The Biosynthesis and Medicinal Properties of Taraxerol
Abstract
1. Introduction
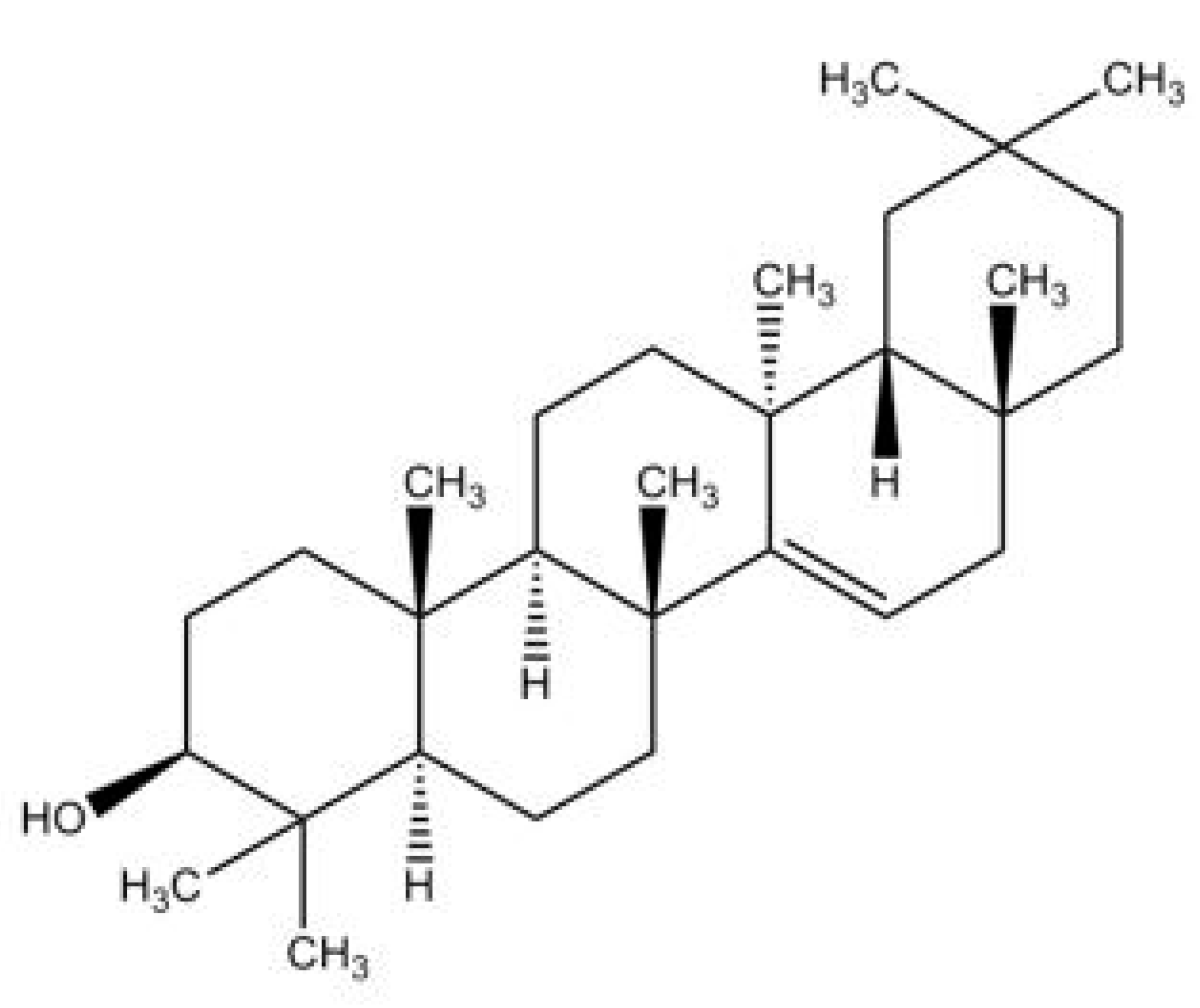
2. Taraxerol
2.1. Distribution of Taraxerol in the Plant Kingdom
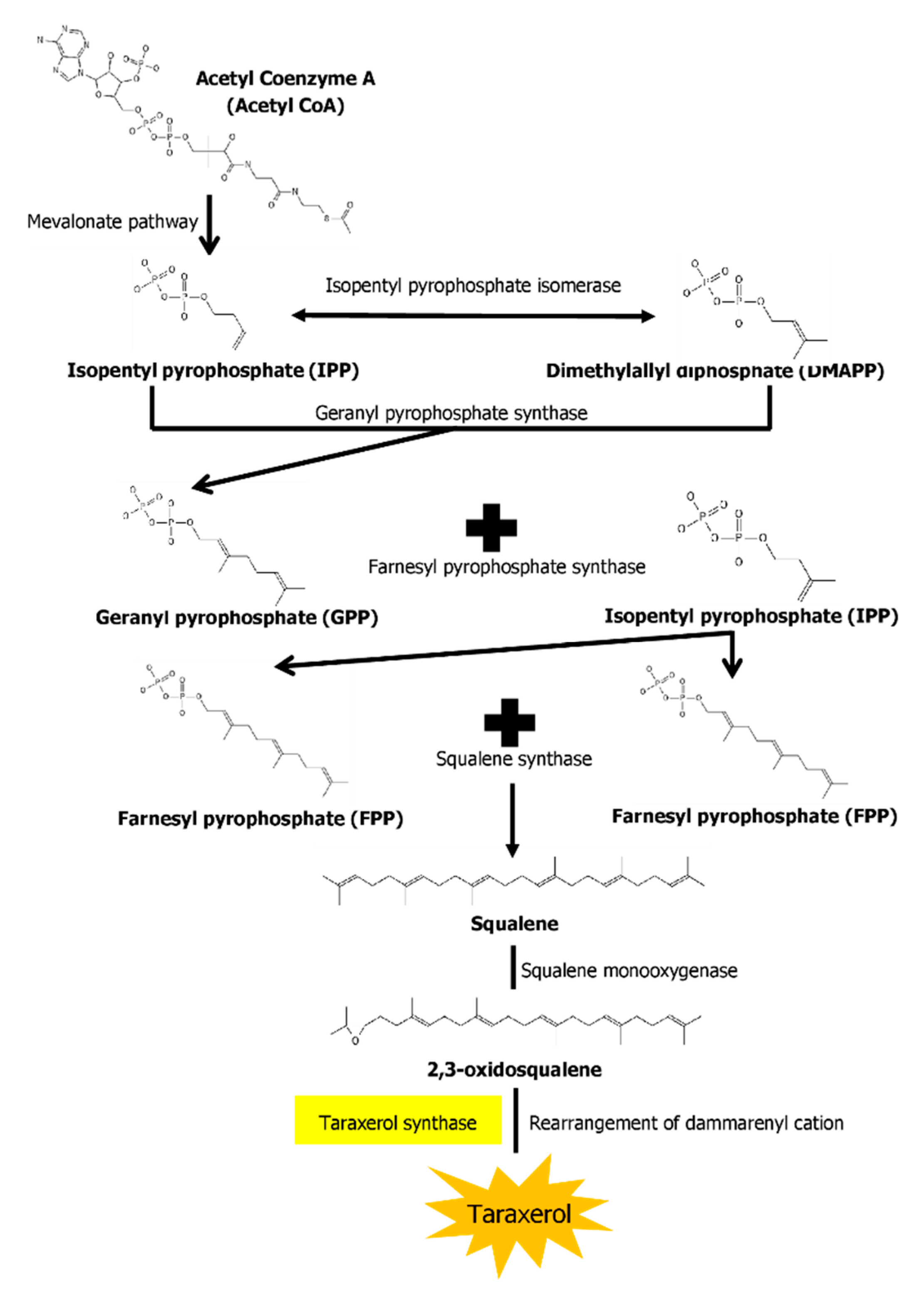
2.2. Biosynthesis Pathway of Taraxerol
3. Medicinal Properties of Taraxerol
3.1. Antioxidative Properties
3.2. Antimicrobial Properties
3.3. Anti-Fungal Properties
3.4. Cytotoxic Properties
3.5. Anti-Diabetic Properties
3.6. Anti-Inflammatory Properties
3.7. Treatment for Neurodegenerative Diseases
3.8. Other Notable Pharmacological Properties of Taraxerol
4. In Vitro Production of Taraxerol
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salim, A.A.; Chin, Y.-W.; Kinghorn, A.D. Drug Discovery from Plants. In Bioactive Molecules and Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–24. [Google Scholar] [CrossRef]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. Natural Products to Drugs: Natural Product-Derived Compounds in Clinical Trials. Nat. Prod. Rep. 2008, 25, 475. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The Pentacyclic Triterpenoids in Herbal Medicines and Their Pharmacological Activities in Diabetes and Diabetic Complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Sun, H. Synthesis, Biology and Clinical Significance of Pentacyclic Triterpenes: A Multi-Target Approach to Prevention and Treatment of Metabolic and Vascular Diseases. Nat. Prod. Rep. 2011, 28, 543. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, Y.; Chanev, C.; Dentchev, T.; Vitanova, D. Triterpenoids and sterols from Hypericum perforatum. Comptes Rendus Acad. Bulg. 2003, 56, 37–40. [Google Scholar]
- Kumar, V.; Mukherjee, K.; Kumar, S.; Mal, M.; Mukherjee, P.K. Validation of HPTLC Method for the Analysis of Taraxerol in Clitoria ternatea. Phytochem. Anal. 2007, 19, 244–250. [Google Scholar] [CrossRef]
- Sangeetha, K.N.; Shilpa, K.; Jyothi Kumari, P.; Lakshmi, B.S. Reversal of Dexamethasone Induced Insulin Resistance in 3T3L1 Adipocytes by 3β-Taraxerol of Mangifera Indica. Phytomedicine 2013, 20, 213–220. [Google Scholar] [CrossRef]
- Koay, Y.C.; Wong, K.C.; Hasnah, O.; Ibrahim, E.M.S.; Mohammad, Z.A. Chemical constituents and biological activities of Strobilanthes crispus L. Rec. Nat. Prod. 2013, 7, 59–64. [Google Scholar]
- Takasaki, M.; Konoshima, T.; Tokuda, H.; Masuda, K.; Arai, Y.; Shiojima, K.; Ageta, H. Anti-Carcinogenic Activity of Taraxacum Plant. I. Biol. Pharm. Bull. 1999, 22, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sahu, P.M.; Sharma, M.K. Anti-Inflammatory and Antimicrobial Activities of Triterpenoids from Strobilanthes Callosus Nees. Phytomedicine 2002, 9, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Biswas, K.; Ghosh, A.K.; Haldar, P.K. A pentacyclic triterpenoid possessing anti-inflammatory activity from the fruits of Dregea volubilis. Pharmacogn. Mag. 2009, 5, 64–68. [Google Scholar] [CrossRef]
- Ngo, S.T.; Li, M.S. Top-Leads from Natural Products for Treatment of Alzheimer’s Disease: Docking and Molecular Dynamics Study. Mol. Simul. 2013, 39, 279–291. [Google Scholar] [CrossRef]
- Sharma, K.; Zafar, R. Optimization of Methyl Jasmonate and β-Cyclodextrin for Enhanced Production of Taraxerol and Taraxasterol in (Taraxacum officinale Weber) Cultures. Plant Physiol. Biochem. 2016, 103, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Beaton, J.M.; Spring, F.S.; Stevenson, R.; Stewart, J.L. Triterpenoids. Part XXXVII. The Constitution of Taraxerol. J. Chem. Soc. (Resumed) 1955, 2131–2137. [Google Scholar] [CrossRef]
- Gururaja, G.; Mundkinajeddu, D.; Dethe, S.; Sangli, G.; Abhilash, K.; Agarwal, A. Cholesterol Esterase Inhibitory Activity of Bioactives from Leaves of Mangifera indica L. Pharmacogn. Res. 2015, 7, 355. [Google Scholar] [CrossRef]
- Du, J.; Gao, L. Chemical constituents of the leaves of Acanthopanax trifoliatus (Linn) Merr. Zhongguo Zhong Yao Za Zhi 1992, 17, 356–357. [Google Scholar]
- Phan, M.G.; Chinh Truong, T.T.; Phan, T.S.; Matsunami, K.; Otsuka, H. Mangiferonic Acid, 22-Hydroxyhopan-3-One, and Physcion as Specific Chemical Markers for Alnus nepalensis. Biochem. Syst. Ecol. 2010, 38, 1065–1068. [Google Scholar] [CrossRef]
- Correia Da Silva, T.B.; Souza, V.K.T.; Da Silva, A.P.F.; Lyra Lemos, R.P.; Conserva, L.M. Determination of the Phenolic Content and Antioxidant Potential of Crude Extracts and Isolated Compounds from Leaves of Cordia multispicata and Tournefortia bicolor. Pharm. Biol. 2009, 48, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.d.C.C.; Machado, D.C.; da Silva, J.M.; Conegundes, J.L.M.; Gualberto, A.C.M.; Gameiro, J.; Moreira Chedier, L.; Castañon, M.C.M.N.; Scio, E. Pereskia aculeata Miller Leaves Present In Vivo Topical Anti-Inflammatory Activity in Models of Acute and Chronic Dermatitis. J. Ethnopharmacol. 2015, 173, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Mokoka, T.A.; McGaw, L.J.; Mdee, L.K.; Bagla, V.P.; Iwalewa, E.O.; Eloff, J.N. Antimicrobial Activity and Cytotoxicity of Triterpenes Isolated from Leaves of Maytenus undata (Celastraceae). BMC Complement. Altern. Med. 2013, 13, 111. [Google Scholar] [CrossRef]
- Tsao, C.-C.; Shen, Y.-C.; Su, C.-R.; Li, C.-Y.; Liou, M.-J.; Dung, N.-X.; Wu, T.-S. New Diterpenoids and the Bioactivity of Erythrophleum fordii. Bioorg. Med. Chem. 2008, 16, 9867–9870. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Jiménez-Estrada, M.; Cruz-Ortega, R.; Anaya, A.L. Pentacyclic Triterpenes with Selective Bioactivity from Sebastiania adenophora Leaves, Euphorbiaceae. J. Chem. Ecol. 2006, 33, 147–156. [Google Scholar] [CrossRef]
- Falodun, A.; Qadir, M.I.; Chouldary, M.I. Isolation and characterization of xanthine oxidase inhibitory constituents of Pyrenacantha staudtii. Yao Xue Xue Bao 2009, 44, 390–394. [Google Scholar] [PubMed]
- Hu, H.; Liu, Q.; Yang, Y.; Yang, L.; Wang, Z. Chemical constituents of Clerodendrum trichotomum Leaves. Zhong Yao Cai 2014, 37, 1590–1593. [Google Scholar]
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Sahu, R.; Gangopadhyay, M.; De Feo, V.; Zia-Ul-Haq, M. Abroma Augusta L. (Malvaceae) Leaf Extract Attenuates Diabetes Induced Nephropathy and Cardiomyopathy via Inhibition of Oxidative Stress and Inflammatory Response. J. Transl. Med. 2015, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Liu, D. Cloning and Characterization of Farnesyl Diphosphate Synthase Gene Involved in Triterpenoids Biosynthesis from Poria cocos. Int. J. Mol. Sci. 2014, 15, 22188–22202. [Google Scholar] [CrossRef]
- Cao, S.; Brodie, P.; Miller, J.S.; Birkinshaw, C.; Rakotondrafara, A.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Antiproliferative Compounds of Helmiopsis sphaerocarpa from the Madagascar Rainforest. Nat. Prod. Res. 2009, 23, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Naik, D.G.; Mujumdar, A.M.; Waghole, R.J.; Misar, A.V.; Bligh, S.W.; Bashall, A.; Crowder, J. Taraxer-14-En-3β-Ol, an Anti-Inflammatory Compound from Sterculia foetida L. Planta Med. 2004, 70, 68–69. [Google Scholar] [CrossRef]
- Manguro, L.O.A.; Onyango Okwiri, S.; Lemmen, P. Oleanane-Type Triterpenes of Embelia schimperi Leaves. Phytochemistry 2006, 67, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.E.; Cechinel Filho, V.; Cruz, R.C.B.; Meyre-Silva, C.; Cruz, A.B. Antifungal activity of Eugenia umbelliflora against dermatophytes. Nat. Prod. Commun. 2009, 4, 1181–1184. [Google Scholar] [CrossRef]
- Raja Naika, H.; Krishna, V.; Lingaraju, K.; Chandramohan, V.; Dammalli, M.; Navya, P.N.; Suresh, D. Molecular Docking and Dynamic Studies of Bioactive Compounds from Naravelia zeylanica (L.) DC against Glycogen Synthase Kinase-3β Protein. J. Taibah Univ. Sci. 2015, 9, 41–49. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Li, H.; Chen, H.; Li, P.; Ye, B. Chemical constituents in the leave of Rhizophora stylosa L and their biological activities. Yao Xue Xue Bao 2008, 43, 974–978. [Google Scholar]
- Williams, L.A.D. Rhizophora mangle (Rhizophoraceae) Triterpenoids with Insecticidal Activity. Naturwissenschaften 1999, 86, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Pensec, F.; Szakiel, A.; Pączkowski, C.; Woźniak, A.; Grabarczyk, M.; Bertsch, C.; Fischer, M.J.C.; Chong, J. Characterization of Triterpenoid Profiles and Triterpene Synthase Expression in the Leaves of Eight Vitis vinifera Cultivars Grown in the Upper Rhine Valley. J. Plant Res. 2016, 129, 499–512. [Google Scholar] [CrossRef]
- Okoth, D.A.; Koorbanally, N.A. Cardanols, Long Chain Cyclohexenones and Cyclohexenols from Lannea schimperi (Anacardiaceae). Nat. Prod. Commun. 2015, 10, 1934578X1501000. [Google Scholar] [CrossRef]
- Padmaja, V.; Thankamany, V.; Hisham, A. Antibacterial, Antifungal and Anthelmintic Activities of Root Barks of Uvaria hookeri and Uvaria narum. J. Ethnopharmacol. 1993, 40, 181–186. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, S.; Zeng, F.; Xie, L.; Shen, Z. Antinociceptive and Anti-Inflammatory Activities of Schefflera octophylla Extracts. J. Ethnopharmacol. 2015, 171, 42–50. [Google Scholar] [CrossRef]
- Rashid, M.-U.; Alamzeb, M.; Ali, S.; Ahmad Khan, A.; Igoli, J.O.; Ferro, V.A.; Gray, A.I.; Rafiullah Khan, M. A new ceramide along with eight known compounds from the roots of Artemisia incisa pamp. Rec. Nat. Prod. 2014, 9, 294–304. [Google Scholar]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Vasas, A.; Kele, Z.; Hohmann, J. Antiproliferative Constituents of the Roots of Conyza canadensis. Planta Med. 2011, 77, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-M.; Liu, X.-K.; Qing, C.; Wu, D.-G.; Zhu, D.-Y. Chemical constituents from the roots of Homonoia riparia. Yao Xue Xue Bao 2007, 42, 292–296. [Google Scholar] [PubMed]
- Duan, J.; Wang, L.; Qian, S.; Su, S.; Tang, Y. A New Cytotoxic Prenylated Dihydrobenzofuran Derivative and Other Chemical Constituents from the Rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.P.; Lee, H.J.; Lee, D.-U.; Lee, S.K.; Hong, J.-H.; Lee, C.J. Effects of Lupenone, Lupeol, and Taraxerol Derived From Adenophora triphyllaon the Gene Expression and Production of Airway MUC5AC Mucin. Tuberc. Respir. Dis. 2015, 78, 210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.-W. Studies on chemical constituents of root tuber of cultivated Pseudostellaria heterophylla (Zheshen No. 1). Zhongguo Zhong Yao Za Zhi 2008, 33, 2353–2355. [Google Scholar]
- Xiang, Y.; Zhang, C.; Zheng, Y. Studies on the chemical constituents of the roots of Rhododendron molle G. Don. J. Huazhong Univ. Sci. Technol. 2004, 24, 202–204. [Google Scholar]
- Liu, Y.-M.; Tian, D.; Bao, H.; Zhao, G.-L.; Wang, J.-X. Study on chemical constituents of root bark of Discocleidion rufescens. Zhong Yao Cai 2012, 35, 1795–1798. [Google Scholar]
- Kaennakam, S.; Sichaem, J.; Khumkratok, S.; Siripong, P.; Tip-pyang, S. A New Taraxerol Derivative from the Roots of Microcos tomentosa. Nat. Prod. Commun. 2013, 8, 1934578X1300801. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Shang, X.-Y.; Cui, B.-S.; Yuan, Y.; Chen, X.-G.; Yang, Y.-C.; Shi, J.-G. Triterpenoids from the Roots of Pterospermum heterophyllum Hance. J. Asian Nat. Prod. Res. 2009, 11, 652–657. [Google Scholar] [CrossRef]
- Ango, P.Y.; Kapche, D.W.F.G.; Fotso, G.W.; Fozing, C.D.; Yeboah, E.M.O.; Mapitse, R.; Demirtas, I.; Ngadjui, B.T.; Yeboah, S.O. Thonningiiflavanonol A and Thonningiiflavanonol B, Two Novel Flavonoids, and Other Constituents of Ficus thonningii Blume (Moraceae). Z. Naturforsch. 2016, 71, 65–71. [Google Scholar] [CrossRef]
- Paul, B.D.; Subba Rao, G.; Kapadia, G.J. Isolation of Myricadiol, Myricitrin, Taraxerol, and Taraxerone from Myrica cerifera L. Root Bark. J. Pharm. Sci. 1974, 63, 958–959. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Cai, X.F.; Na, M.; Lee, J.J.; Bae, K. Triterpenoids and Diarylheptanoids from Alnus hirsuta Inhibit HIF-1 in Ags Cells. Arch. Pharm. Res. 2007, 30, 412–418. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Chen, Z.; Min, Z.; Lou, F. Two Novel C29-5β-Sterols from the Stems of Opuntia dillenii. Steroids 2006, 71, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Al Muqarrabun, L.M.R.; Ahmat, N.; Aris, S.R.S.; Norizan, N.; Shamsulrijal, N.; Yusof, F.Z.M.; Suratman, M.N.; Yusof, M.I.M.; Salim, F. A New Triterpenoid from Sapium baccatum (Euphorbiaceae). Former. Nat. Prod. Res. 2014, 28, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Cornelio, K.B. Triterpenes from Euphorbia hirta and Their Cytotoxicity. Chin. J. Nat. Med. 2013, 11, 528–533. [Google Scholar] [CrossRef]
- Mawa, S.; Jantan, I.; Husain, K. Isolation of Terpenoids from the Stem of Ficus aurantiaca Griff and Their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils. Molecules 2016, 21, 9. [Google Scholar] [CrossRef]
- Somwong, P.; Suttisri, R.; Buakeaw, A. New Sesquiterpenes and Phenolic Compound from Ficus foveolata. Fitoterapia 2013, 85, 1–7. [Google Scholar] [CrossRef]
- Lin, L.-C.; Chou, C.-J.; Kuo, Y.-C. Cytotoxic Principles from Ventilago leiocarpa. J. Nat. Prod. 2001, 64, 674–676. [Google Scholar] [CrossRef]
- Si, X.; Wei, S.; Xu, X.; Fang, X.; Wu, W. Chemical constituents in the leaves of Mangifera persiciformis C.Y. Wu et Y.L. Ming. Zhongguo Zhong Yao Za Zhi 1995, 20, 295–296. [Google Scholar]
- Lu, R.-M.; Su, X.; Zhou, Y.-Y.; Wei, J.-H. Study on the chemical constituents of Uvaria microcarpa. Zhong Yao Cai 2009, 32, 1056–1059. [Google Scholar]
- Zhang, H.; Wang, S.; Chen, R.; Yu, D. Studies on chemical constituents of Uvaria macrophylia. Yao Xue Xue Bao 2002, 37, 124–127. [Google Scholar] [PubMed]
- Marzouk, A.M.; Osman, S.M.; Gohar, A.A. A New Pregnane Glycoside from Gomphocarpus fruticosus Growing in Egypt. Nat. Prod. Res. 2015, 30, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Rios, M.Y. Active Compounds against Tinea Pedis Dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Gawrońska-Grzywacz, M.; Krzaczek, T. Identification and Determination of Triterpenoids in Hieracium pilosella L. J. Sep. Sci. 2007, 30, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene Alcohols from the Flowers of Compositae and Their Anti-Inflammatory Effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Saeecd, M.T.; Agarwal, R.; Khan, M.W.Y.; Ahmad, F.; Osman, S.M.; Akihisa, T.; Suzuki, K.; Matsumoto, T. Unsaponifiable Lipid Constituents of Ten Indian Seed Oils. J. Am. Oil Chem. Soc. 1991, 68, 193–197. [Google Scholar] [CrossRef]
- Dharmaratne, H.R.W.; Sajeevani, J.R.D.M.; Marasinghe, G.P.K.; Ekanayake, E.M.H.G.S. Distribution of Pyranocoumarins in Calophyllum Cordato-Oblongum. Phytochemistry 1998, 49, 995–998. [Google Scholar] [CrossRef]
- Qi, H.; Wang, R.; Liu, Y.; Shi, Y. Studies on the chemical constituents of Codonopsis pilosula. Zhong Yao Cai 2011, 34, 546–548. [Google Scholar]
- Chen, Y.; Zhu, Y.; Wei, J.; Liang, N. Chemical components of Codonopsis pilosula (Franch.) Nannf. var. volubilis (Nannf.) L.T. Shen. Zhongguo Zhong Yao Za Zhi 1995, 20, 611–612. [Google Scholar]
- Jamila, N.; Khairuddean, M.; Yeong, K.K.; Osman, H.; Murugaiyah, V. Cholinesterase Inhibitory Triterpenoids from the Bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2014, 30, 133–139. [Google Scholar] [CrossRef]
- Wang, Z.; Yeats, T.; Han, H.; Jetter, R. Cloning and Characterization of Oxidosqualene Cyclases from Kalanchoe daigremontiana. J. Biol. Chem. 2010, 285, 29703–29712. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, H.; Shen, D.; Yang, B.; Yang, X. Studies on the chemical constituents of Vaccinium iteophyllum. Zhong Yao Cai 2007, 30, 47–49. [Google Scholar] [PubMed]
- Feng, Z.; Wang, Y.; Zhang, P. The chemical constituents of Rhododendron ovatum Planch. Yao Xue Xue Bao 2005, 40, 150–152. [Google Scholar]
- Lee, J.H.; Lee, K.T.; Yang, J.H.; Baek, N.I.; Kim, D.K. Acetylcholinesterase Inhibitors from the Twigs of Vaccinium Oldhami Miquel. Arch. Pharm. Res. 2004, 27, 53–56. [Google Scholar] [CrossRef]
- Khiev, P.; Oh, S.-R.; Chae, H.-S.; Kwon, O.-K.; Ahn, K.-S.; Chin, Y.-W.; Lee, H.-K. Anti-Inflammatory Diterpene from Thyrsanthera suborbicularis. Chem. Pharm. Bull. 2011, 59, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, X.-J.; Li, Y.-F.; Yang, G.-Z. Terpenoids from Euphorbia antiquorum L. Yao Xue Xue Bao 2009, 44, 1118–1122. [Google Scholar] [PubMed]
- Jiang, C.; Mu, S.; Deng, B.; Ge, Y.; Zhang, J.; Hao, X. Studies on the chemical constituents from Euphorbia chrysocoma. Zhong Yao Cai 2009, 32, 1390–1392. [Google Scholar]
- Jang, D.S.; Cuendet, M.; Pawlus, A.D.; Kardono, L.B.S.; Kawanishi, K.; Farnsworth, N.R.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Potential Cancer Chemopreventive Constituents of the Leaves of Macaranga triloba. Phytochemistry 2004, 65, 345–350. [Google Scholar] [CrossRef]
- Setzer, W.N.; Shen, X.; Bates, R.B.; Burns, J.R.; MCClure, K.J.; Zhang, P.; Moriarity, D.M.; Lawton, R.O. A Phytochemical Investigation of Alchornea latifolia. Fitoterapia 2000, 71, 195–198. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Xie, J.-M.; Yao, H.; Lin, X.-Y.; Zhang, Y.-H. Studies on the triterpenoids of Vitex trifolia. Zhong Yao Cai 2010, 33, 908–910. [Google Scholar]
- Gao, L.; Wei, X.; He, Y. Studies on chemical constituents of Clerodendrum bungei. Zhongguo Zhong Yao Za Zhi 2003, 28, 1042–1044. [Google Scholar] [PubMed]
- Yang, Y.; Deng, Z.; Proksch, P.; Lin, W. Two new 18-en-oleane derivatives from marine mangrove plant, Barringtonia racemosa. Pharmazie 2006, 61, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.G.; Tavares, G.L.; Thomaz, L.D.; Sabino, J.R.; Borges, K.B.; Vieira, P.C.; Veiga, T.A.M.; de Souza Borges, W. Taraxerol 4-Methoxybenzoate, Anin Vitro Inhibitor of Photosynthesis Isolated from Pavonia multiflora A. St-Hil. (Malvaceae). Chem. Biodivers. 2016, 13, 284–292. [Google Scholar] [CrossRef]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; de Koning, C.B. A New Cinnamoylglycoflavonoid, Antimycobacterial and Antioxidant Constituents from Heritiera littoralis leaf Extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef]
- Xu, L.-R.; Zhang, S.; Wu, J.; Qi, S.-H.; Huang, J.-S.; Xiao, Z.-H.; Zhang, D.-J.; Yang, J.; Tian, Y. A new triterpenoid: Taraxerol-3-beta-O-tridecyl ether from Derris triofoliata. Pharmazie 2004, 59, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Zhang, B.; Xu, Q.; Li, L.; Hao, X. Study on the chemical constituents of Mitragyna rotundifolia. Zhong Yao Cai 2006, 29, 557–560. [Google Scholar]
- Chaturvedula, V.S.P.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New Cytotoxic Terpenoids from the Wood of Vepris punctata from the Madagascar Rainforest. J. Nat. Prod. 2004, 67, 895–898. [Google Scholar] [CrossRef]
- Gachet, M.S.; Kunert, O.; Kaiser, M.; Brun, R.; Zehl, M.; Keller, W.; Muñoz, R.A.; Bauer, R.; Schuehly, W. Antiparasitic Compounds from Cupania cinerea with Activities against Plasmodium falciparum and Trypanosoma bruceirhodesiense. J. Nat. Prod. 2011, 74, 559–566. [Google Scholar] [CrossRef]
- Misra, G.; Mitra, C.R. Mimusops Hexandra—II. Phytochemistry 1966, 5, 535–538. [Google Scholar] [CrossRef]
- Haliński, Ł.P.; Paszkiewicz, M.; Gołębiowski, M.; Stepnowski, P. The Chemical Composition of Cuticular Waxes from Leaves of the Gboma Eggplant (Solanum macrocarpon L.). J. Food Compost. Anal. 2012, 25, 74–78. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Chang, M.-J.; Seo, H.-W.; Lee, J.-H.; Min, B.-S.; Na, M.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Lee, H.K.; et al. Triterpenoids and a Sterol from the Stem-Bark of Styrax japonicaand Their Protein Tyrosine Phosphatase 1B Inhibitory Activities. Phytother. Res. 2008, 22, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, J. A study on chemical components of Tetrastigma hemsleyanum Diels et Gilg. Native to China. Zhongguo Zhong Yao Za Zhi 1999, 24, 611–612. [Google Scholar] [PubMed]
- Swain, S.S.; Rout, K.K.; Chand, P.K. Production of Triterpenoid Anti-Cancer Compound Taraxerol in Agrobacterium-Transformed Root Cultures of Butterfly Pea (Clitoria ternatea L.). Appl. Biochem. Biotechnol. 2012, 168, 487–503. [Google Scholar] [CrossRef]
- Holstein, S.A.; Hohl, R.J. Isoprenoids: Remarkable Diversity of Form and Function. Lipids 2004, 39, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Miziorko, H.M. Enzymes of the Mevalonate Pathway of Isoprenoid Biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef]
- Delourme, D.; Lacroute, F.; Karst, F. Cloning of an Arabidopsis thaliana CDNA Coding for Farnesyl Diphosphate Synthase by Functional Complementation in Yeast. Plant Mol. Biol. 1994, 26, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.J.S.; Ruiz, J.M. Mechanism of the Biosynthesis of Squalene from Farnesyl Pyrophosphate. Tetrahedron 1987, 43, 2661–2674. [Google Scholar] [CrossRef]
- Tansey, T. Structure and Regulation of Mammalian Squalene Synthase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1529, 49–62. [Google Scholar] [CrossRef]
- Abe, I. Enzymatic Synthesis of Cyclic Triterpenes. Nat. Prod. Rep. 2007, 24, 1311. [Google Scholar] [CrossRef]
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic Cyclization of Squalene and Oxidosqualene to Sterols and Triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Basyuni, M.; Oku, H.; Tsujimoto, E.; Kinjo, K.; Baba, S.; Takara, K. Triterpene Synthases from the Okinawan Mangrove Tribe, Rhizophoraceae. FEBS J. 2007, 274, 5028–5042. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-S.; Na, M.-K.; Oh, S.-R.; Ahn, K.-S.; Jeong, G.-S.; Li, G.; Lee, S.-K.; Joung, H.; Lee, H.-K. New Furofuran and Butyrolactone Lignans with Antioxidant Activity from the Stem Bark of Styrax japonica. J. Nat. Prod. 2004, 67, 1980–1984. [Google Scholar] [CrossRef]
- Hernández-Chávez, I.; Torres-Tapia, L.W.; Simá-Polanco, P.; Cedillo-Rivera, R.; Moo-Puc, R.; Peraza-Sánchez, S.R. Antigiardial Activity of Cupania dentata Bark and Its Constituents. J. Mex. Chem. Soc. 2017, 56, 105–108. [Google Scholar] [CrossRef]
- Simelane, M.; Shonhai, A.; Shode, F.; Smith, P.; Singh, M.; Opoku, A. Anti-Plasmodial Activity of Some Zulu Medicinal Plants and of Some Triterpenes Isolated from Them. Molecules 2013, 18, 12313–12323. [Google Scholar] [CrossRef] [PubMed]
- Warfield, J.; Setzer, W.N.; Ogungbe, I.V. Interactions of Antiparasitic Sterols with Sterol 14α-Demethylase (CYP51) of Human Pathogens. SpringerPlus 2014, 3, 679. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; Beck, R.C.R.; da Silva, C.d.B. Essential Oils for Treatment for Onychomycosis: A Mini-Review. Mycopathologia 2015, 181, 9–15. [Google Scholar] [CrossRef]
- Tan, B.; Shi, H.-L.; Ji, G.; Xie, J.Q. Effects of Taraxerol and Taraxeryl Acetate on Cell Cycle and Apoptosis of Human Gastric Epithelial Cell Line AGS. Chin. J. Integr. Med. 2011, 9, 638–642. [Google Scholar] [CrossRef]
- Sangeetha, K.N.; Sujatha, S.; Muthusamy, V.S.; Anand, S.; Nithya, N.; Velmurugan, D.; Balakrishnan, A.; Lakshmi, B.S. 3β-Taraxerol of Mangifera Indica, a PI3K Dependent Dual Activator of Glucose Transport and Glycogen Synthesis in 3T3-L1 Adipocytes. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 359–366. [Google Scholar] [CrossRef]
- Yao, X.; Li, G.; Bai, Q.; Xu, H.; Lü, C. Taraxerol Inhibits LPS-Induced Inflammatory Responses through Suppression of TAK1 and Akt Activation. Int. Immunopharmacol. 2013, 15, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Godyń, J.; Jończyk, J.; Panek, D.; Malawska, B. Therapeutic Strategies for Alzheimer’s Disease in Clinical Trials. Pharmacol. Rep. 2016, 68, 127–138. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Sharma, N.R.; Singh, B.; Sen, A.; Roy, A. Natural Compounds from Clerodendrum Spp. as Possible Therapeutic Candidates against SARS-CoV-2: An In Silico Investigation. J. Biomol. Struct. Dyn. 2020, 39, 4774–4785. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Sharma, K. Occurrence of Taraxerol and Taraxasterol in Medicinal Plants. Pharmacogn. Rev. 2015, 9, 19. [Google Scholar] [CrossRef] [PubMed]
| Family | Genus | Species | Parts Extracted | Taraxerol Accumulation | Authors, [Ref.] |
|---|---|---|---|---|---|
| Acanthaceae | Strobilanthes | S. callosus | Aerial parts | 0.69% for 5.0 Kg of plant material | [13] |
| S. crispus | Leaves | N/A 1 | [11] | ||
| Anacardiaceae | Lannea | L. schimperi | Stems, bark, and roots | 299 mg/Kg dry weight | [38] |
| Mangifera | M. indica | Leaves | 0.4–0.9% yield 2 | [18] | |
| M. persiciformis | Not specified | N/A | [60] | ||
| Annonaceae | Uvaria | U. microcarpa | Not specified | N/A | [61] |
| U. macrophylla | Not specified | N/A | [62] | ||
| U. hookeri | Bark of the roots | 75 mg/Kg dry weight | [39] | ||
| U. narum | Bark of the roots | 0.04 mg/g dry weight | [39] | ||
| Apocynaceae | Gomphocarpus | G. fruticosus | Aerial parts | 80 mg/Kg dry weight | [63] |
| Araliaceae | Schefflera | S. octophylla | Bark of the roots | N/A | [40] |
| Araliaceae | Acanthopanax | A. trifoliatus | Leaves | N/A | [19] |
| Asteraceae | Artemisia | A. incisa | Roots | 36.67 mg/Kg dry weight | [41] |
| Conyza | C. canadensis | Roots | 4.27 mg/Kg dry weight | [42] | |
| Ageratina | A. pichinchensis var. bustamenta | Aerial parts | 23.88 mg/Kg dry weight | [64] | |
| Crossostephium | C. chinense | Whole plants | N/A | [43] | |
| Atractylodes | A. lancea | Rhizome | N/A | [44] | |
| Hieracum | H. pilosella | Inflorescences | 0.37% of 100 g of plant material | [65] | |
| Taraxacum | T. officinale | Roots | N/A | [12] | |
| Chrysanthemum | C. morifolium (I) | Flowers | 0.2% yield 2 | [66] | |
| C. morifolium (II) | Flowers | 0.4% yield 2 | [66] | ||
| Matricarcia | M. matricarioides | Flowers | 0.1% yield 2 | [66] | |
| Cosmos | C. bipinnatus | Flowers | 1.6% yield 2 | [66] | |
| Carthamus | C. tinctorius | Flowers | 0.6% yield 2 | [66] | |
| Taraxacum | T. platycarpum | Flowers | 0.5% yield 2 | [66] | |
| Betulaceae | Alnus | A. nepalensis | Leaves and twigs | 19.7 mg (leaves) 2 6 mg (twigs) 2 | [20] |
| A. hirsuta | Bark of the stems | 3.03 mg/Kg dry weight | [53] | ||
| Braganiceae | Cordia | C. multispicata | Leaves | 19.05 mg/Kg dry weight | [21] |
| Cactaceae | Pereskia | P. aculeata | Leaves | 7.12% total abundance 3 | [22] |
| Opuntia | O. dillenii | Stems | N/A | [54] | |
| Caesalpiniaceae | Acrocarpus | A. faxinifolius | Seed oils | N/A | [67] |
| Calophyllaceae | Calophyllum | C. cordato-oblongum | Twigs | N/A | [68] |
| Campanulaceae | Adenophora | A. triphylla | Roots | 0.04 mg/g dry weight | [41] |
| Codonopsis | C. pilosula | Not specified | N/A | [69] | |
| C. pilosula var. volubilis | Not specified | N/A | [70] | ||
| caryophyllales | Pseudostellaria | P. heteraphylla | Root tuber | N/A | [46] |
| Casuarinaceae | Casuarina | C. equisetifolia | Seed oils | N/A | [45,67] |
| Celastraceae | Maytenus | M. undata | Leaves | 0.26 mg/g dry weight | [23] |
| Clusiaceae | Garcinia | G. hombroniana | Bark | 2.31 mg/Kg dry weight | [71] |
| Crassulaceae | Kalanchoe | K. daigremontiana | Leaf | N/A | [72] |
| Ericaceae | Vaccinium | V. iteophyllum | Not specified | N/A | [73] |
| Rhododendron | R. ovatum | Not specified | N/A | [74] | |
| Vaccinium | V. oldhami | Twigs | 22 mg/Kg dry weight | [75] | |
| Rhododendron | R. molle | Roots | 30 mg/Kg dry weight | [47] | |
| Euphorbiaceae | Sapium | S. baccatum | Bark of the stems | 3.25 mg/Kg dry weight | [55] |
| Euphorbia | E. hirta | Stems | 0.03 mg/g dry weight | [56] | |
| Discocleidion | D. rufescens | Bark of the roots | N/A | [48] | |
| Thyrsanthera | T. suborbicularis | Whole plant | 13.67 mg/Kg dry weight | [76] | |
| Euphorbia | E. antiquorum | Not specified | N/A | [77] | |
| E. chrysocoma | Not specified | N/A | [78] | ||
| Excoecaria | E. agallocha | Not specified | N/A | [24] | |
| Sebastiana | S. adenophora | Leaves | 1.6–13.0 mg/Kg dry weight | [25] | |
| Homonoia | H. riparia | Roots | N/A | [43] | |
| Macaranga | M. triloba | Leaves | 0.19 mg/g dry weight | [79] | |
| Alchorneae | A. latifolia | Leaves | 0.0007% 3 | [80] | |
| Fabaceae | Prosopsis | P. juliflora | Seed oils | N/A | [67] |
| Clitoria | C. ternatea | Roots | 12.4 mg/g dry weight | [9] | |
| Erythrophleum | E. fordii | Leaves | 3.01 mg/Kg dry weight | [24] | |
| Icacinaceae | Pyrenacantha | P. staudtii | Leaves | N/A | [26] |
| Lamiaceae | Clerodendrum | C. trichotomum | Leaves | N/A | [27] |
| Vitex | V. trifolia | Not specified | N/A | [81] | |
| Clerodendrum | C. bungei | Not specified | N/A | [82] | |
| Lecythidaceae | Barringtonia | B. racemosa | Bark of the stems | N/A | [83] |
| Malvaceae | Pavonia | P. multiflora | Not specified | N/A | [84] |
| Abroma | A. augusta L. | Leaf | 28.80 mg/Kg dry weight | [28] | |
| Heritiera | H. littoralis | Leaf | N/A | [85] | |
| Bombax | B. ceiba (II) | Leaf | N/A | [29] | |
| Microcos | M. tomentosa | Roots | 10.08 mg/Kg dry weight | [49] | |
| helmiopsis | H. sphaerrocarpa | Leaves | 6.56 mg/Kg dry weight | [30] | |
| Sterculia | S. foetida | Leaves | 0.11 mg/g dry weight | [31] | |
| Pterospermum | P. heterophyllum | Roots | 12.88 mg/Kg dry weight | [50] | |
| Moraceae | Ficus | F. thonningii Blume | Roots | 0.04 mg/g dry weight | [51] |
| F. aurantiaca | Stem | N/A | [57] | ||
| F. foveolata | Stem | 2.9 mg/Kg dry weight | [58] | ||
| Myricaceae | Myrica | M. rubra | Bark | 141.00 mg/Kg dry weight | [52] |
| M. cerifera | Root | N/A | [52] | ||
| Myrsinaceae | Embelia | E. schimperi | Leaves | 35 mg/Kg dry weight | [32] |
| Myrtaceae | Eugenia | E. umbelliflora | Leaves | N/A | [33] |
| Ranunculaceae | Naravelia | N. Zeylanica | Leaves | N/A | [34] |
| Rhamnaceae | Ventilago | V. leiocarpa | Stems | N/A | [59] |
| Sageretia | S. theezans | Not specified | N/A | [86] | |
| Rhizophoraceae | Rhizophora | R. stylosa | Leaves | N/A | [35] |
| R. mangle | Leaves and stems | 0.77 mg/g dry weight | [36] | ||
| Rubiaceae | Mitragyna | M. rotundifolia | Bark | N/A | [87] |
| Rutaceae | Vepris | V. punctata | Wood | 2.2 mg 2 | [88] |
| Sapindaceae | Cupania | C. cinerea | Bark | 0.08 mg/g dry weight | [89] |
| Sapotaceae | Mimusops | M. elengi | Seed oils | N/A | [45] |
| M. hexandra | Bark | 14.14 mg/Kg dry weight | [90] | ||
| Solanaceae | Solanum | S. macrocarpon | Cuticular waxes of the leaves | 3.5–7.4 ng cm−2 * | [91] |
| Styracaceae | Styrax | S. japonica | Stem-bark | 28.08 mg/Kg dry weight | [92] |
| Vitaceae | Vitis | V. vinifera | Leaf | N/A | [37] |
| Tetrastigma | T. hemsleyanum | Not specified | N/A | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mus, A.A.; Goh, L.P.W.; Marbawi, H.; Gansau, J.A. The Biosynthesis and Medicinal Properties of Taraxerol. Biomedicines 2022, 10, 807. https://doi.org/10.3390/biomedicines10040807
Mus AA, Goh LPW, Marbawi H, Gansau JA. The Biosynthesis and Medicinal Properties of Taraxerol. Biomedicines. 2022; 10(4):807. https://doi.org/10.3390/biomedicines10040807
Chicago/Turabian StyleMus, Ahmad Asnawi, Lucky Poh Wah Goh, Hartinie Marbawi, and Jualang Azlan Gansau. 2022. "The Biosynthesis and Medicinal Properties of Taraxerol" Biomedicines 10, no. 4: 807. https://doi.org/10.3390/biomedicines10040807
APA StyleMus, A. A., Goh, L. P. W., Marbawi, H., & Gansau, J. A. (2022). The Biosynthesis and Medicinal Properties of Taraxerol. Biomedicines, 10(4), 807. https://doi.org/10.3390/biomedicines10040807






