Alteration of Neural Pathways and Its Implications in Alzheimer’s Disease
Abstract
1. Introduction
2. Neural Circuits Associated with AD
2.1. Hippocampal Pathways
2.1.1. Hippocampal Pathways in Healthy Brains
2.1.2. Hippocampal Pathways in the Brains with AD
2.2. Septal Pathways
2.2.1. Septal Pathways in Healthy Brains
2.2.2. Septal Pathways in the Brain with AD
2.3. Locus Coeruleus Pathways
2.3.1. Locus Coeruleus Pathways in Healthy Brains
2.3.2. Locus Coeruleus Pathways in the Brains with AD
2.4. Substantia Nigral Pathways
2.4.1. Substantia Nigral Pathways in Healthy Brains
2.4.2. Substantia Nigral Pathways in the Brains with AD
2.5. Visual Pathways
2.5.1. Visual Pathways in Healthy Brains
2.5.2. Visual Pathways in the Brains with AD
2.6. Olfactory Pathways
2.6.1. Olfactory Pathways in Healthy Brains
2.6.2. Olfactory Pathways in the Brain with AD
3. Targeting the Neural Circuits for Treatment of AD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Albers, M.W.; Gilmore, G.C.; Kaye, J.; Murphy, C.; Wingfield, A.; Bennett, D.A.; Boxer, A.L.; Buchman, A.S.; Cruickshanks, K.J.; Devanand, D.P.; et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2015, 11, 70–98. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Epperly, T.; Dunay, M.A.; Boice, J.L. Alzheimer Disease: Pharmacologic and Nonpharmacologic Therapies for Cognitive and Functional Symptoms. Am. Fam. Physician 2017, 95, 771–778. [Google Scholar]
- Mahase, E. FDA approves controversial Alzheimer’s drug despite uncertainty over effectiveness. BMJ 2021, 373, n1462. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Busche, M.A.; Konnerth, A. Impairments of neural circuit function in Alzheimer’s disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150429. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Nishioka, C.; Poh, C.; Sun, S.W. Diffusion tensor imaging reveals visual pathway damage in patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 97–107. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kim, Y.J.; Kim, K.A.; Mook-Jung, I.; Moon, M. Visualization of Altered Hippocampal Connectivity in an Animal Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 7886–7899. [Google Scholar] [CrossRef]
- Zott, B.; Busche, M.A.; Sperling, R.A.; Konnerth, A. What Happens with the Circuit in Alzheimer’s Disease in Mice and Humans? Annu. Rev. Neurosci. 2018, 41, 277–297. [Google Scholar] [CrossRef]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- Devanand, D.P.; Liu, X.; Tabert, M.H.; Pradhaban, G.; Cuasay, K.; Bell, K.; de Leon, M.J.; Doty, R.L.; Stern, Y.; Pelton, G.H. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol. Psychiatry 2008, 64, 871–879. [Google Scholar] [CrossRef]
- Gates, G.A.; Anderson, M.L.; Feeney, M.P.; McCurry, S.M.; Larson, E.B. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 771–777. [Google Scholar] [CrossRef]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef]
- Sporns, O.; Tononi, G.; Kotter, R. The human connectome: A structural description of the human brain. PLoS Comput. Biol. 2005, 1, e42. [Google Scholar] [CrossRef]
- Sporns, O. Connectome Networks: From Cells to Systems. In Micro-, Meso- and Macro-Connectomics of the Brain; Kennedy, H., Van Essen, D.C., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 107–127. [Google Scholar] [CrossRef]
- Craddock, R.C.; Jbabdi, S.; Yan, C.G.; Vogelstein, J.T.; Castellanos, F.X.; Di Martino, A.; Kelly, C.; Heberlein, K.; Colcombe, S.; Milham, M.P. Imaging human connectomes at the macroscale. Nat. Methods 2013, 10, 524–539. [Google Scholar] [CrossRef]
- Sporns, O. The human connectome: Origins and challenges. Neuroimage 2013, 80, 53–61. [Google Scholar] [CrossRef]
- Zeng, H. Mesoscale connectomics. Curr. Opin. Neurobiol. 2018, 50, 154–162. [Google Scholar] [CrossRef]
- Oh, S.W.; Harris, J.A.; Ng, L.; Winslow, B.; Cain, N.; Mihalas, S.; Wang, Q.; Lau, C.; Kuan, L.; Henry, A.M.; et al. A mesoscale connectome of the mouse brain. Nature 2014, 508, 207–214. [Google Scholar] [CrossRef]
- Knierim, J.J. The hippocampus. Curr. Biol. 2015, 25, R1116–R1121. [Google Scholar] [CrossRef]
- Lisman, J.; Buzsaki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Tzakis, N.; Holahan, M.R. Social Memory and the Role of the Hippocampal CA2 Region. Front. Behav. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef]
- Liu, M.G.; Chen, J. Roles of the hippocampal formation in pain information processing. Neurosci. Bull. 2009, 25, 237–266. [Google Scholar] [CrossRef]
- Machado, C.J.; Bachevalier, J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 2006, 120, 761–786. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Holmes, T.C.; Lopez, A.J. Noncanonical connections between the subiculum and hippocampal CA1. J. Comp. Neurol. 2016, 524, 3666–3673. [Google Scholar] [CrossRef]
- Lehr, A.B.; Kumar, A.; Tetzlaff, C.; Hafting, T.; Fyhn, M.; Stober, T.M. CA2 beyond social memory: Evidence for a fundamental role in hippocampal information processing. Neurosci. Biobehav. Rev. 2021, 126, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Rowland, D.C.; Weible, A.P.; Wickersham, I.R.; Wu, H.; Mayford, M.; Witter, M.P.; Kentros, C.G. Transgenically targeted rabies virus demonstrates a major monosynaptic projection from hippocampal area CA2 to medial entorhinal layer II neurons. J. Neurosci. 2013, 33, 14889–14898. [Google Scholar] [CrossRef]
- Kesner, R.P. Behavioral functions of the CA3 subregion of the hippocampus. Learn. Mem. 2007, 14, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Sampath, D.; Sathyanesan, M.; Newton, S.S. Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatr. Dis. Treat. 2017, 13, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Witter, M.P.; Doan, T.P.; Jacobsen, B.; Nilssen, E.S.; Ohara, S. Architecture of the Entorhinal Cortex A Review of Entorhinal Anatomy in Rodents with Some Comparative Notes. Front. Syst. Neurosci. 2017, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Katayama, Y.; Kawakami, Y. Hippocampal CA1/subiculum-prefrontal cortical pathways induce plastic changes of nociceptive responses in cingulate and prelimbic areas. BMC Neurosci. 2010, 11, 100. [Google Scholar] [CrossRef]
- Conde, F.; Maire-Lepoivre, E.; Audinat, E.; Crepel, F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J. Comp. Neurol. 1995, 352, 567–593. [Google Scholar] [CrossRef]
- Verwer, R.W.; Meijer, R.J.; Van Uum, H.F.; Witter, M.P. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus 1997, 7, 397–402. [Google Scholar] [CrossRef]
- Thierry, A.M.; Gioanni, Y.; Degenetais, E.; Glowinski, J. Hippocampo-prefrontal cortex pathway: Anatomical and electrophysiological characteristics. Hippocampus 2000, 10, 411–419. [Google Scholar] [CrossRef]
- Cui, Z.; Gerfen, C.R.; Young, W.S., 3rd. Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol. 2013, 521, 1844–1866. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Jeong, Y.O.; Park, H.H.; Lee, S.K.; Shin, S.J.; Jung, H.; Kim, B.H.; Hong, S.B.; Park, Y.H.; et al. Topographical Visualization of the Reciprocal Projection between the Medial Septum and the Hippocampus in the 5XFAD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 63992. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.Z. From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits. Front. Neural Circuits 2017, 11, 86. [Google Scholar] [CrossRef]
- Jankowski, M.M.; Ronnqvist, K.C.; Tsanov, M.; Vann, S.D.; Wright, N.F.; Erichsen, J.T.; Aggleton, J.P.; O’Mara, S.M. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front. Syst. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Bubb, E.J.; Kinnavane, L.; Aggleton, J.P. Hippocampal—Diencephalic—Cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci. Adv. 2017, 1, 2398212817723443. [Google Scholar] [CrossRef]
- Mathiasen, M.L.; O’Mara, S.M.; Aggleton, J.P. The anterior thalamic nuclei and nucleus reuniens: So similar but so different. Neurosci. Biobehav. Rev. 2020, 119, 268–280. [Google Scholar] [CrossRef]
- Jimenez, J.C.; Su, K.; Goldberg, A.R.; Luna, V.M.; Biane, J.S.; Ordek, G.; Zhou, P.; Ong, S.K.; Wright, M.A.; Zweifel, L.; et al. Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron 2018, 97, 670–683.e6. [Google Scholar] [CrossRef]
- Sweeney, P.; Yang, Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat. Commun. 2015, 6, 10188. [Google Scholar] [CrossRef]
- Dolleman-van der Weel, M.J.; Griffin, A.L.; Ito, H.T.; Shapiro, M.L.; Witter, M.P.; Vertes, R.P.; Allen, T.A. The nucleus reuniens of the thalamus sits at the nexus of a hippocampus and medial prefrontal cortex circuit enabling memory and behavior. Learn. Mem. 2019, 26, 191–205. [Google Scholar] [CrossRef]
- Aggleton, J.P.; Vann, S.D.; Saunders, R.C. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur. J. Neurosci. 2005, 22, 2519–2530. [Google Scholar] [CrossRef]
- Christiansen, K.; Dillingham, C.M.; Wright, N.F.; Saunders, R.C.; Vann, S.D.; Aggleton, J.P. Complementary subicular pathways to the anterior thalamic nuclei and mammillary bodies in the rat and macaque monkey brain. Eur. J. Neurosci. 2016, 43, 1044–1061. [Google Scholar] [CrossRef]
- Vann, S.D.; Nelson, A.J. The mammillary bodies and memory: More than a hippocampal relay. Prog. Brain Res. 2015, 219, 163–185. [Google Scholar] [CrossRef]
- Cordella, A.; Krashia, P.; Nobili, A.; Pignataro, A.; La Barbera, L.; Viscomi, M.T.; Valzania, A.; Keller, F.; Ammassari-Teule, M.; Mercuri, N.B.; et al. Dopamine loss alters the hippocampus-nucleus accumbens synaptic transmission in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol. Dis. 2018, 116, 142–154. [Google Scholar] [CrossRef]
- Aqrabawi, A.J.; Kim, J.C. Hippocampal projections to the anterior olfactory nucleus differentially convey spatiotemporal information during episodic odour memory. Nat. Commun. 2018, 9, 2735. [Google Scholar] [CrossRef]
- Swanson, L.W.; Kohler, C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J. Neurosci. 1986, 6, 3010–3023. [Google Scholar] [CrossRef]
- Allen, G.; Barnard, H.; McColl, R.; Hester, A.L.; Fields, J.A.; Weiner, M.F.; Ringe, W.K.; Lipton, A.M.; Brooker, M.; McDonald, E.; et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch. Neurol. 2007, 64, 1482–1487. [Google Scholar] [CrossRef]
- Wang, L.; Zang, Y.; He, Y.; Liang, M.; Zhang, X.; Tian, L.; Wu, T.; Jiang, T.; Li, K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage 2006, 31, 496–504. [Google Scholar] [CrossRef]
- Yakushev, I.; Schreckenberger, M.; Muller, M.J.; Schermuly, I.; Cumming, P.; Stoeter, P.; Gerhard, A.; Fellgiebel, A. Functional implications of hippocampal degeneration in early Alzheimer’s disease: A combined DTI and PET study. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2219–2227. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, B.; Yao, H.; Xie, S.; Feng, F.; Zhang, Z.; Guo, Y.; An, N.; Zhou, Y.; Zhang, X.; et al. Aberrant Hippocampal Functional Connectivity Is Associated with Fornix White Matter Integrity in Alzheimer’s Disease and Mild Cognitive Impairment. J. Alzheimers Dis. 2020, 75, 1153–1168. [Google Scholar] [CrossRef]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef]
- Mondragon-Rodriguez, S.; Gu, N.; Fasano, C.; Pena-Ortega, F.; Williams, S. Functional Connectivity between Hippocampus and Lateral Septum is Affected in Very Young Alzheimer’s Transgenic Mouse Model. Neuroscience 2019, 401, 96–105. [Google Scholar] [CrossRef]
- Lin, T.W.; Shih, Y.H.; Chen, S.J.; Lien, C.H.; Chang, C.Y.; Huang, T.Y.; Chen, S.H.; Jen, C.J.; Kuo, Y.M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef]
- Alvarez, R.P.; Biggs, A.; Chen, G.; Pine, D.S.; Grillon, C. Contextual fear conditioning in humans: Cortical-hippocampal and amygdala contributions. J. Neurosci. 2008, 28, 6211–6219. [Google Scholar] [CrossRef]
- Comery, T.A.; Martone, R.L.; Aschmies, S.; Atchison, K.P.; Diamantidis, G.; Gong, X.; Zhou, H.; Kreft, A.F.; Pangalos, M.N.; Sonnenberg-Reines, J.; et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 2005, 25, 8898–8902. [Google Scholar] [CrossRef] [PubMed]
- Aggleton, J.P.; Pralus, A.; Nelson, A.J.; Hornberger, M. Thalamic pathology and memory loss in early Alzheimer’s disease: Moving the focus from the medial temporal lobe to Papez circuit. Brain 2016, 139, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Kinnavane, L.; Vann, S.D.; Nelson, A.J.D.; O’Mara, S.M.; Aggleton, J.P. Collateral Projections Innervate the Mammillary Bodies and Retrosplenial Cortex: A New Category of Hippocampal Cells. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Baloyannis, S.J.; Mavroudis, I.; Baloyannis, I.S.; Costa, V.G. Mammillary Bodies in Alzheimer’s Disease: A Golgi and Electron Microscope Study. Am. J. Alzheimers Dis. Other Demen. 2016, 31, 247–256. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.B.; Paek, S.H.; Cho, Z.H. Papez Circuit Observed by in vivo Human Brain With 7.0T MRI Super-Resolution Track Density Imaging and Track Tracing. Front. Neuroanat. 2019, 13, 17. [Google Scholar] [CrossRef]
- Hara, Y.; Motoi, Y.; Hikishima, K.; Mizuma, H.; Onoe, H.; Matsumoto, S.E.; Elahi, M.; Okano, H.; Aoki, S.; Hattori, N. Involvement of the Septo-Hippocampal Cholinergic Pathway in Association with Septal Acetylcholinesterase Upregulation in a Mouse Model of Tauopathy. Curr. Alzheimer Res. 2017, 14, 94–103. [Google Scholar] [CrossRef]
- Belarbi, K.; Schindowski, K.; Burnouf, S.; Caillierez, R.; Grosjean, M.E.; Demeyer, D.; Hamdane, M.; Sergeant, N.; Blum, D.; Buee, L. Early Tau pathology involving the septo-hippocampal pathway in a Tau transgenic model: Relevance to Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 152–157. [Google Scholar] [CrossRef]
- Rubio, S.E.; Vega-Flores, G.; Martinez, A.; Bosch, C.; Perez-Mediavilla, A.; del Rio, J.; Gruart, A.; Delgado-Garcia, J.M.; Soriano, E.; Pascual, M. Accelerated aging of the GABAergic septohippocampal pathway and decreased hippocampal rhythms in a mouse model of Alzheimer’s disease. FASEB J. 2012, 26, 4458–4467. [Google Scholar] [CrossRef]
- Soler, H.; Dorca-Arevalo, J.; Gonzalez, M.; Rubio, S.E.; Avila, J.; Soriano, E.; Pascual, M. The GABAergic septohippocampal connection is impaired in a mouse model of tauopathy. Neurobiol. Aging 2017, 49, 40–51. [Google Scholar] [CrossRef]
- Chiasseu, M.; Alarcon-Martinez, L.; Belforte, N.; Quintero, H.; Dotigny, F.; Destroismaisons, L.; Vande Velde, C.; Panayi, F.; Louis, C.; Di Polo, A. Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 58. [Google Scholar] [CrossRef]
- Khakpai, F.; Nasehi, M.; Haeri-Rohani, A.; Eidi, A.; Zarrindast, M.R. Septo-hippocampo-septal loop and memory formation. Basic Clin. Neurosci. 2013, 4, 5–23. [Google Scholar]
- Khakpai, F.; Zarrindast, M.R.; Nasehi, M.; Haeri-Rohani, A.; Eidi, A. The role of glutamatergic pathway between septum and hippocampus in the memory formation. EXCLI J. 2013, 12, 41–51. [Google Scholar]
- Zhao, C.; Eisinger, B.; Gammie, S.C. Characterization of GABAergic neurons in the mouse lateral septum: A double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PLoS ONE 2013, 8, e73750. [Google Scholar] [CrossRef]
- Tsanov, M. Differential and complementary roles of medial and lateral septum in the orchestration of limbic oscillations and signal integration. Eur. J. Neurosci. 2018, 48, 2783–2794. [Google Scholar] [CrossRef]
- Siegel, A.; Tassoni, J.P. Differential efferent projections of the lateral and medial septal nuclei to the hippocampus in the cat. Brain Behav. Evol. 1971, 4, 201–219. [Google Scholar] [CrossRef]
- Deng, K.; Yang, L.; Xie, J.; Tang, H.; Wu, G.S.; Luo, H.R. Whole-brain mapping of projection from mouse lateral septal nucleus. Biol. Open 2019, 8, bio043554. [Google Scholar] [CrossRef]
- Swanson, L.W.; Cowan, W.M. The connections of the septal region in the rat. J. Comp. Neurol. 1979, 186, 621–655. [Google Scholar] [CrossRef]
- Gong, Y.; Xu, L.; Wang, H.; Guo, F.; Sun, X.; Gao, S. Involvements of the lateral hypothalamic area in gastric motility and its regulation by the lateral septum. Gen. Comp. Endocrinol. 2013, 194, 275–285. [Google Scholar] [CrossRef]
- Simerly, R.B.; Swanson, L.W. The organization of neural inputs to the medial preoptic nucleus of the rat. J. Comp. Neurol. 1986, 246, 312–342. [Google Scholar] [CrossRef]
- Wong, L.C.; Wang, L.; D’Amour, J.A.; Yumita, T.; Chen, G.; Yamaguchi, T.; Chang, B.C.; Bernstein, H.; You, X.; Feng, J.E.; et al. Effective Modulation of Male Aggression through Lateral Septum to Medial Hypothalamus Projection. Curr. Biol. 2016, 26, 593–604. [Google Scholar] [CrossRef]
- Vega-Quiroga, I.; Yarur, H.E.; Gysling, K. Lateral septum stimulation disinhibits dopaminergic neurons in the antero-ventral region of the ventral tegmental area: Role of GABA-A alpha 1 receptors. Neuropharmacology 2018, 128, 76–85. [Google Scholar] [CrossRef]
- Kiss, J.; Patel, A.J.; Baimbridge, K.G.; Freund, T.F. Topographical localization of neurons containing parvalbumin and choline acetyltransferase in the medial septum-diagonal band region of the rat. Neuroscience 1990, 36, 61–72. [Google Scholar] [CrossRef]
- Hajszan, T.; Alreja, M.; Leranth, C. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: Novel glutamatergic local circuit cells. Hippocampus 2004, 14, 499–509. [Google Scholar] [CrossRef]
- Gu, Z.; Alexander, G.M.; Dudek, S.M.; Yakel, J.L. Hippocampus and Entorhinal Cortex Recruit Cholinergic and NMDA Receptors Separately to Generate Hippocampal Theta Oscillations. Cell Rep. 2017, 21, 3585–3595. [Google Scholar] [CrossRef]
- Teles-Grilo Ruivo, L.M.; Mellor, J.R. Cholinergic modulation of hippocampal network function. Front. Synaptic Neurosci. 2013, 5, 2. [Google Scholar] [CrossRef]
- Muller, C.; Remy, S. Septo-hippocampal interaction. Cell Tissue Res. 2018, 373, 565–575. [Google Scholar] [CrossRef]
- Colom, L.V.; Castaneda, M.T.; Reyna, T.; Hernandez, S.; Garrido-Sanabria, E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse 2005, 58, 151–164. [Google Scholar] [CrossRef]
- Senut, M.C.; Menetrey, D.; Lamour, Y. Cholinergic and peptidergic projections from the medial septum and the nucleus of the diagonal band of Broca to dorsal hippocampus, cingulate cortex and olfactory bulb: A combined wheatgerm agglutinin-apohorseradish peroxidase-gold immunohistochemical study. Neuroscience 1989, 30, 385–403. [Google Scholar] [CrossRef]
- Gaykema, R.P.; Luiten, P.G.; Nyakas, C.; Traber, J. Cortical projection patterns of the medial septum-diagonal band complex. J. Comp. Neurol. 1990, 293, 103–124. [Google Scholar] [CrossRef]
- Desikan, S.; Koser, D.E.; Neitz, A.; Monyer, H. Target selectivity of septal cholinergic neurons in the medial and lateral entorhinal cortex. Proc. Natl. Acad. Sci. USA 2018, 115, E2644–E2652. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, K.; Li, D.; Yuan, Q.; Yao, Z. Special function of nestin(+) neurons in the medial septum-diagonal band of Broca in adult rats. Neural Regen. Res. 2014, 9, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kesner, A.J.; Shin, R.; Calva, C.B.; Don, R.F.; Junn, S.; Potter, C.T.; Ramsey, L.A.; Abou-Elnaga, A.F.; Cover, C.G.; Wang, D.V.; et al. Supramammillary neurons projecting to the septum regulate dopamine and motivation for environmental interaction in mice. Nat. Commun. 2021, 12, 2811. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Sun, H.; Wu, M.; Xie, D.; Hu, S.W.; Ding, H.L.; Cao, J.L. Medial septum glutamatergic neurons control wakefulness through a septo-hypothalamic circuit. Curr. Biol. 2021, 31, 1379–1392.e4. [Google Scholar] [CrossRef] [PubMed]
- Cazala, P.; Galey, D.; Durkin, T. Electrical self-stimulation in the medial and lateral septum as compared to the lateral hypothalamus: Differential intervention of reward and learning processes? Physiol. Behav. 1988, 44, 53–59. [Google Scholar] [CrossRef]
- Schmitz, T.W.; Mur, M.; Aghourian, M.; Bedard, M.A.; Spreng, R.N.; Alzheimer’s Disease Neuroimaging, I. Longitudinal Alzheimer’s Degeneration Reflects the Spatial Topography of Cholinergic Basal Forebrain Projections. Cell Rep. 2018, 24, 38–46. [Google Scholar] [CrossRef]
- Kesner, R.P.; Adelstein, T.B.; Crutcher, K.A. Equivalent spatial location memory deficits in rats with medial septum or hippocampal formation lesions and patients with dementia of the Alzheimer’s type. Brain Cogn. 1989, 9, 289–300. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kawashima, S. Effects of amyloid-beta-(25-35) on passive avoidance, radial-arm maze learning and choline acetyltransferase activity in the rat. Eur. J. Pharmacol. 2001, 412, 265–272. [Google Scholar] [CrossRef]
- Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.J.; Desmercieres, J.; Troquier, L.; Brouillette, J.; Tsambou, L.; Grosjean, M.E.; Caillierez, R.; Demeyer, D.; et al. Loss of medial septum cholinergic neurons in THY-Tau22 mouse model: What links with tau pathology? Curr. Alzheimer Res. 2011, 8, 633–638. [Google Scholar] [CrossRef]
- Pang, K.C.; Jiao, X.; Sinha, S.; Beck, K.D.; Servatius, R.J. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: Effects on proactive interference. Hippocampus 2011, 21, 835–846. [Google Scholar] [CrossRef]
- Colom, L.V. Septal networks: Relevance to theta rhythm, epilepsy and Alzheimer’s disease. J. Neurochem. 2006, 96, 609–623. [Google Scholar] [CrossRef]
- Pentkowski, N.S.; Rogge-Obando, K.K.; Donaldson, T.N.; Bouquin, S.J.; Clark, B.J. Anxiety and Alzheimer’s disease: Behavioral analysis and neural basis in rodent models of Alzheimer’s-related neuropathology. Neurosci. Biobehav. Rev. 2021, 127, 647–658. [Google Scholar] [CrossRef]
- Hartig, W.; Stieler, J.; Boerema, A.S.; Wolf, J.; Schmidt, U.; Weissfuss, J.; Bullmann, T.; Strijkstra, A.M.; Arendt, T. Hibernation model of tau phosphorylation in hamsters: Selective vulnerability of cholinergic basal forebrain neurons—Implications for Alzheimer’s disease. Eur. J. Neurosci. 2007, 25, 69–80. [Google Scholar] [CrossRef]
- Marien, M.R.; Colpaert, F.C.; Rosenquist, A.C. Noradrenergic mechanisms in neurodegenerative diseases: A theory. Brain Res. Rev. 2004, 45, 38–78. [Google Scholar] [CrossRef]
- Samuels, E.R.; Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part I: Principles of functional organisation. Curr. Neuropharmacol. 2008, 6, 235–253. [Google Scholar] [CrossRef]
- Uematsu, A.; Tan, B.Z.; Johansen, J.P. Projection specificity in heterogeneous locus coeruleus cell populations: Implications for learning and memory. Learn. Mem. 2015, 22, 444–451. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Wiltgen, B.J.; Brown, R.A.; Talton, L.E.; Silva, A.J. New circuits for old memories: The role of the neocortex in consolidation. Neuron 2004, 44, 101–108. [Google Scholar] [CrossRef]
- Chandler, D.; Waterhouse, B.D. Evidence for broad versus segregated projections from cholinergic and noradrenergic nuclei to functionally and anatomically discrete subregions of prefrontal cortex. Front. Behav. Neurosci. 2012, 6, 20. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Miyamichi, K.; Gao, X.J.; Beier, K.T.; Weissbourd, B.; DeLoach, K.E.; Ren, J.; Ibanes, S.; Malenka, R.C.; Kremer, E.J.; et al. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 2015, 524, 88–92. [Google Scholar] [CrossRef]
- Vitrac, C.; Benoit-Marand, M. Monoaminergic Modulation of Motor Cortex Function. Front. Neural Circuits 2017, 11, 72. [Google Scholar] [CrossRef]
- Hansen, N. The Longevity of Hippocampus-Dependent Memory Is Orchestrated by the Locus Coeruleus-Noradrenergic System. Neural Plast. 2017, 2017, 2727602. [Google Scholar] [CrossRef]
- Lipski, W.J.; Grace, A.A. Activation and inhibition of neurons in the hippocampal ventral subiculum by norepinephrine and locus coeruleus stimulation. Neuropsychopharmacology 2013, 38, 285–292. [Google Scholar] [CrossRef]
- Oleskevich, S.; Descarries, L.; Lacaille, J.C. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J. Neurosci. 1989, 9, 3803–3815. [Google Scholar] [CrossRef]
- McCall, J.G.; Siuda, E.R.; Bhatti, D.L.; Lawson, L.A.; McElligott, Z.A.; Stuber, G.D.; Bruchas, M.R. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife 2017, 6, e18247. [Google Scholar] [CrossRef]
- Llorca-Torralba, M.; Suarez-Pereira, I.; Bravo, L.; Camarena-Delgado, C.; Garcia-Partida, J.A.; Mico, J.A.; Berrocoso, E. Chemogenetic Silencing of the Locus Coeruleus-Basolateral Amygdala Pathway Abolishes Pain-Induced Anxiety and Enhanced Aversive Learning in Rats. Biol. Psychiatry 2019, 85, 1021–1035. [Google Scholar] [CrossRef]
- Giustino, T.F.; Ramanathan, K.R.; Totty, M.S.; Miles, O.W.; Maren, S. Locus Coeruleus Norepinephrine Drives Stress-Induced Increases in Basolateral Amygdala Firing and Impairs Extinction Learning. J. Neurosci. 2020, 40, 907–916. [Google Scholar] [CrossRef]
- Bergado, J.A.; Frey, S.; Lopez, J.; Almaguer-Melian, W.; Frey, J.U. Cholinergic afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. Neurobiol. Learn. Mem. 2007, 88, 331–341. [Google Scholar] [CrossRef]
- Irle, E.; Markowitsch, H.J. Afferent connections of the substantia innominata/basal nucleus of Meynert in carnivores and primates. J. Hirnforsch. 1986, 27, 343–367. [Google Scholar]
- Jones, B.E.; Yang, T.Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985, 242, 56–92. [Google Scholar] [CrossRef]
- Brun, P.; Suaud-Chagny, M.F.; Gonon, F.; Buda, M. In vivo noradrenaline release evoked in the anteroventral thalamic nucleus by locus coeruleus activation: An electrochemical study. Neuroscience 1993, 52, 961–972. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.Q.; Qiao, J.T.; Dafny, N. Locus coeruleus modulates thalamic nociceptive responses via adrenoceptors. Brain Res. 1998, 784, 116–122. [Google Scholar] [CrossRef]
- Westlund, K.N.; Zhang, D.; Carlton, S.M.; Sorkin, L.S.; Willis, W.D. Noradrenergic innervation of somatosensory thalamus and spinal cord. Prog. Brain Res. 1991, 88, 77–88. [Google Scholar] [CrossRef]
- Voisin, D.L.; Guy, N.; Chalus, M.; Dallel, R. Nociceptive stimulation activates locus coeruleus neurones projecting to the somatosensory thalamus in the rat. J. Physiol. 2005, 566, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Lee, H.S.; Lee, B.Y.; Waterhouse, B.D. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004, 1026, 56–67. [Google Scholar] [CrossRef]
- Khanday, M.A.; Somarajan, B.I.; Mehta, R.; Mallick, B.N. Noradrenaline from Locus Coeruleus Neurons Acts on Pedunculo-Pontine Neurons to Prevent REM Sleep and Induces Its Loss-Associated Effects in Rats. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kodama, T.; Koyama, Y. Nitric oxide from the laterodorsal tegmental neurons: Its possible retrograde modulation on norepinephrine release from the axon terminal of the locus coeruleus neurons. Neuroscience 2006, 138, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; McElligott, J.G. Cerebellar norepinephrine depletion and impaired acquisition of specific locomotor tasks in rats. Brain Res. 1984, 296, 129–138. [Google Scholar] [CrossRef]
- Mravec, B.; Lejavova, K.; Cubinkova, V. Locus (coeruleus) minoris resistentiae in pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 992–1001. [Google Scholar] [CrossRef]
- Ross, J.A.; McGonigle, P.; Van Bockstaele, E.J. Locus Coeruleus, norepinephrine and Abeta peptides in Alzheimer’s disease. Neurobiol. Stress 2015, 2, 73–84. [Google Scholar] [CrossRef]
- Bondareff, W.; Mountjoy, C.Q.; Roth, M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 1982, 32, 164–168. [Google Scholar] [CrossRef]
- Simic, G.; Babic Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milosevic, N.; Bazadona, D.; Buee, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef]
- Kaushal, R.; Taylor, B.K.; Jamal, A.B.; Zhang, L.; Ma, F.; Donahue, R.; Westlund, K.N. GABA-A receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of NAalpha1 receptors in the medial prefrontal cortex. Neuroscience 2016, 334, 148–159. [Google Scholar] [CrossRef]
- Martins, A.R.; Froemke, R.C. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci. 2015, 18, 1483–1492. [Google Scholar] [CrossRef]
- Jiang, M.; Griff, E.R.; Ennis, M.; Zimmer, L.A.; Shipley, M.T. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J. Neurosci. 1996, 16, 6319–6329. [Google Scholar] [CrossRef]
- Osaka, T.; Matsumura, H. Noradrenergic inputs to sleep-related neurons in the preoptic area from the locus coeruleus and the ventrolateral medulla in the rat. Neurosci. Res. 1994, 19, 39–50. [Google Scholar] [CrossRef]
- Sherin, J.E.; Elmquist, J.K.; Torrealba, F.; Saper, C.B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 1998, 18, 4705–4721. [Google Scholar] [CrossRef]
- Sonne, J.; Reddy, V.; Beato, M.R. Neuroanatomy, Substantia Nigra; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Morello, F.; Partanen, J. Diversity and development of local inhibitory and excitatory neurons associated with dopaminergic nuclei. FEBS Lett. 2015, 589, 3693–3701. [Google Scholar] [CrossRef]
- York, D.H. Possible dopaminergic pathway from substantia nigra to putamen. Brain Res. 1970, 20, 233–249. [Google Scholar] [CrossRef]
- Hattori, T. Conceptual history of the nigrostriatal dopamine system. Neurosci. Res. 1993, 16, 239–262. [Google Scholar] [CrossRef]
- French, I.T.; Muthusamy, K.A. A Review of the Pedunculopontine Nucleus in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 99. [Google Scholar] [CrossRef]
- Beckstead, R.M.; Frankfurter, A. The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience 1982, 7, 2377–2388. [Google Scholar] [CrossRef]
- Beckstead, R.M. Long collateral branches of substantia nigra pars reticulata axons to thalamus, superior colliculus and reticular formation in monkey and cat. Multiple retrograde neuronal labeling with fluorescent dyes. Neuroscience 1983, 10, 767–779. [Google Scholar] [CrossRef]
- Vilela Filho, O. Thalamic ventrobasal stimulation for pain relief. Probable mechanisms, pathways and neurotransmitters. Arq. Neuropsiquiatr. 1994, 52, 578–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Straughan, D.W.; MacLeod, N.K.; James, T.A.; Kilpatrick, I.C. GABA and the nigrothalamic pathway. Brain Res. Bull. 1980, 5, 7–11. [Google Scholar] [CrossRef]
- Gulcebi, M.I.; Ketenci, S.; Linke, R.; Hacioglu, H.; Yanali, H.; Veliskova, J.; Moshe, S.L.; Onat, F.; Cavdar, S. Topographical connections of the substantia nigra pars reticulata to higher-order thalamic nuclei in the rat. Brain Res. Bull. 2012, 87, 312–318. [Google Scholar] [CrossRef]
- Tsumori, T.; Yokota, S.; Ono, K.; Yasui, Y. Synaptic organization of GABAergic projections from the substantia nigra pars reticulata and the reticular thalamic nucleus to the parafascicular thalamic nucleus in the rat. Brain Res. 2002, 957, 231–241. [Google Scholar] [CrossRef]
- Francois, C.; Tande, D.; Yelnik, J.; Hirsch, E.C. Distribution and morphology of nigral axons projecting to the thalamus in primates. J. Comp. Neurol. 2002, 447, 249–260. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takada, M.; Mizuno, N. Topographic distribution and collateral projections of the two major populations of nigrothalamic neurons. A retrograde labeling study in the rat. Neurosci. Res. 1997, 28, 1–9. [Google Scholar] [CrossRef]
- Carpenter, M.B.; Nakano, K.; Kim, R. Nigrothalamic projections in the monkey demonstrated by autoradiographic technics. J. Comp. Neurol. 1976, 165, 401–415. [Google Scholar] [CrossRef]
- Bodor, A.L.; Giber, K.; Rovo, Z.; Ulbert, I.; Acsady, L. Structural correlates of efficient GABAergic transmission in the basal ganglia-thalamus pathway. J. Neurosci. 2008, 28, 3090–3102. [Google Scholar] [CrossRef]
- Zhou, F. The substantia nigra pars reticulata. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2016; Volume 24, pp. 293–316. [Google Scholar]
- Paz, J.T.; Chavez, M.; Saillet, S.; Deniau, J.M.; Charpier, S. Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J. Neurosci. 2007, 27, 929–941. [Google Scholar] [CrossRef]
- Aoki, S.; Smith, J.B.; Li, H.; Yan, X.; Igarashi, M.; Coulon, P.; Wickens, J.R.; Ruigrok, T.J.; Jin, X. An open cortico-basal ganglia loop allows limbic control over motor output via the nigrothalamic pathway. eLife 2019, 8, e49995. [Google Scholar] [CrossRef]
- Haber, S.N.; Calzavara, R. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Res. Bull. 2009, 78, 69–74. [Google Scholar] [CrossRef]
- Ikemoto, S.; Yang, C.; Tan, A. Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behav. Brain Res. 2015, 290, 17–31. [Google Scholar] [CrossRef]
- McHaffie, J.G.; Jiang, H.; May, P.J.; Coizet, V.; Overton, P.G.; Stein, B.E.; Redgrave, P. A direct projection from superior colliculus to substantia nigra pars compacta in the cat. Neuroscience 2006, 138, 221–234. [Google Scholar] [CrossRef]
- Beckstead, R.M.; Domesick, V.B.; Nauta, W.J. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979, 175, 191–217. [Google Scholar] [CrossRef]
- Chronister, R.B.; Walding, J.S.; Aldes, L.D.; Marco, L.A. Interconnections between substantia nigra reticulata and medullary reticular formation. Brain Res. Bull. 1988, 21, 313–317. [Google Scholar] [CrossRef]
- Perez, S.E.; Lazarov, O.; Koprich, J.B.; Chen, E.Y.; Rodriguez-Menendez, V.; Lipton, J.W.; Sisodia, S.S.; Mufson, E.J. Nigrostriatal dysfunction in familial Alzheimer’s disease-linked APPswe/PS1DeltaE9 transgenic mice. J. Neurosci. 2005, 25, 10220–10229. [Google Scholar] [CrossRef]
- Colloby, S.J.; McParland, S.; O’Brien, J.T.; Attems, J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain 2012, 135, 2798–2808. [Google Scholar] [CrossRef]
- Iaccarino, L.; Sala, A.; Caminiti, S.P.; Presotto, L.; Perani, D.; Alzheimer’s Disease Neuroimaging, I. In vivo MRI Structural and PET Metabolic Connectivity Study of Dopamine Pathways in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 75, 1003–1016. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Hamasaki, H.; Honda, H.; Suzuki, S.O.; Shijo, M.; Ohara, T.; Hatabe, Y.; Okamoto, T.; Ninomiya, T.; Iwaki, T. Tauopathy in basal ganglia involvement is exacerbated in a subset of patients with Alzheimer’s disease: The Hisayama study. Alzheimers Dement. 2019, 11, 415–423. [Google Scholar] [CrossRef]
- Fu, Y.; Liao, H.W.; Do, M.T.; Yau, K.W. Non-image-forming ocular photoreception in vertebrates. Curr. Opin. Neurobiol. 2005, 15, 415–422. [Google Scholar] [CrossRef]
- Matynia, A. Blurring the boundaries of vision: Novel functions of intrinsically photosensitive retinal ganglion cells. J. Exp. Neurosci. 2013, 7, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Martersteck, E.M.; Hirokawa, K.E.; Evarts, M.; Bernard, A.; Duan, X.; Li, Y.; Ng, L.; Oh, S.W.; Ouellette, B.; Royall, J.J.; et al. Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Rep. 2017, 18, 2058–2072. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.P.; Studholme, K.M. Retinofugal projections in the mouse. J. Comp. Neurol. 2014, 522, 3733–3753. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.A.; Kastner, S. Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J. Neurosci. 2009, 29, 1784–1795. [Google Scholar] [CrossRef]
- Sanes, J.R.; Masland, R.H. The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef]
- Gaillard, F.; Karten, H.J.; Sauve, Y. Retinorecipient areas in the diurnal murine rodent Arvicanthis niloticus: A disproportionally large superior colliculus. J. Comp. Neurol. 2013, 521, 1699–1726. [Google Scholar] [CrossRef]
- Kastner, S.; O’Connor, D.H.; Fukui, M.M.; Fehd, H.M.; Herwig, U.; Pinsk, M.A. Functional imaging of the human lateral geniculate nucleus and pulvinar. J. Neurophysiol. 2004, 91, 438–448. [Google Scholar] [CrossRef]
- Ahmadlou, M.; Zweifel, L.S.; Heimel, J.A. Functional modulation of primary visual cortex by the superior colliculus in the mouse. Nat. Commun. 2018, 9, 3895. [Google Scholar] [CrossRef]
- Doron, N.N.; Ledoux, J.E. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J. Comp. Neurol. 1999, 412, 383–409. [Google Scholar] [CrossRef]
- Ito, S.; Feldheim, D.A. The Mouse Superior Colliculus: An Emerging Model for Studying Circuit Formation and Function. Front. Neural Circuits 2018, 12, 10. [Google Scholar] [CrossRef]
- Beltramo, R.; Scanziani, M. A collicular visual cortex: Neocortical space for an ancient midbrain visual structure. Science 2019, 363, 64–69. [Google Scholar] [CrossRef]
- Dhande, O.S.; Estevez, M.E.; Quattrochi, L.E.; El-Danaf, R.N.; Nguyen, P.L.; Berson, D.M.; Huberman, A.D. Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. J. Neurosci. 2013, 33, 17797–17813. [Google Scholar] [CrossRef]
- Simpson, J.I. The accessory optic system. Annu. Rev. Neurosci. 1984, 7, 13–41. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Kofuji, P. Novel insights into non-image forming visual processing in the retina. Cellscience 2008, 5, 77–83. [Google Scholar]
- Baver, S.B.; Pickard, G.E.; Sollars, P.J.; Pickard, G.E. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur. J. Neurosci. 2008, 27, 1763–1770. [Google Scholar] [CrossRef]
- Guler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.W.; Berson, D.M. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef]
- Sonoda, T.; Li, J.Y.; Hayes, N.W.; Chan, J.C.; Okabe, Y.; Belin, S.; Nawabi, H.; Schmidt, T.M. A noncanonical inhibitory circuit dampens behavioral sensitivity to light. Science 2020, 368, 527–531. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Syed, A.B. Alzheimer’s disease and the eye. Ophthalmic Physiol. Opt. 1996, 16 (Suppl. S1), S2–S8. [Google Scholar] [CrossRef]
- Javaid, F.Z.; Brenton, J.; Guo, L.; Cordeiro, M.F. Visual and Ocular Manifestations of Alzheimer’s Disease and Their Use as Biomarkers for Diagnosis and Progression. Front. Neurol. 2016, 7, 55. [Google Scholar] [CrossRef]
- Rizzo, M.; Anderson, S.W.; Dawson, J.; Nawrot, M. Vision and cognition in Alzheimer’s disease. Neuropsychologia 2000, 38, 1157–1169. [Google Scholar] [CrossRef]
- Tales, A.; Troscianko, T.; Lush, D.; Haworth, J.; Wilcock, G.K.; Butler, S.R. The pupillary light reflex in aging and Alzheimer’s disease. Aging 2001, 13, 473–478. [Google Scholar]
- Weldemichael, D.A.; Grossberg, G.T. Circadian rhythm disturbances in patients with Alzheimer’s disease: A review. Int. J. Alzheimers Dis. 2010, 2010, 716453. [Google Scholar] [CrossRef]
- Kusne, Y.; Wolf, A.B.; Townley, K.; Conway, M.; Peyman, G.A. Visual system manifestations of Alzheimer’s disease. Acta Ophthalmol. 2017, 95, e668–e676. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Criscuolo, C.; Cerri, E.; Fabiani, C.; Capsoni, S.; Cattaneo, A.; Domenici, L. The retina as a window to early dysfunctions of Alzheimer’s disease following studies with a 5xFAD mouse model. Neurobiol. Aging 2018, 67, 181–188. [Google Scholar] [CrossRef]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2, e93621. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; Rodrigues-Neves, A.C.; Ramirez, A.I.; de Hoz, R.; Fernandez-Albarral, J.A.; Lopez-Cuenca, I.; Ramirez, J.M.; Ambrosio, A.F.; Salazar, J.J. Microglial Activation in the Retina of a Triple-Transgenic Alzheimer’s Disease Mouse Model (3xTg-AD). Int. J. Mol. Sci. 2020, 21, 30816. [Google Scholar] [CrossRef]
- Lodovichi, C. Topographic organization in the olfactory bulb. Cell Tissue Res. 2021, 383, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Scalia, F.; Winans, S.S. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J. Comp. Neurol. 1975, 161, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Taniguchi, K. Phylogenic studies on the olfactory system in vertebrates. J. Vet. Med. Sci. 2014, 76, 781–788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shipley, M.T.; Ennis, M.; Puche, A.C. The olfactory system. In Neuroscience in Medicine; Springer: New York, NY, USA, 2003; pp. 579–593. [Google Scholar]
- Mutlu, A.S.; Gao, S.M.; Zhang, H.; Wang, M.C. Olfactory specificity regulates lipid metabolism through neuroendocrine signaling in Caenorhabditis elegans. Nat. Commun. 2020, 11, 1450. [Google Scholar] [CrossRef]
- Hintiryan, H.; Gou, L.; Zingg, B.; Yamashita, S.; Lyden, H.M.; Song, M.Y.; Grewal, A.K.; Zhang, X.; Toga, A.W.; Dong, H.W. Comprehensive connectivity of the mouse main olfactory bulb: Analysis and online digital atlas. Front. Neuroanat. 2012, 6, 30. [Google Scholar] [CrossRef]
- Giessel, A.J.; Datta, S.R. Olfactory maps, circuits and computations. Curr. Opin. Neurobiol. 2014, 24, 120–132. [Google Scholar] [CrossRef]
- Igarashi, K.M.; Ieki, N.; An, M.; Yamaguchi, Y.; Nagayama, S.; Kobayakawa, K.; Kobayakawa, R.; Tanifuji, M.; Sakano, H.; Chen, W.R.; et al. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 2012, 32, 7970–7985. [Google Scholar] [CrossRef]
- Nagayama, S.; Takahashi, Y.K.; Yoshihara, Y.; Mori, K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J. Neurophysiol. 2004, 91, 2532–2540. [Google Scholar] [CrossRef]
- Nagayama, S.; Enerva, A.; Fletcher, M.L.; Masurkar, A.V.; Igarashi, K.M.; Mori, K.; Chen, W.R. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front. Neural Circuits 2010, 4, 120. [Google Scholar] [CrossRef]
- Suryanarayana, S.M.; Perez-Fernandez, J.; Robertson, B.; Grillner, S. Olfaction in Lamprey Pallium Revisited-Dual Projections of Mitral and Tufted Cells. Cell Rep. 2021, 34, 108596. [Google Scholar] [CrossRef]
- Kang, N.; Baum, M.J.; Cherry, J.A. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem. Senses 2011, 36, 251–260. [Google Scholar] [CrossRef]
- Cadiz-Moretti, B.; Otero-Garcia, M.; Martinez-Garcia, F.; Lanuza, E. Afferent projections to the different medial amygdala subdivisions: A retrograde tracing study in the mouse. Brain Struct. Funct. 2016, 221, 1033–1065. [Google Scholar] [CrossRef]
- Martinez-Marcos, A.; Halpern, M. Differential projections from the anterior and posterior divisions of the accessory olfactory bulb to the medial amygdala in the opossum, Monodelphis domestica. Eur. J. Neurosci. 1999, 11, 3789–3799. [Google Scholar] [CrossRef]
- Billing, A.; Henrique Correia, M.; Kelly, D.A.; Li, G.L.; Bergan, J.F. Synaptic Connections of Aromatase Circuits in the Medial Amygdala Are Sex Specific. eNeuro 2020, 7, ENEURO.0489-19.2020. [Google Scholar] [CrossRef]
- D’Aniello, B.; Semin, G.R.; Scandurra, A.; Pinelli, C. The Vomeronasal Organ: A Neglected Organ. Front. Neuroanat. 2017, 11, 70. [Google Scholar] [CrossRef]
- Trotier, D. Vomeronasal organ and human pheromones. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 184–190. [Google Scholar] [CrossRef]
- Williams, S.S.; Williams, J.; Combrinck, M.; Christie, S.; Smith, A.D.; McShane, R. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 667–670. [Google Scholar] [CrossRef]
- Franks, K.H.; Chuah, M.I.; King, A.E.; Vickers, J.C. Connectivity of Pathology: The Olfactory System as a Model for Network-Driven Mechanisms of Alzheimer’s Disease Pathogenesis. Front. Aging Neurosci. 2015, 7, 234. [Google Scholar] [CrossRef]
- Al Koborssy, D.; Palouzier-Paulignan, B.; Canova, V.; Thevenet, M.; Fadool, D.A.; Julliard, A.K. Modulation of olfactory-driven behavior by metabolic signals: Role of the piriform cortex. Brain Struct. Funct. 2019, 224, 315–336. [Google Scholar] [CrossRef]
- Barnes, D.C.; Hofacer, R.D.; Zaman, A.R.; Rennaker, R.L.; Wilson, D.A. Olfactory perceptual stability and discrimination. Nat. Neurosci. 2008, 11, 1378–1380. [Google Scholar] [CrossRef]
- Aqrabawi, A.J.; Kim, J.C. Olfactory memory representations are stored in the anterior olfactory nucleus. Nat. Commun. 2020, 11, 1246. [Google Scholar] [CrossRef]
- Xu, W.; Wilson, D.A. Odor-evoked activity in the mouse lateral entorhinal cortex. Neuroscience 2012, 223, 12–20. [Google Scholar] [CrossRef]
- Cao, L.; Schrank, B.R.; Rodriguez, S.; Benz, E.G.; Moulia, T.W.; Rickenbacher, G.T.; Gomez, A.C.; Levites, Y.; Edwards, S.R.; Golde, T.E.; et al. Abeta alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat. Commun. 2012, 3, 1009. [Google Scholar] [CrossRef]
- Macknin, J.B.; Higuchi, M.; Lee, V.M.; Trojanowski, J.Q.; Doty, R.L. Olfactory dysfunction occurs in transgenic mice overexpressing human tau protein. Brain Res. 2004, 1000, 174–178. [Google Scholar] [CrossRef]
- Wesson, D.W.; Levy, E.; Nixon, R.A.; Wilson, D.A. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J. Neurosci. 2010, 30, 505–514. [Google Scholar] [CrossRef]
- Saiz-Sanchez, D.; De La Rosa-Prieto, C.; Ubeda-Banon, I.; Martinez-Marcos, A. Interneurons and beta-amyloid in the olfactory bulb, anterior olfactory nucleus and olfactory tubercle in APPxPS1 transgenic mice model of Alzheimer’s disease. Anat. Rec. 2013, 296, 1413–1423. [Google Scholar] [CrossRef]
- He, B.; Zheng, M.; Liu, Q.; Shi, Z.; Long, S.; Lu, X.; Pei, Z.; Yuan, T.F.; Su, H.; Yao, X. Injected Amyloid Beta in the Olfactory Bulb Transfers to Other Brain Regions via Neural Connections in Mice. Mol. Neurobiol. 2018, 55, 1703–1713. [Google Scholar] [CrossRef]
- Wesson, D.W.; Wilson, D.A.; Nixon, R.A. Should olfactory dysfunction be used as a biomarker of Alzheimer’s disease? Expert Rev. Neurother. 2010, 10, 633–635. [Google Scholar] [CrossRef]
- Jung, H.J.; Shin, I.S.; Lee, J.E. Olfactory function in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Laryngoscope 2019, 129, 362–369. [Google Scholar] [CrossRef]
- Roberts, R.O.; Christianson, T.J.; Kremers, W.K.; Mielke, M.M.; Machulda, M.M.; Vassilaki, M.; Alhurani, R.E.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol. 2016, 73, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Stamps, J.J.; Bartoshuk, L.M.; Heilman, K.M. A brief olfactory test for Alzheimer’s disease. J. Neurol. Sci. 2013, 333, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Etievant, A.; Monnin, J.; Lihoreau, T.; Tamadazte, B.; Rougeot, P.; Magnin, E.; Tavernier, L.; Pazart, L.; Haffen, E. Comparison of Noninvasive Imagery Methods to Observe Healthy and Degenerated Olfactory Epithelium in Mice for the Early Diagnosis of Neurodegenerative Diseases. Front. Neuroanat. 2020, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, D.; Yin, J.; Wang, Z.; Zhou, Y.; Ni, H.; Liu, Y. Olfactory fMRI Activation Pattern Across Different Concentrations Changes in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 786. [Google Scholar] [CrossRef]
- Yang, Q.; Song, D.; Qing, H. Neural changes in Alzheimer’s disease from circuit to molecule: Perspective of optogenetics. Neurosci. Biobehav. Rev. 2017, 79, 110–118. [Google Scholar] [CrossRef]
- Wang, X.; Ren, P.; Mapstone, M.; Conwell, Y.; Porsteinsson, A.P.; Foxe, J.J.; Raizada, R.D.S.; Lin, F.; and the Alzheimer’s Disease Neuroimaging, I. Identify a shared neural circuit linking multiple neuropsychiatric symptoms with Alzheimer’s pathology. Brain Imaging Behav. 2019, 13, 53–64. [Google Scholar] [CrossRef]
- Harris, S.S.; Wolf, F.; De Strooper, B.; Busche, M.A. Tipping the Scales: Peptide-Dependent Dysregulation of Neural Circuit Dynamics in Alzheimer’s Disease. Neuron 2020, 107, 417–435. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Golde, T.E.; DeKosky, S.T.; Galasko, D. Alzheimer’s disease: The right drug, the right time. Science 2018, 362, 1250–1251. [Google Scholar] [CrossRef]
- Ordaz, J.D.; Wu, W.; Xu, X.M. Optogenetics and its application in neural degeneration and regeneration. Neural Regen. Res. 2017, 12, 1197–1209. [Google Scholar] [CrossRef]
- Yang, X.; Yao, C.; Tian, T.; Li, X.; Yan, H.; Wu, J.; Li, H.; Pei, L.; Liu, D.; Tian, Q.; et al. A novel mechanism of memory loss in Alzheimer’s disease mice via the degeneration of entorhinal-CA1 synapses. Mol. Psychiatry 2018, 23, 199–210. [Google Scholar] [CrossRef]
- Benn, A.; Barker, G.R.; Stuart, S.A.; Roloff, E.V.; Teschemacher, A.G.; Warburton, E.C.; Robinson, E.S. Optogenetic Stimulation of Prefrontal Glutamatergic Neurons Enhances Recognition Memory. J. Neurosci. 2016, 36, 4930–4939. [Google Scholar] [CrossRef]
- Etter, G.; van der Veldt, S.; Manseau, F.; Zarrinkoub, I.; Trillaud-Doppia, E.; Williams, S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat. Commun. 2019, 10, 5322. [Google Scholar] [CrossRef]
- Song, J.; Patel, R.V.; Sharif, M.; Ashokan, A.; Michaelides, M. Chemogenetics as a neuromodulatory approach to treating neuropsychiatric diseases and disorders. Mol. Ther. 2021, 30, 990–1005. [Google Scholar] [CrossRef]
- Yuan, P.; Grutzendler, J. Attenuation of beta-Amyloid Deposition and Neurotoxicity by Chemogenetic Modulation of Neural Activity. J. Neurosci. 2016, 36, 632–641. [Google Scholar] [CrossRef]
- Rorabaugh, J.M.; Chalermpalanupap, T.; Botz-Zapp, C.A.; Fu, V.M.; Lembeck, N.A.; Cohen, R.M.; Weinshenker, D. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 2017, 140, 3023–3038. [Google Scholar] [CrossRef]
- Reed, T.; Cohen Kadosh, R. Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Weiler, M.; Stieger, K.C.; Long, J.M.; Rapp, P.R. Transcranial Magnetic Stimulation in Alzheimer’s Disease: Are We Ready? eNeuro 2020, 7, ENEURO.0235-19.2019. [Google Scholar] [CrossRef]
- Marron, E.M.; Viejo-Sobera, R.; Quintana, M.; Redolar-Ripoll, D.; Rodriguez, D.; Garolera, M. Transcranial magnetic stimulation intervention in Alzheimer’s disease: A research proposal for a randomized controlled trial. BMC Res. Notes 2018, 11, 648. [Google Scholar] [CrossRef]
- Floel, A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage 2014, 85 Pt 3, 934–947. [Google Scholar] [CrossRef]
- Khedr, E.M.; Salama, R.H.; Abdel Hameed, M.; Abo Elfetoh, N.; Seif, P. Therapeutic Role of Transcranial Direct Current Stimulation in Alzheimer Disease Patients: Double-Blind, Placebo-Controlled Clinical Trial. Neurorehabil. Neural Repair 2019, 33, 384–394. [Google Scholar] [CrossRef]
- Fariba, K.; Gupta, V. Deep Brain Stimulation; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Perlmutter, J.S.; Mink, J.W. Deep brain stimulation. Annu. Rev. Neurosci. 2006, 29, 229–257. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, Y.; Tian, X.; Zheng, X.; Wang, X.; Li, W.; Wu, X.; Shu, B.; Hou, W. Deep Brain Stimulation for Alzheimer’s Disease: Stimulation Parameters and Potential Mechanisms of Action. Front. Aging Neurosci. 2021, 13, 619543. [Google Scholar] [CrossRef]
- Jeong, D.U.; Lee, J.E.; Lee, S.E.; Chang, W.S.; Kim, S.J.; Chang, J.W. Improvements in memory after medial septum stimulation are associated with changes in hippocampal cholinergic activity and neurogenesis. Biomed. Res. Int. 2014, 2014, 568587. [Google Scholar] [CrossRef]
- Laxton, A.W.; Tang-Wai, D.F.; McAndrews, M.P.; Zumsteg, D.; Wennberg, R.; Keren, R.; Wherrett, J.; Naglie, G.; Hamani, C.; Smith, G.S.; et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann. Neurol. 2010, 68, 521–534. [Google Scholar] [CrossRef]
- Chan, D.; Suk, H.J.; Jackson, B.; Milman, N.P.; Stark, D.; Beach, S.D.; Tsai, L.H. Induction of specific brain oscillations may restore neural circuits and be used for the treatment of Alzheimer’s disease. J. Intern. Med. 2021, 290, 993–1009. [Google Scholar] [CrossRef]
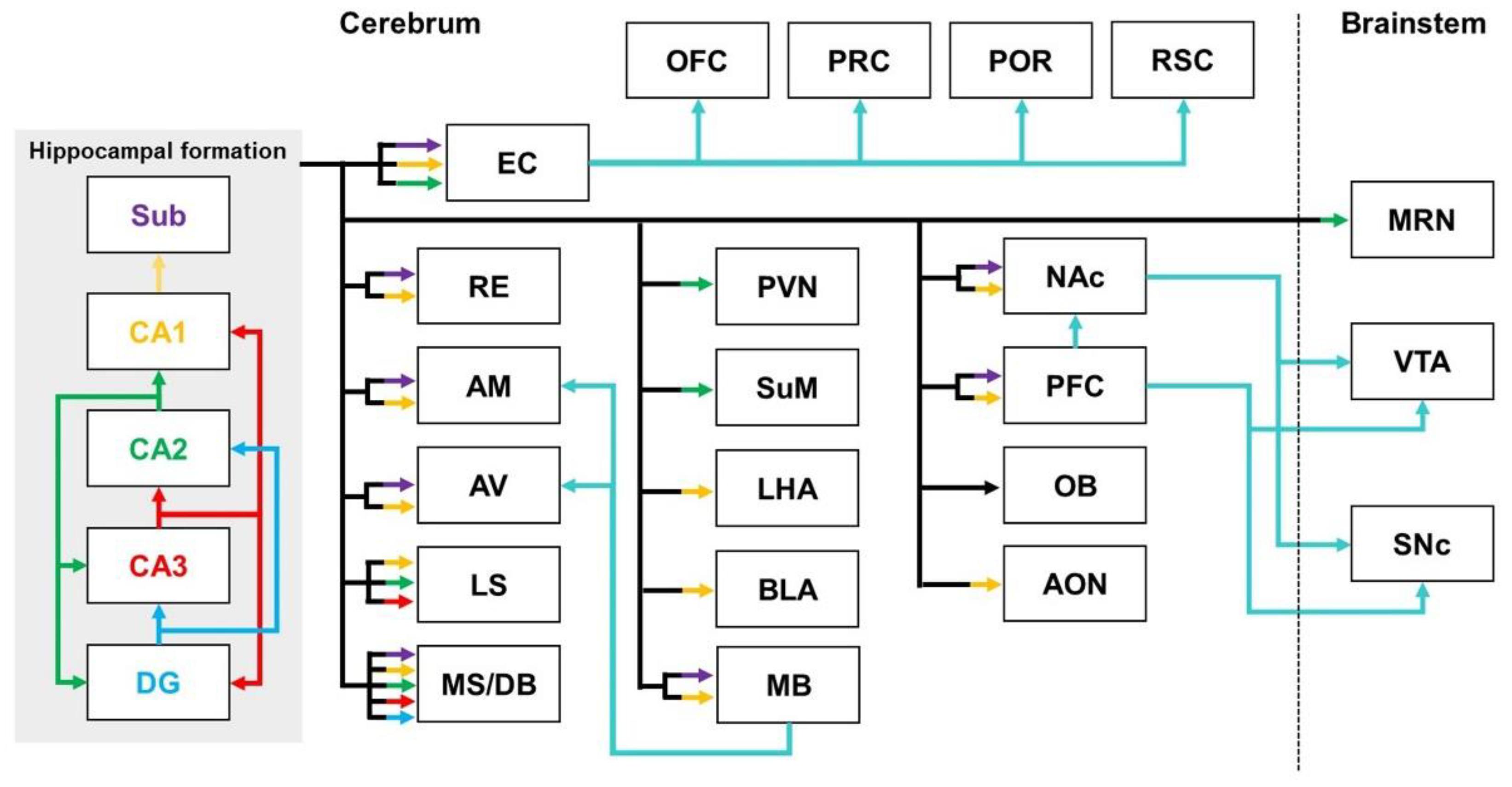
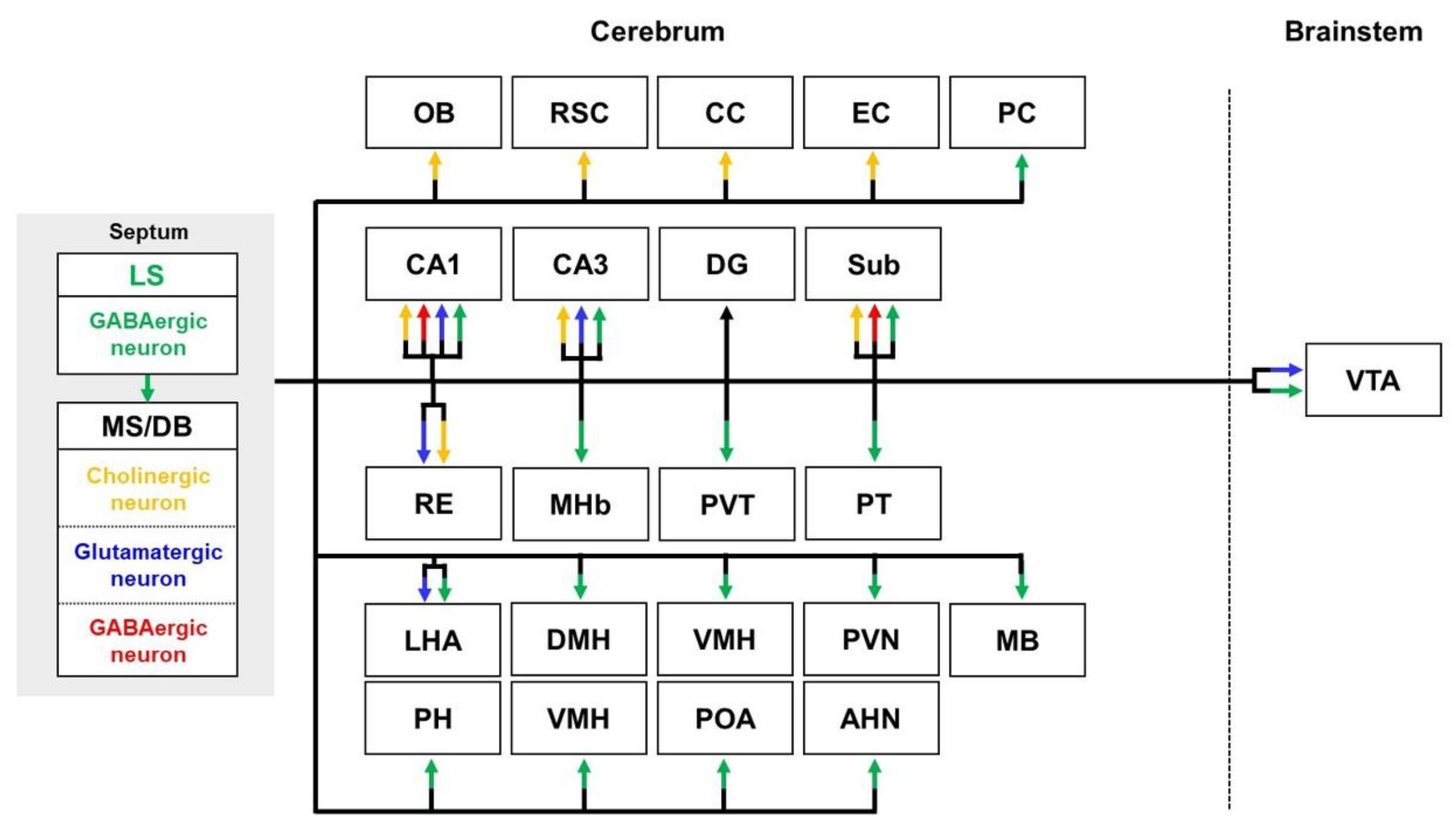

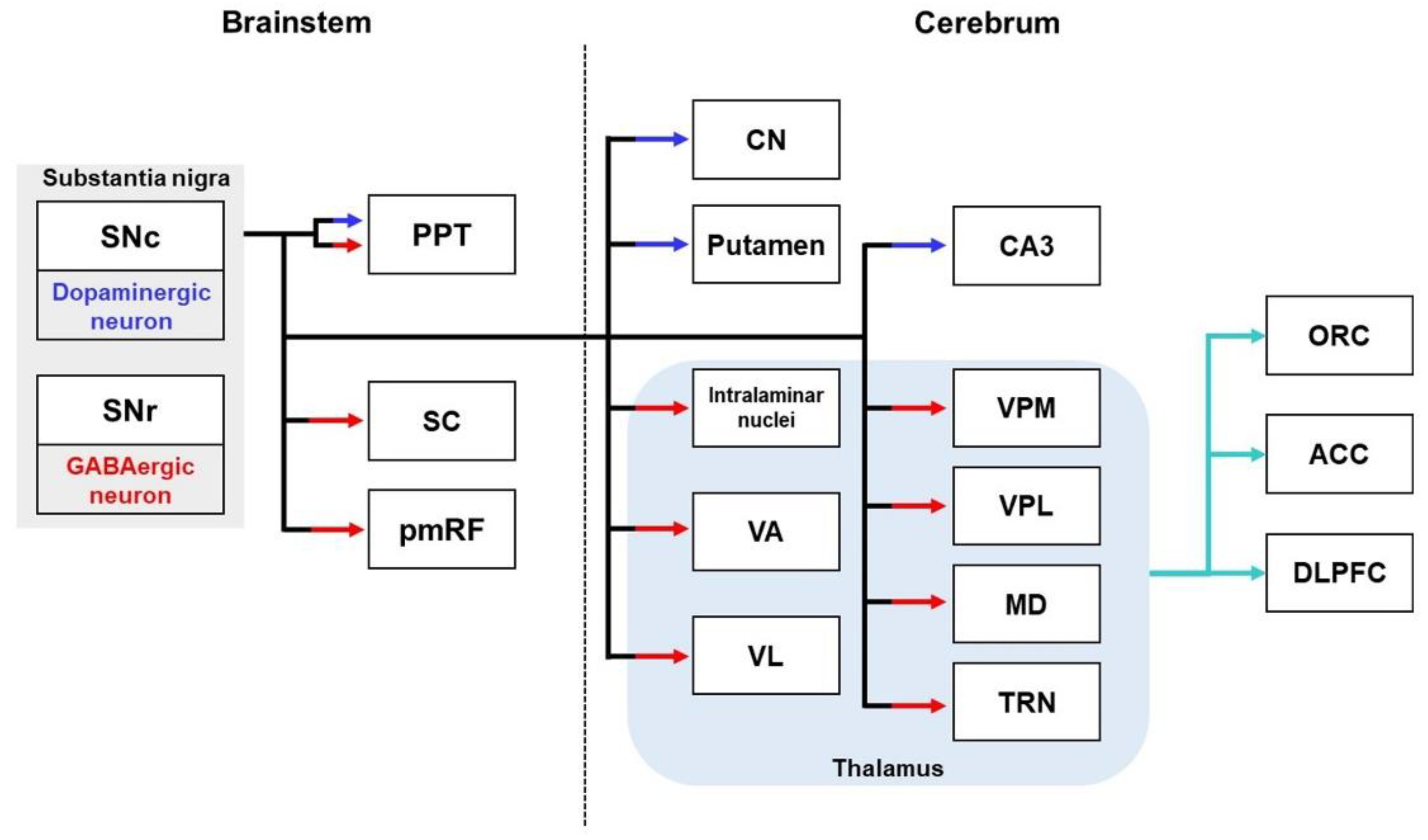



| Regions | Models | Neural Tracers | Findings | References | |
|---|---|---|---|---|---|
| Hippocampal formation | Tg2576 mouse | Fast Blue | Subiculum → NAc ↓ | Decreased glutamatergic transmission from the subiculum to the NAc core. | [51] |
| 5XFAD mice | DiI | Hippocampal formation → MS ↓ | The DG→MS and Sub→MS pathways was degenerated before cognitive decline. | [40] | |
| Septal area | Tg601 mice | DTIDTT | MS → hippocampus ↓ | The connectivity of the septo-hippocampal pathway in the old (16- to 18-month-old) mice was reduced compared to healthy and adult (six- to eight-month-old) mice. | [67] |
| THY-Tau22 mice | FG | MS → hippocampus ↓ | Innervation from the MS to the hippocampus decreased in the 5XFAD mice compared to WT mice. | [68] | |
| J20 mice | BDA | MS → hippocampus ↓ | GABAergic septo-hippocampal connection was reduced in eight-month-old J20 mice compared to WT mice. | [69] | |
| VLW mice | BDA | MS → hippocampus ↓ | The GABAergic septo-hippocampal innervation on parvalbumin-positive interneurons deteriorated in two-month-old VLW mice compared to WT mice. | [70] | |
| 5XFAD mice | DiI | MS → hippocampus ↓ | Innervation from the MS to the hippocampus decreased by about 52% in the 5XFAD mice compared to WT mice. | [12] | |
| 5XFAD mice | BDA | MS → hippocampal formation ↓ | Impairment of the connectivity of the septo-hippocampal pathway occurred before cognitive decline. | [40] | |
| Locus coeruleus | 5XFAD mice | DiI | LC → hippocampus ↓ | Innervation from the LC to the hippocampus decreased by about 69.1% in the 5XFAD mice compared to WT mice. | [12] |
| Substantia nigra | 5XFAD mice | DiI | SN → hippocampus ↓ | Innervation from the SN to the hippocampus decreased by about 41.3% in the 5XFAD mice compared to WT mice. | [12] |
| Visual area | 3xTg mice | Cholera toxin beta subunit | Retina → superior colliculus ↓ | The retino-collicular pathway through which RGCs reach the terminals in the superior colliculus, which is the primary target of RGCs, is impaired in three-month-old 3xTg mice. | [71] |
| Olfactory area | 5XFAD mice | DiI | OB → hippocampus ↓ | Innervation from the OB to the hippocampus decreased by about 52% in the 5XFAD mice compared to WT mice. | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Nam, Y.; Kim, H.s.; Jung, H.; Jeon, S.G.; Hong, S.B.; Moon, M. Alteration of Neural Pathways and Its Implications in Alzheimer’s Disease. Biomedicines 2022, 10, 845. https://doi.org/10.3390/biomedicines10040845
Kim S, Nam Y, Kim Hs, Jung H, Jeon SG, Hong SB, Moon M. Alteration of Neural Pathways and Its Implications in Alzheimer’s Disease. Biomedicines. 2022; 10(4):845. https://doi.org/10.3390/biomedicines10040845
Chicago/Turabian StyleKim, Sujin, Yunkwon Nam, Hyeon soo Kim, Haram Jung, Seong Gak Jeon, Sang Bum Hong, and Minho Moon. 2022. "Alteration of Neural Pathways and Its Implications in Alzheimer’s Disease" Biomedicines 10, no. 4: 845. https://doi.org/10.3390/biomedicines10040845
APA StyleKim, S., Nam, Y., Kim, H. s., Jung, H., Jeon, S. G., Hong, S. B., & Moon, M. (2022). Alteration of Neural Pathways and Its Implications in Alzheimer’s Disease. Biomedicines, 10(4), 845. https://doi.org/10.3390/biomedicines10040845






