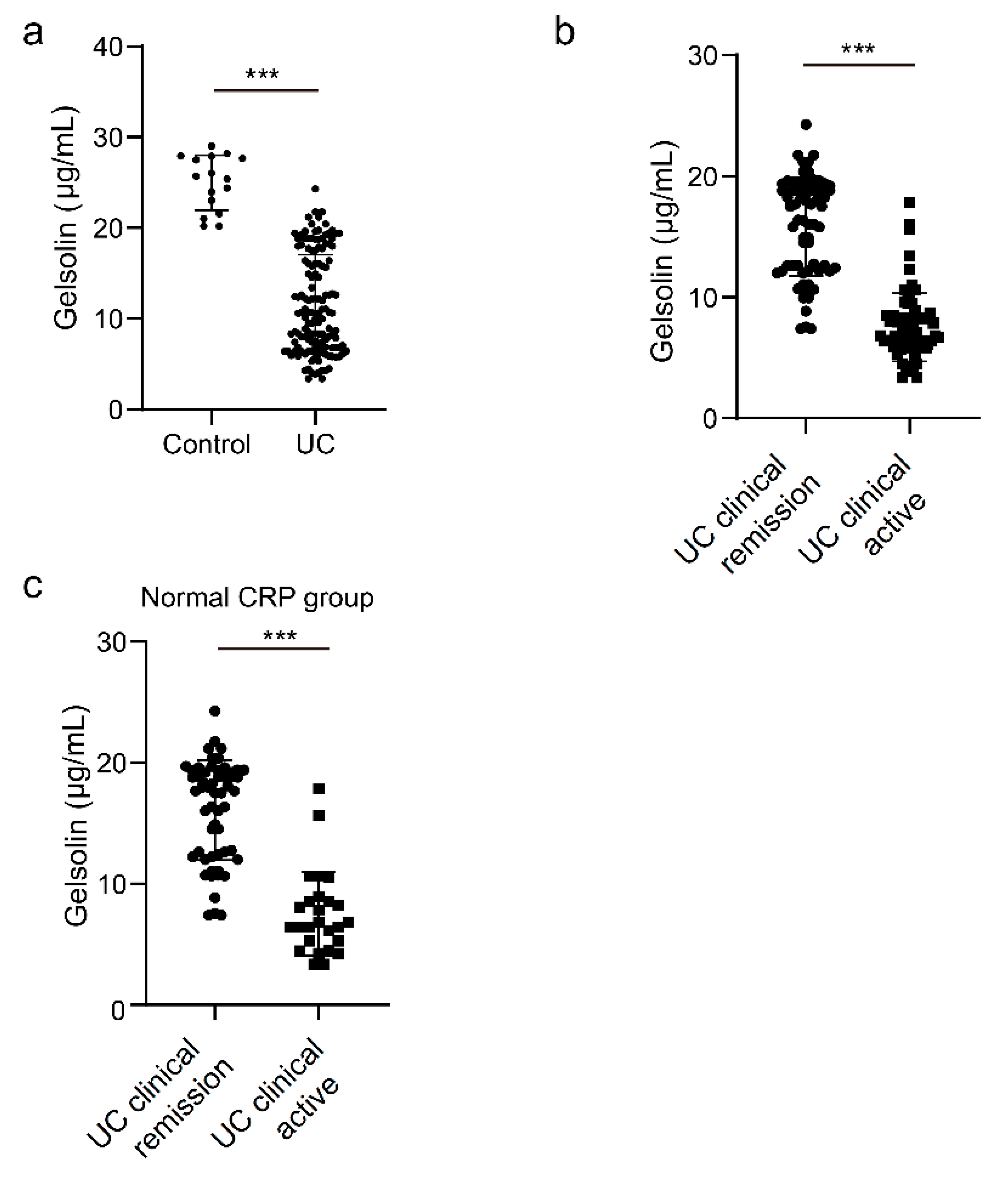
3.1. Downregulation of Serum GSN in Patients with Clinically Active UC
3.2. Inverse Relationship between the Serum GSN Level and Endoscopic Activity in Patients with UC
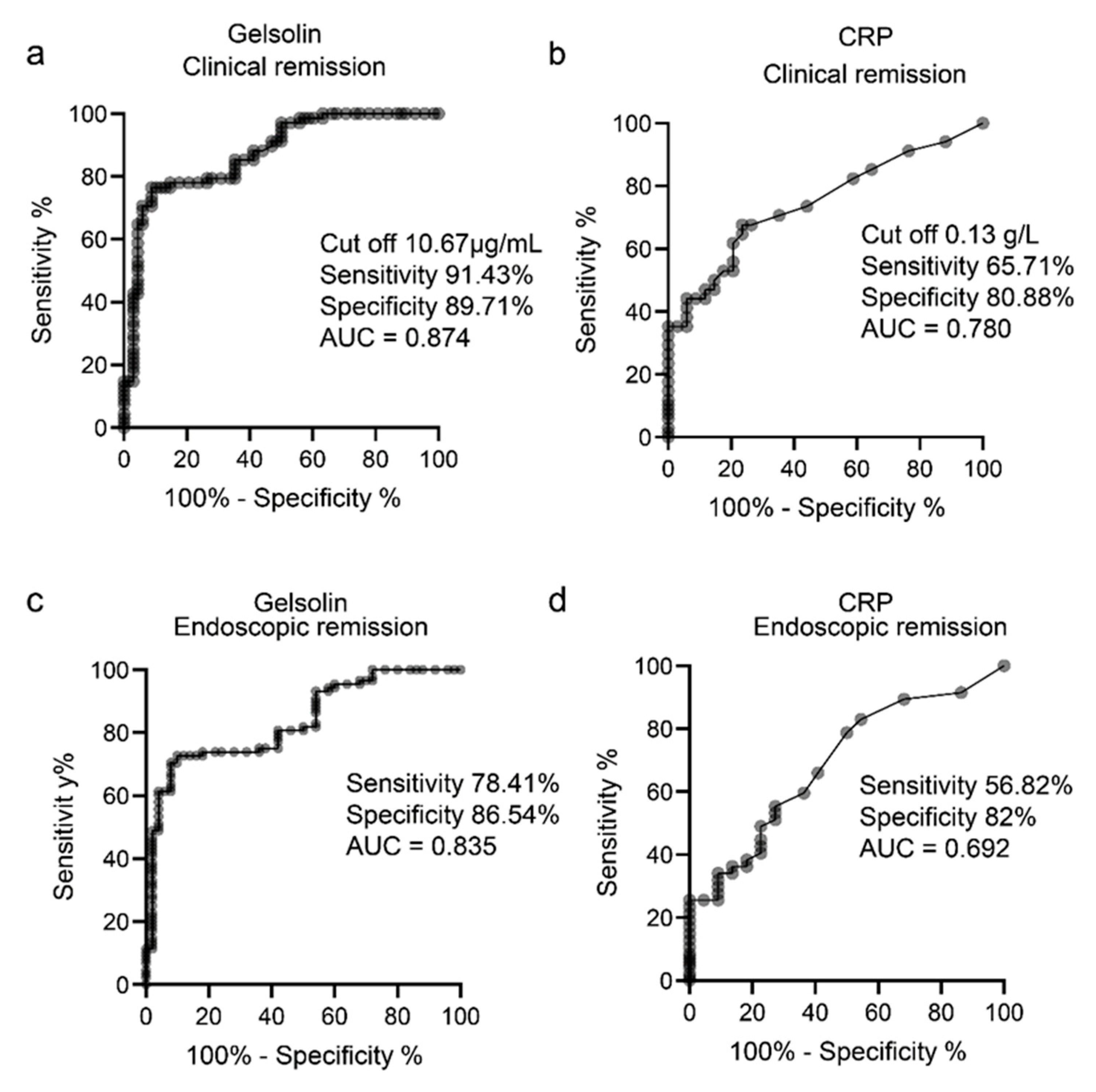
3.3. GSN as a Serological Biomarker of Clinically and Endoscopically Active UC
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Mucosal healing in inflammatory bowel disease: Impossible ideal or therapeutic target? Gut 2007, 56, 453–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, K.; Kobayashi, K.; Mukae, M.; Sada, M.; Koizumi, W. Clinical Study of the Relation between Mucosal Healing and Long-Term Outcomes in Ulcerative Colitis. Gastroenterol. Res. Pract. 2013, 2013, 192794. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, P.B.; de Castro, F.D.; Rosa, B.; Moreira, M.J.; Cotter, J. Mucosal Healing in Ulcerative Colitis—When Zero is Better. J. Crohn’s Colitis 2015, 10, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Colombel, J.-F.; D’Haens, G.; Lee, W.-J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2019, 14, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Fagan, E.A.; Dyck, R.F.; Maton, P.N.; Hodgson, H.J.F.; Chadwick, V.S.; Petrie, A.; Pepys, M.B. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur. J. Clin. Investig. 1982, 12, 351–359. [Google Scholar] [CrossRef]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A Meta-Analysis of the Utility of C-Reactive Protein, Erythrocyte Sedimentation Rate, Fecal Calprotectin, and Fecal Lactoferrin to Exclude Inflammatory Bowel Disease in Adults With IBS. Am. J. Gastroenterol. 2015, 110, 444–454. [Google Scholar] [CrossRef]
- Shinzaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J. Crohn’s Colitis 2016, 11, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Shinzaki, S.; Matsuoka, K.; Tanaka, H.; Takeshima, F.; Kato, S.; Torisu, T.; Ohta, Y.; Watanabe, K.; Nakamura, S.; Yoshimura, N.; et al. Leucine-rich alpha-2 glycoprotein is a potential biomarker to monitor disease activity in inflammatory bowel disease receiving adalimumab: PLANET study. J. Gastroenterol. 2021, 56, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Yasutomi, E.; Inokuchi, T.; Hiraoka, S.; Takei, K.; Igawa, S.; Yamamoto, S.; Ohmori, M.; Oka, S.; Yamasaki, Y.; Kinugasa, H.; et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Epstein, F.H.; Lee, W.M.; Galbraith, R.M. The Extracellular Actin-Scavenger System and Actin Toxicity. N. Engl. J. Med. 1992, 326, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Q.; Yamamoto, M.; Mejillano, M.; Yin, H.L. Gelsolin, a Multifunctional Actin Regulatory Protein. J. Biol. Chem. 1999, 274, 33179–33182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldt, J.; Schicht, M.; Garreis, F.; Welss, J.; Schneider, U.W.; Paulsen, F. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 2018, 20, e7. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Levental, I.; Durnaś, B.; Janmey, P.A.; Bucki, R. Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target. Int. J. Mol. Sci. 2018, 19, 2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborn, T.M.; Verdrengh, M.; Stossel, T.P.; Tarkowski, A.; Bokarewa, M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R117. [Google Scholar] [CrossRef] [Green Version]
- Esawy, M.; Makram, W.K.; Albalat, W.; Shabana, M.A. Plasma gelsolin levels in patients with psoriatic arthritis: A possible novel marker. Clin. Rheumatol. 2020, 39, 1881–1888. [Google Scholar] [CrossRef]
- Silacci, P.; Mazzolai, L.; Gauci, C.; Stergiopulos, N.; Yin, H.L.; Hayoz, D. Gelsolin Superfamily Proteins: Key Regulators of Cellular Functions. Cell Mol. Life Sci. 2004, 61, 2614–2623. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, D.J.; Stossel, T.P.; Orkin, S.H.; Mole, J.E.; Coltens, H.R.; Yin, H.L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature 1986, 323, 455–458. [Google Scholar] [CrossRef]
- Lind, S.E.; Janmey, P.A. Human plasma gelsolin binds to fibronectin. J. Biol. Chem. 1984, 259, 13262–13266. [Google Scholar] [CrossRef]
- Osborn, T.M.; Dahlgren, C.; Hartwig, J.H.; Stossel, T.P. Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin. Am. J. Physiol. Physiol. 2007, 292, C1323–C1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucki, R.; Byfield, F.J.; Kulakowska, A.; McCormick, M.E.; Drozdowski, W.; Namiot, Z.; Hartung, T.; Janmey, P.A. Extracellular Gelsolin Binds Lipoteichoic Acid and Modulates Cellular Response to Proinflammatory Bacterial Wall Components. J. Immunol. 2008, 181, 4936–4944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucki, R.; Kułakowska, A.; Byfield, F.J.; Żendzian-Piotrowska, M.; Baranowski, M.; Marzec, M.; Winer, J.P.; Ciccarelli, N.J.; Górski, J.; Drozdowski, W.; et al. Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am. J. Physiol. Physiol. 2010, 299, C1516–C1523. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, A.; Lennon, G.; O’Sullivan, O.; Docherty, N.; Balfe, A.; Maguire, A.; Mulcahy, H.E.; Doherty, G.; O’Donoghue, D.; Hyland, J.; et al. Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 2015, 64, 1553–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef] [Green Version]
- Pepys, M.B.; Hirschfield, G. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Shine, B.; Berghouse, L.; Jones, J.; Landon, J. C-Reactive protein as an aid in the differentiation of functional and inflammatory bowel disorders. Clin. Chim. Acta 1985, 148, 105–109. [Google Scholar] [CrossRef]
- A Solem, C.; Loftus, E.V.; Tremaine, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. Correlation of C-Reactive Protein with Clinical, Endoscopic, Histologic, and Radiographic Activity in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2005, 11, 707–712. [Google Scholar] [CrossRef]
- Acosta, M.B.-D.; Vallejo, N.; De La Iglesia, D.; Uribarri, L.; Bastón, I.; Ferreiro-Iglesias, R.; Lorenzo, A.; Dominguez-Munoz, J.E. Evaluation of the Risk of Relapse in Ulcerative Colitis According to the Degree of Mucosal Healing (Mayo 0 vs 1): A Longitudinal Cohort Study. J. Crohn’s Colitis 2015, 10, 13–19. [Google Scholar] [CrossRef]
- Liao, H.; Wu, J.; Kuhn, E.; Chin, W.; Chang, B.; Jones, M.D.; O’Neil, S.; Clauser, K.R.; Karl, J.; Hasler, F.; et al. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Care Res. 2004, 50, 3792–3803. [Google Scholar] [CrossRef] [PubMed]
- Genre, F.; López-Mejías, R.; Miranda-Filloy, J.A.; Ubilla, B.; Carnero-López, B.; Gómez-Acebo, I.; Blanco, R.; Ochoa, R.; Rueda-Gotor, J.; González-Juanatey, C.; et al. Gelsolin levels are decreased in ankylosing spondylitis patients undergoing an-ti-TNF-alpha therapy. Clin. Exp. Rheumatol. 2014, 32, 218–224. [Google Scholar] [PubMed]
- Hu, Y.; Li, H.; Li, W.H.; Meng, H.X.; Fan, Y.Z.; Li, W.J.; Ji, Y.T.; Zhao, H.; Zhang, L.; Jin, X.M.; et al. The value of decreased plasma gelsolin levels in patients with systemic lupus erythematosus and rheumatoid arthritis in diagnosis and disease activity evaluation. Lupus 2013, 22, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Kandur, Y.; Celik, A.; Gözübenli, F.; Çetinkaya, A.; Olgar, Ş. Plasma gelsolin as a potential biomarker for Henoch–Schoenlein purpura. Scand. J. Rheumatol. 2016, 46, 166–168. [Google Scholar] [CrossRef]
- Roy, S.; Esmaeilniakooshkghazi, A.; Patnaik, S.; Wang, Y.; George, S.P.; Ahrorov, A.; Hou, J.K.; Herron, A.J.; Sesaki, H.; Khurana, S. Villin-1 and Gelsolin Regulate Changes in Actin Dynamics That Affect Cell Survival Signaling Pathways and Intestinal Inflammation. Gastroenterology 2018, 154, 1405–1420.e2. [Google Scholar] [CrossRef]

| Patient Characteristic | N = 138 |
|---|---|
| Sex, female/male, N | 84/54 |
| Age, years, median (range) | 47 (20–82) |
| Duration of disease, months (range) | 143 (7–372) |
| Disease location, N Extensive/left-sided/proctitis | 94/36/8 |
| Treatment, N Oral 5-aminosalicylic acid, N (%) Corticosteroids, N (%) Biologic agents, N (%) Immunomodulators, N (%) Calcineurin inhibitors, N (%) Topical agents, N (%) | 103 (74.6) 18 (13.0) 45 (32.6) 27 (19.6) 3 (2.2) 45 (32.6) |
| C-reactive protein, mg/dL, median (range) | 0.08 (0–8.4) |
| Albumin, g/dL, median (range) | 4.1 (1.8–4.9) |
| Mayo score median (range) | 3 (0–12) |
| Accession | Description | p |
|---|---|---|
| Q9HC84 | Mucin-5B | 0.03349 |
| Q9H3R2 | Mucin-13 | 0.004309 |
| P06396 | Gelsolin | 0.03639 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, K.; Nakamura, M.; Yamamura, T.; Sawada, T.; Ishikawa, E.; Oishi, A.; Ikegami, S.; Kakushima, N.; Furukawa, K.; Iida, T.; et al. Gelsolin as a Potential Biomarker for Endoscopic Activity and Mucosal Healing in Ulcerative Colitis. Biomedicines 2022, 10, 872. https://doi.org/10.3390/biomedicines10040872
Maeda K, Nakamura M, Yamamura T, Sawada T, Ishikawa E, Oishi A, Ikegami S, Kakushima N, Furukawa K, Iida T, et al. Gelsolin as a Potential Biomarker for Endoscopic Activity and Mucosal Healing in Ulcerative Colitis. Biomedicines. 2022; 10(4):872. https://doi.org/10.3390/biomedicines10040872
Chicago/Turabian StyleMaeda, Keiko, Masanao Nakamura, Takeshi Yamamura, Tsunaki Sawada, Eri Ishikawa, Akina Oishi, Shuji Ikegami, Naomi Kakushima, Kazuhiro Furukawa, Tadashi Iida, and et al. 2022. "Gelsolin as a Potential Biomarker for Endoscopic Activity and Mucosal Healing in Ulcerative Colitis" Biomedicines 10, no. 4: 872. https://doi.org/10.3390/biomedicines10040872
APA StyleMaeda, K., Nakamura, M., Yamamura, T., Sawada, T., Ishikawa, E., Oishi, A., Ikegami, S., Kakushima, N., Furukawa, K., Iida, T., Mizutani, Y., Ishikawa, T., Ohno, E., Honda, T., Ishigami, M., & Kawashima, H. (2022). Gelsolin as a Potential Biomarker for Endoscopic Activity and Mucosal Healing in Ulcerative Colitis. Biomedicines, 10(4), 872. https://doi.org/10.3390/biomedicines10040872







