Diagnostic and Clinical Value of Specific Autoantibodies against Kelch-like 12 Peptide and Nuclear Envelope Proteins in Patients with Primary Biliary Cholangitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Detection of Antibodies
2.2.1. Detection of Anti-gp210 Antibodies and AMA M2
2.2.2. Detection of Anti-Nucleoporin p62 Antibodies
2.2.3. Detection of Anti-LBR Antibodies
2.2.4. Detection of Anti-KLHL12 Antibodies
2.3. Statistical Analysis
3. Results
3.1. Clinical, Histological, and Laboratory Features of PBC Patients and Control Groups
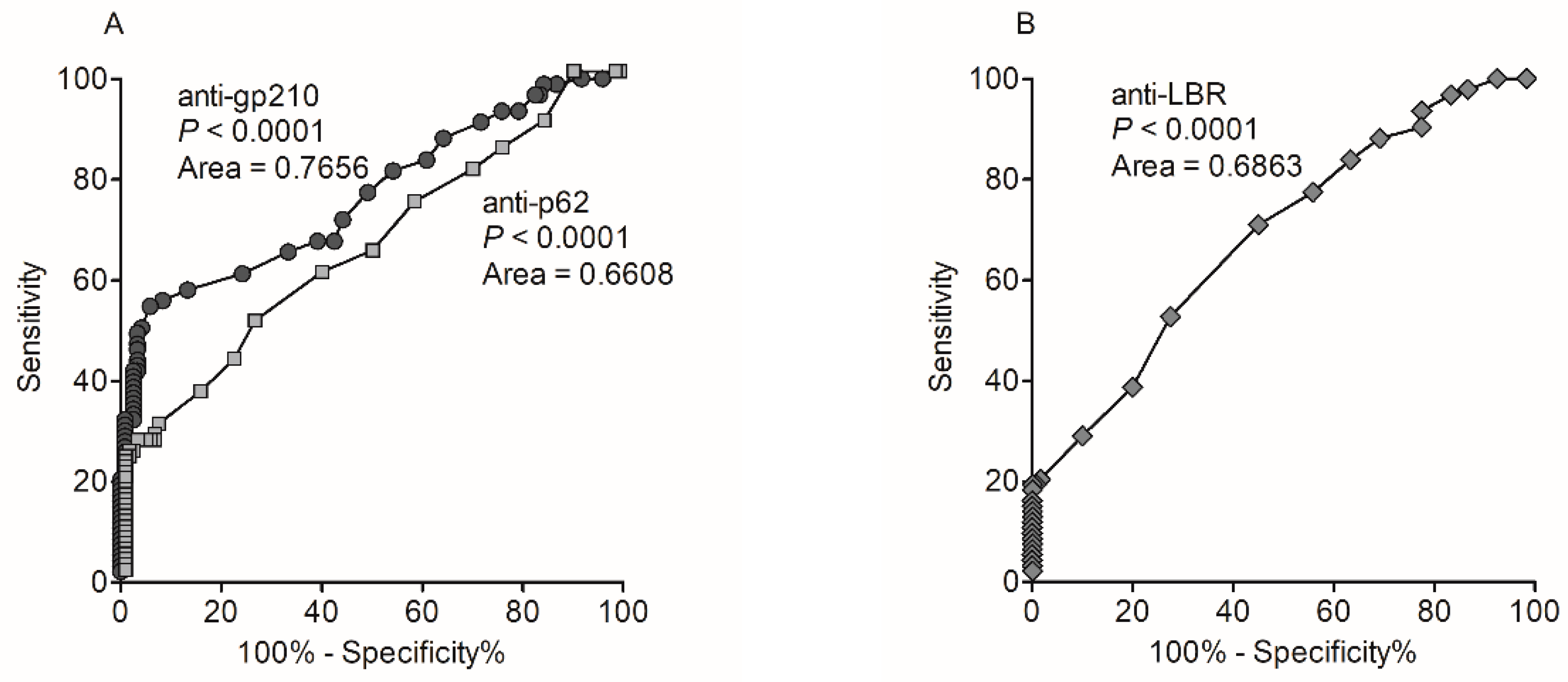
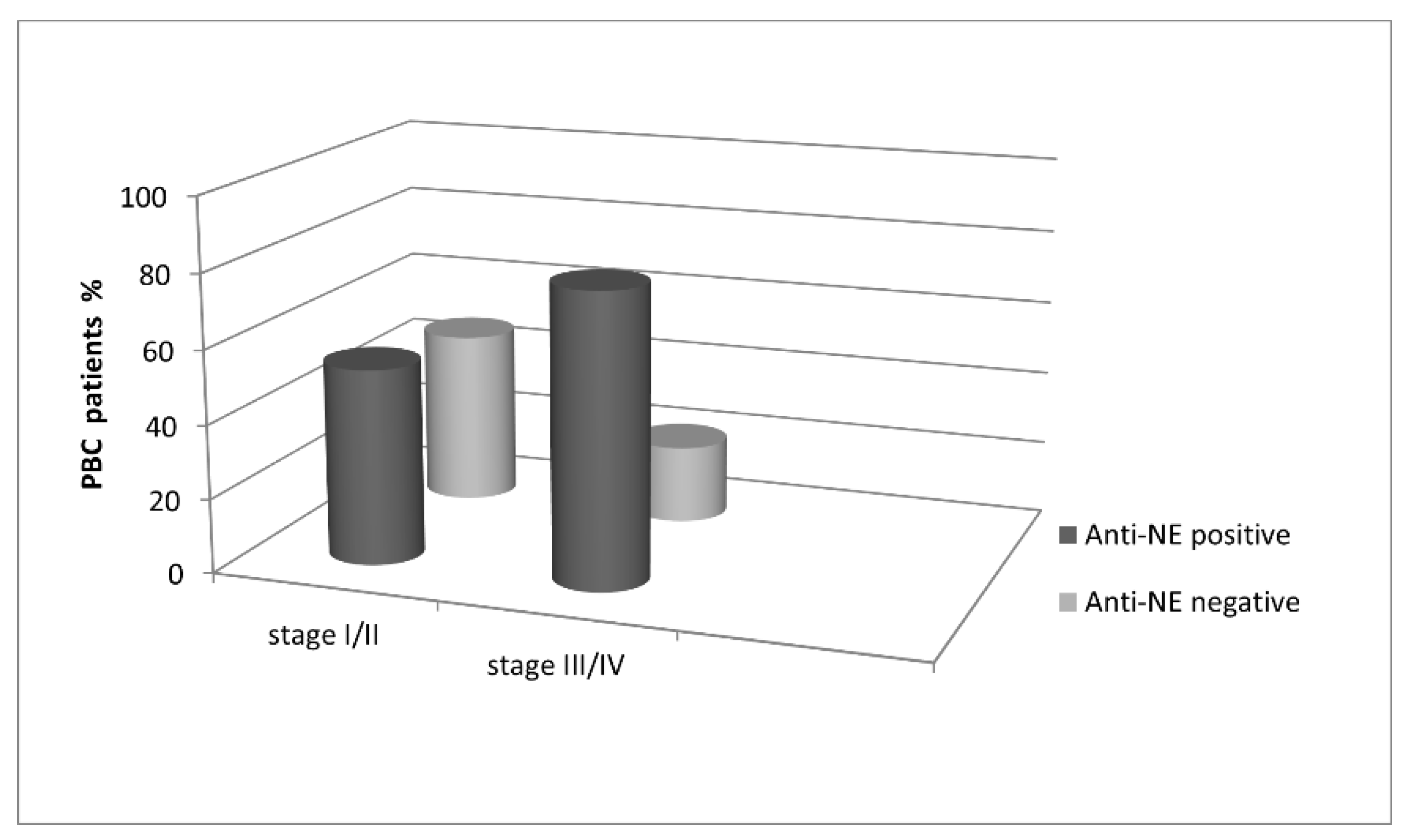
3.2. Occurrence and Diagnostic Value of Anti-Nuclear Envelope Antibodies
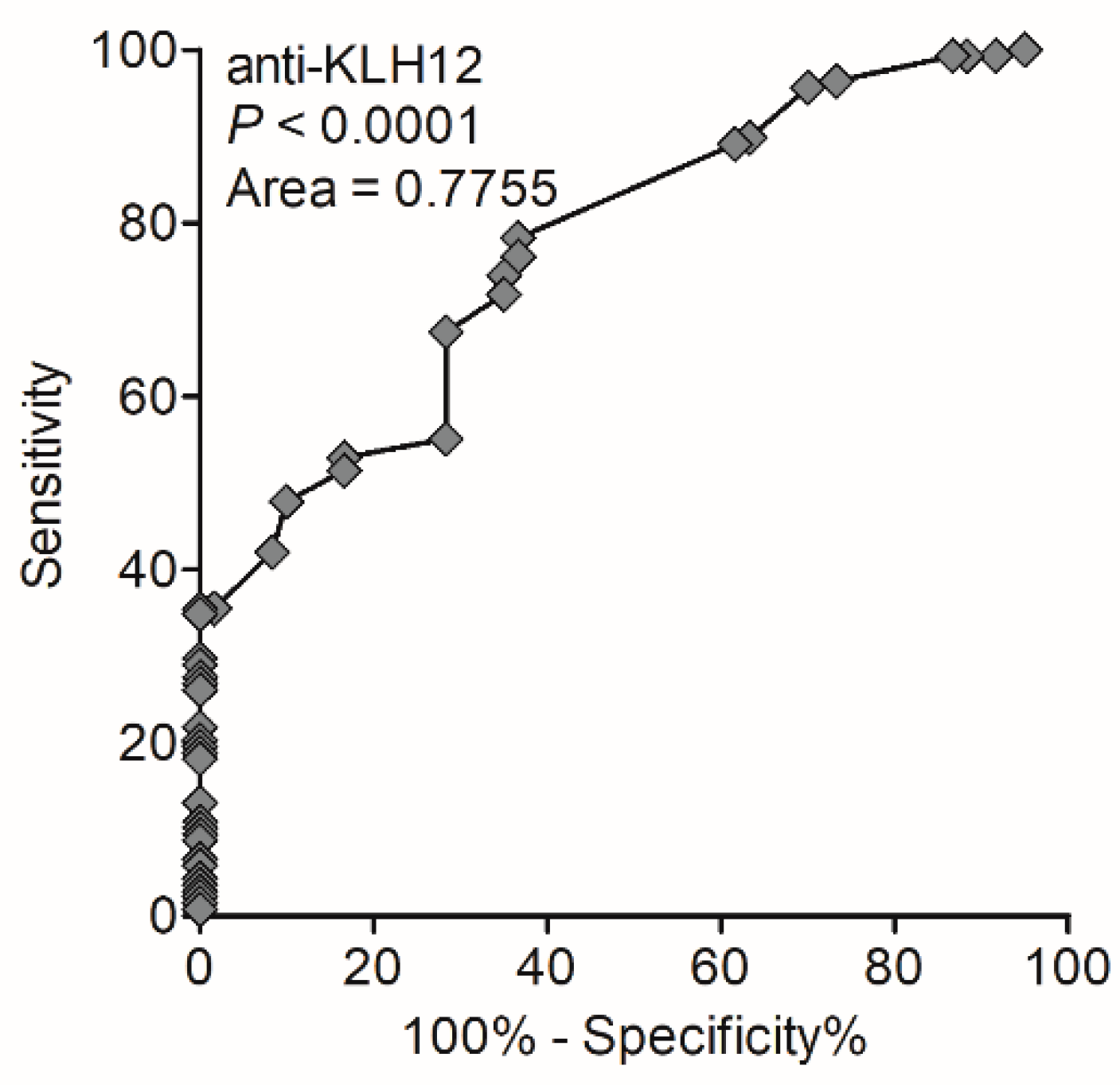
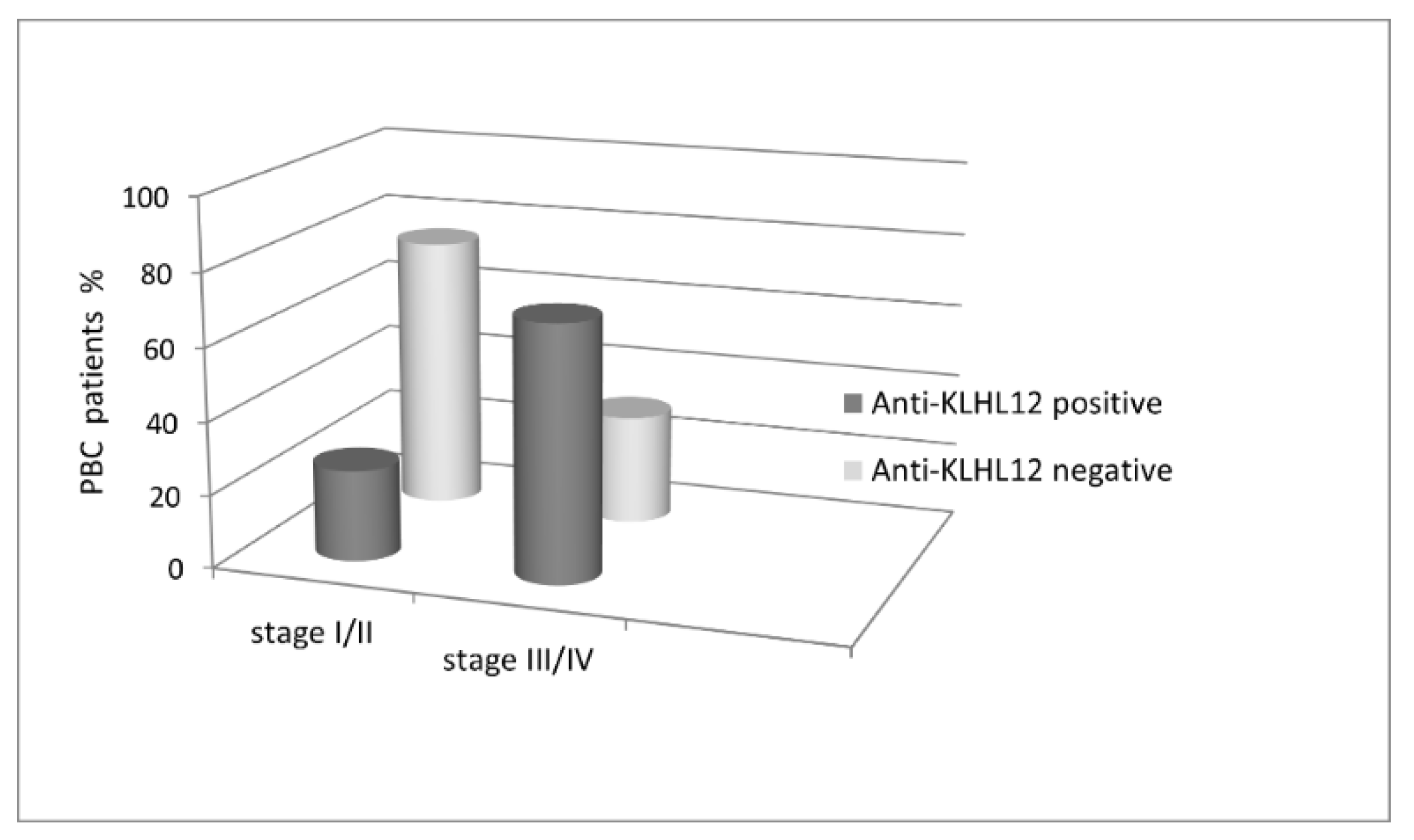
3.3. Occurrence and Diagnostic Value of Anti-KLHL12 Antibodies
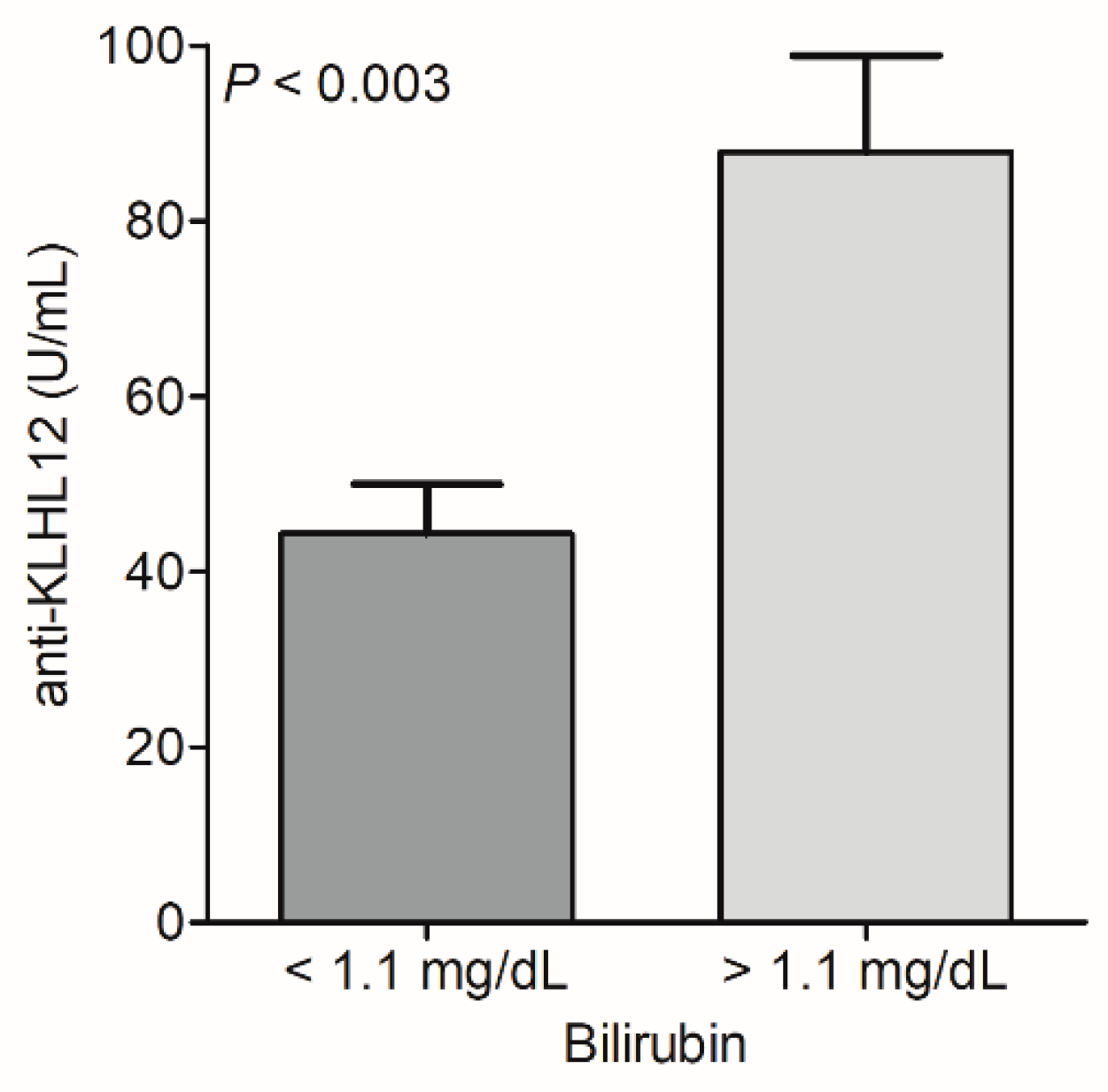
3.4. Biochemical Features of PBC Patients According to the Status of Anti-NE and Anti-KLHL12 Antibodies
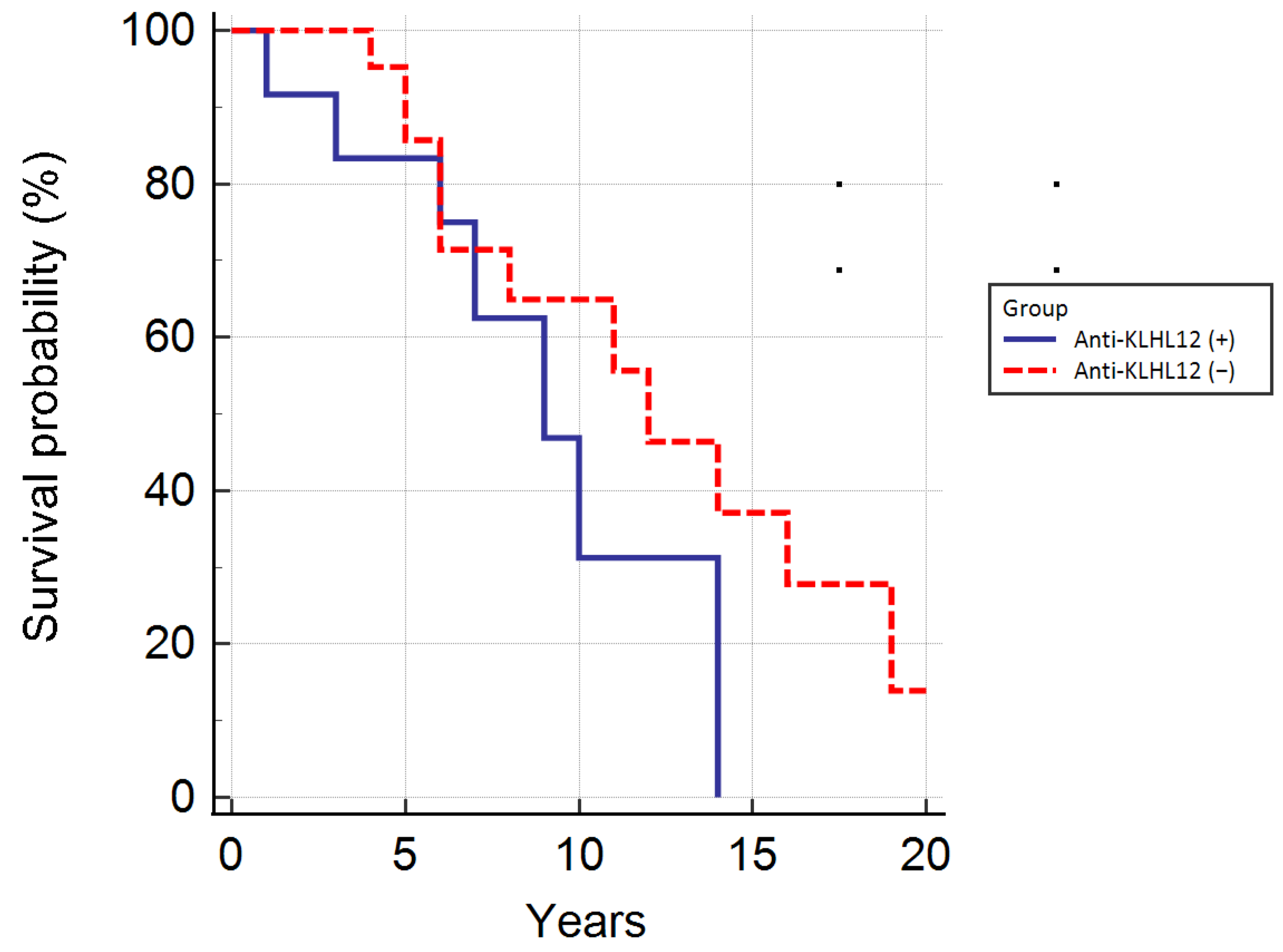
3.5. Autoantibodies Directed against Nuclear Envelope Proteins and KLHL12 Antibodies, and the Survival of Patients
3.6. Analysis of the Correlation between Histological Parameters of PBC Patients, and Autoantibodies Directed against Nuclear Envelope Proteins and the KLHL12 Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Carey, E.J.; Ali, A.H.; Lindor, K.D. Primary biliary cirrhosis. Lancet 2015, 386, 1565–1575. [Google Scholar] [CrossRef]
- Tanaka, A.; Leung, P.S.; Young, H.A.; Gershwin, M.E. Toward solving the etiological mystery of primary biliary cholangitis. Hepatol. Commun. 2017, 1, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.; Bowlus, C.L. Primary biliary cholangitis: Diagnosis and treatment. Liver Res. 2018, 2, 81–86. [Google Scholar] [CrossRef]
- Beretta-Piccoli, B.T.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M.; Adams, D.; Alpini, G.; Banales, J.M.; Beuers, U.; Björnsson, E.; Bowlus, C.; et al. The challenges of primary biliary cholangitis: What is new and what needs to be done. J. Autoimmun. 2019, 105, 102328. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyama, K.; Baba, H.; Morimoto, Y.; Tsunematsu, T.; Ogawa, H. Primary Biliary Cholangitis: Its Pathological Characteristics and Immunopathological Mechanisms. J. Med Investig. 2017, 64, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Grywalska, E.; Michalak, A.; Kowalik, A.; Mielnik, M.; Roliński, J. Deviations in Peripheral Blood Cell Populations are Associated with the Stage of Primary Biliary Cholangitis and Presence of Itching. Arch. Immunol. Ther. Exp. 2018, 66, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Chazouillères, O.; Cortez-Pinto, H.; Macedo, G.; de Lédinghen, V.; Adekunle, F.; Carbone, M. A consensus integrated care pathway for patients with primary biliary cholangitis: A guideline-based approach to clinical care of patients. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 929–939. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Bernstein, D.; Shiffman, M.L.; Kwo, P.; Kim, W.R.; Kowdley, K.V.; Jacobson, I.M. Diagnosis and Management of Primary Biliary Cholangitis. Am. J. Gastroenterol. 2019, 114, 48–63. [Google Scholar] [CrossRef]
- Paziewska, A.; Habior, A.; Rogowska, A.; Zych, W.; Goryca, K.; Karczmarski, J.; Dabrowska, M.; Ambrozkiewicz, F.; Walewska-Zielecka, B.; Krawczyk, M.; et al. A novel approach to genome-wide association analysis identifies genetic asso-ciations with primary biliary cholangitis and primary sclerosing cholangitis in Polish patients. BMC Med. Genomics 2017, 10, 2. [Google Scholar] [CrossRef]
- Gazda, J.; Drazilova, S.; Janicko, M.; Jarcuska, P. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Beretta-Piccoli, B.T.; Mieli-Vergani, G.; Vergani, D. The clinical usage and definition of autoantibodies in immune-mediated liver disease: A comprehensive overview. J. Autoimmun. 2018, 95, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Kouroumalis, E.; Samonakis, D.; Voumvouraki, A. Biomarkers for primary biliary cholangitis: Current perspectives. Hepatic Med. Évid. Res. 2018, 10, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 69, 394–419. [Google Scholar] [CrossRef] [PubMed]
- Cancado, E.L.R.; Harriz, M. The Importance of Autoantibody Detection in Primary Biliary Cirrhosis. Front. Immunol. 2015, 6, 309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Zheng, X.; Jiang, P.; Tang, R.; Gong, Y.; Dai, Y.; Wang, L.; Xu, P.; Sun, W.; Wang, L.; et al. Genome-wide Association Studies of Specific Antinuclear Autoantibody Subphenotypes in Primary Biliary Cholangitis. Hepatology 2019, 70, 294–307. [Google Scholar] [CrossRef]
- Cristoferi, L.; Gerussi, A.; Invernizzi, P. Anti-gp210 and other anti-nuclear pore complex autoantibodies in primary biliary cholangitis: What we know and what we should know. Liver Int. 2021, 41, 432–435. [Google Scholar] [CrossRef]
- Rigopoulou, E.I.; Davies, E.T.; Pares, A.; Zachou, K.; Liaskos, C.; Bogdanos, D.; Rodés, J.; Dalekos, G.N.; Vergani, D. Prevalence and clinical significance of isotype specific antinuclear antibodies in primary biliary cirrhosis. Gut 2005, 54, 528–532. [Google Scholar] [CrossRef]
- Vermeersch, P.; Bossuyt, X. Prevalence and clinical significance of rare antinuclear antibody patterns. Autoimmun. Rev. 2013, 12, 998–1003. [Google Scholar] [CrossRef]
- Granito, A.; Yang, W.-H.; Muratori, L.; Lim, M.J.; Nakajima, A.; Ferri, S.; Pappas, G.; Quarneti, C.; Bianchi, F.B.; Bloch, D.; et al. PML Nuclear Body Component Sp140 Is a Novel Autoantigen in Primary Biliary Cirrhosis. Am. J. Gastroenterol. 2010, 105, 125–131. [Google Scholar] [CrossRef]
- Han, E.; Jo, S.J.; Lee, H.; Choi, A.-R.; Lim, J.; Jung, E.-S.; Oh, E.-J. Clinical relevance of combined anti-mitochondrial M2 detection assays for primary biliary cirrhosis. Clin. Chim. Acta 2016, 464, 113–117. [Google Scholar] [CrossRef]
- Hu, C.J.; Zhang, F.C.; Li, Y.Z.; Zhang, X. Primary biliary cirrhosis: What do autoantibodies tell us? World J. Gastroenterol. 2010, 16, 3616–3629. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Muratori, P.; Quarneti, C.; Pappas, G.; Cicola, R.; Muratori, L. Antinuclear antibodies as ancillary markers in primary biliary cirrhosis. Expert Rev. Mol. Diagn. 2012, 12, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bogdanos, D.P.; Komorowski, L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin. Chim. Acta 2011, 412, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Habior, A.; Wieszczy, P.; Gawel, D. Analysis of Autoantibodies against Promyelocytic Leukemia Nuclear Body Components and Biochemical Parameters in Sera of Patients with Primary Biliary Cholangitis. Diagnostics 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Milkiewicz, P.; Buwaneswaran, H.; Coltescu, C. Value of antibody analysis in the differential diagnosis of chronic cholestatic liver disease. Dig. Dis. 2009, 7, 1355–1360. [Google Scholar]
- Liu, B.; Shi, X.H.; Zhang, F.C.; Zhang, W.; Gao, L.X. Anti-mitochondrial antibody-negative primary biliary cirrhosis: A subset of primary biliary cirrhosis. Liver Int. 2008, 2, 233–239. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Shapira, Y.; Selmi, C.; Barzilai, O.; Ram, M.; Szyper-Kravitz, M.; Sella, S.; Katz, B.-S.P.; Youinou, P.; Renaudineau, Y.; et al. A comprehensive evaluation of serum autoantibodies in primary biliary cirrhosis. J. Autoimmun. 2010, 34, 55–58. [Google Scholar] [CrossRef]
- Liu, H.; Norman, G.L.; Shums, Z.; Worman, H.J.; Krawitt, E.L.; Bizzaro, N.; Vergani, D.; Bogdanos, D.; Dalekos, G.N.; Milkiewicz, P.; et al. PBC Screen: An IgG/IgA dual isotype ELISA detecting multiple mitochondrial and nuclear autoantibodies specific for primary biliary cirrhosis. J. Autoimmun. 2010, 35, 436–442. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Tovoli, F.; Muratori, P. Autoantibodies to speckled protein family in primary biliary cholangitis. Allergy Asthma Clin. Immunol. 2021, 17, 1–4. [Google Scholar] [CrossRef]
- Villalta, D.; Sorrentino, M.C.; Girolami, E.; Tampoia, M.; Alessio, M.G.; Brusca, I.; Daves, M.; Porcelli, B.; Barberio, G.; Bizzaro, N. Autoantibody profiling of patients with primary biliary cirrhosis using a multiplexed line-blot assay. Clin. Chim. Acta 2015, 438, 135–138. [Google Scholar] [CrossRef]
- Huang, C.; Han, W.; Wang, C.; Liu, Y.; Chen, Y.; Duan, Z. Early Prognostic Utility of Gp210 Antibody-Positive Rate in Primary Biliary Cholangitis: A Meta-Analysis. Dis. Markers 2019, 2019, 9121207. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Habior, A. Measurement of gp210 autoantibodies in sera of patients with primary biliary cirrhosis. J. Clin. Lab. Anal. 2007, 21, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Rey, C.; Bogdanos, D.; Yang, C.-Y.; Roberts, K.; Leung, P.S.C.; Anaya, J.-M.; Worman, H.J.; Gershwin, M.E. Primary biliary cirrhosis and the nuclear pore complex. Autoimmun. Rev. 2012, 11, 898–902. [Google Scholar] [CrossRef]
- Chantran, Y.; Ballot, E.; Johanet, C. Autoantibodies in primary biliary cirrhosis: Antinuclear envelope autoantibodies. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 256–258. [Google Scholar] [CrossRef] [PubMed]
- de Liso, F.; Matinato, C.; Ronchi, M.; Maiavacca, R. The diagnostic accuracy of biomarkers for diagnosis of primary biliary cholangitis (PBC) in anti-mitochondrial antibody (AMA)-negative PBC patients: A review of literature. Clin. Chem. Lab. Med. (CCLM) 2017, 56, 25–31. [Google Scholar] [CrossRef]
- Hu, S.-L.; Zhao, F.-R.; Hu, Q.; Chen, W.-X. Meta-Analysis Assessment of GP210 and SP100 for the Diagnosis of Primary Biliary Cirrhosis. PLoS ONE 2014, 9, e101916. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Wu, S.; Duan, W.; Chen, S.; Ou, X.; You, H.; Kong, Y.; Jia, J. Meta-Analysis of Antinuclear Antibodies in the Diagnosis of Antimitochondrial Antibody-Negative Primary Biliary Cholangitis. Gastroenterol. Res. Pr. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Wesierska-Gadek, J.; Penner, E.; Battezzati, P.M.; Selmi, C.; Zuin, M.; Hitchman, E.; Worman, H.J.; Gershwin, M.E.; Podda, M.; Invernizzi, P. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology 2006, 43, 1135–1144. [Google Scholar] [CrossRef]
- Yamagiwa, S.; Kamimura, H.; Takamura, M.; Aoyagi, Y. Autoantibodies in primary biliary cirrhosis: Recent progress in research on the pathogenetic and clinical significance. World J. Gastroenterol. 2014, 20, 2606–2612. [Google Scholar] [CrossRef]
- Nakamura, M. Clinical Significance of Autoantibodies in Primary Biliary Cirrhosis. Semin. Liver Dis. 2014, 34, 334–340. [Google Scholar] [CrossRef]
- Wesierska-Gadek, J.; Klima, A.; Ranftler, C.; Komina, O.; Hanover, J.; Invernizzi, P.; Penner, E. Characterization of antibodies to p62 nucleoporin in primary biliary cirrhosis using human recombinant antigen. J. Cell. Biochem. 2008, 104, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wesierska-Gadek, J.; Klima, A.; Komina, O.; Ranftler, C.; Invernizzi, P.; Penner, E. Characterization of autoantibodies against components of the nuclear pore complex, high frequency of anti-p62 nucleoporin antibodies. Ann. N. Y. Acad. Sci. 2007, 1109, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, K.; Hankins, R.W.; Matsushima, H.; Kikuchi, F.; Inomata, T.; Horigome, T.; Shibata, M.; Onozuka, Y.; Ueno, Y.; Hashimoto, E.; et al. Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: A multicenter study. J. Autoimmun. 2003, 20, 247–254. [Google Scholar] [CrossRef]
- Norman, G.L.; Yang, C.-Y.; Ostendorff, H.P.; Shums, Z.; Lim, M.J.; Wang, J.; Awad, A.; Hirschfield, G.; Milkiewicz, P.; Bloch, D.; et al. Anti-kelch-like 12 and anti-hexokinase 1: Novel autoantibodies in primary biliary cirrhosis. Liver Int. 2014, 35, 642–651. [Google Scholar] [CrossRef]
- Norman, G.L.; Reig, A.; Viñas, O.; Mahler, M.; Wunsch, E.; Milkiewicz, P.; Swain, M.G.; Mason, A.; Stinton, L.M.; Aparicio, M.B.; et al. The Prevalence of Anti-Hexokinase-1 and Anti-Kelch-Like 12 Peptide Antibodies in Patients with Primary Biliary Cholangitis Is Similar in Europe and North America: A Large International, Multi-Center Study. Front. Immunol. 2019, 10, 662. [Google Scholar] [CrossRef]
- Gupta, V.A.; Beggs, A.H. Kelch proteins: Emerging roles in skeletal muscle development and diseases. Skelet. Muscle 2014, 4, 11. [Google Scholar] [CrossRef]
- Dhanoa, B.S.; Cogliati, T.; Satish, A.G.; Bruford, E.A.; Friedman, J.S. Update on the Kelch-like (KLHL) gene family. Hum. Genom. 2013, 7, 13. [Google Scholar] [CrossRef]
- Jin, L.; Pahuja, K.B.; Wickliffe, K.E.; Gorur, A.; Baumgärtel, C.; Schekman, R.; Rape, M. Ubiquitin-dependent regulation of COPII coat size and function. Nature 2012, 482, 495–500. [Google Scholar] [CrossRef]
- Hu, C.-J.; Song, G.; Huang, W.; Liu, G.-Z.; Deng, C.-W.; Zeng, H.-P.; Wang, L.; Zhang, F.-C.; Zhang, X.; Jeong, J.S.; et al. Identification of New Autoantigens for Primary Biliary Cirrhosis Using Human Proteome Microarrays. Mol. Cell. Proteom. 2012, 11, 669–680. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines. Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines. The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar]
- Bauer, A.; Habior, A. Detection of Autoantibodies Against Nucleoporin p62 in Sera of Patients with Primary Biliary Cholangitis. Ann. Lab. Med. 2019, 39, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Komori, A.; Ito, M.; Kondo, H.; Aiba, Y.; Migita, K.; Nagaoka, S.; Ohata, K.; Yano, K.; Abiru, S.; et al. Predictive role of anti-gp210 and anticentromere antibodies in long-term outcome of primary biliary cirrhosis. Hepatol. Res. 2007, 37, S412–S419. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Ki, M.; Choi, H.Y.; Kim, B.H.; Jang, E.S.; Jeong, S.-H. Population-based epidemiology of primary biliary cirrhosis in South Korea. Aliment. Pharmacol. Ther. 2015, 43, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, P.; Podda, M.; Battezzati, P.M.; Crosignani, A.; Zuin, M.; Hitchman, E.; Maggioni, M.; Meroni, P.L.; Penner, E.; Wesierska-Gadek, J. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J. Hepatol. 2001, 34, 366–372. [Google Scholar] [CrossRef]
- Bogdanos, D.; Pares, A.; Rodes, J. Vergani D Primary biliary cirrhosis specific antinuclear antibodies in patients from Spain. Am. J. Gastroenterol. 2003, 99, 763–764. [Google Scholar] [CrossRef]
- Nakamura, M.; Shimizu-Yoshida, Y.; Takii, Y.; Komori, A.; Yokoyama, T.; Ueki, T.; Daikoku, M.; Yano, K.; Matsumoto, T.; Migita, K.; et al. Antibody titer to gp210-C terminal peptide as a clinical parameter for monitoring primary biliary cirrhosis. J. Hepatol. 2005, 42, 386–392. [Google Scholar] [CrossRef]
- Bogdanos, D.P.; Liaskos, C.; Pares, A.; Norman, G.; Rigopoulou, E.I.; Caballeria, L.; Dalekos, G.N.; Rodes, J.; Vergani, D. Anti-gp210 antibody mirrors disease severity in primary biliary cirrhosis. Hepatology 2007, 45, 1583. [Google Scholar] [CrossRef]
- Haldar, D.; Janmohamed, A.; Plant, T.; Davidson, M.; Norman, H.; Russell, E.; Serevina, O.; Chung, K.; Qamar, K.; Gunson, B.; et al. Antibodies to gp210 and understanding risk in patients with primary biliary cholangitis. Liver Int. 2020, 41, 535–544. [Google Scholar] [CrossRef]
- Yang, W.-H.; Yu, J.H.; Nakajima, A.; Neuberg, D.; Lindor, K.; Bloch, D.B. Do antinuclear antibodies in primary biliary cirrhosis patients identify increased risk for liver failure? Clin. Gastroenterol. Hepatol. 2004, 2, 1116–1122. [Google Scholar] [CrossRef]
- Sfakianaki, O.; Koulentaki, M.; Tzardi, M.; Tsangaridou, E.; Theodoropoulos, P.A.; Castanas, E.; Kouroumalis, E.A. Peri-nuclear antibodies correlate with survival in Greek primary biliary cirrhosis patients. World J. Gastroenterol. 2010, 16, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kondo, H.; Tanaka, A.; Komori, A.; Ito, M.; Yamamoto, K.; Ohira, H.; Zeniya, M.; Hashimoto, E.; Honda, M.; et al. Autoantibody status and histological variables influence biochemical response to treatment and long-term outcomes in Japanese patients with primary biliary cirrhosis. Hepatol. Res. 2014, 45, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Tsangaridou, E.; Polioudaki, H.; Sfakianaki, R.; Samiotaki, M.; Tzardi, M.; Koulentaki, M.; Panayotou, G.; Kouroumalis, E.; Castanas, E.; Theodoropoulos, P.A. Differential detection of nuclear envelope autoantibodies in primary biliary cirrhosis using routine and alternative methods. BMC Gastroenterol. 2010, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Vergani, D.; Alvarez, F.; Bianchi, F.B.; Cancado, E.L.; Mackay, I.R.; Manns, M.P.; Nishioka, M.; Penner, E. Liver autoimmune serology, a consensus statement from the committee for of the International Autoimmune Hepatitis Group. J. Hepatol. 2004, 41, 677–683. [Google Scholar] [PubMed]
- Dahlqvist, G.; Gaouar, F.; Carrat, F.; Meurisse, S.; Chazouillères, O.; Poupon, R.; Johanet, C.; Corpechot, C.; The French Network of Immunology Laboratories. Large-scale characterization study of patients with antimitochondrial antibodies but nonestablished primary biliary cholangitis. Hepatology 2016, 65, 152–163. [Google Scholar] [CrossRef]





| Primary Biliary Cholangitis Patients (n = 138) | Autoimmune Hepatitis Patients (n = 20) | Primary Sclerosing Cholangitis Patients (n = 40) | Healthy Adult Blood Donors (n = 30) | |
|---|---|---|---|---|
| Age, years (range) | 50 (26–70) | 47 (19–67) | 47 (23–67) | 33 (19–53) |
| Females/males | 131/7 | 15/5 | 16/24 | 22/8 |
| Bilirubin (total), mg/dL | 2.4 (2.2) | 2.3 (2.1) | 1.4 (2.6) | 0.7 (0.6) |
| AST, U/L | 81.4 (51.2) | 44.3 (71.0) | 97.4 (70.1) | 22.5 (21.6) |
| ALT, U/L | 93.6 (72.5) | 61.7 (52.8) | 86.9 (66.0) | 15.1 (26.2) |
| AP, U/L | 506.5 (429.2) | 223.3 (175.4) | 345.5 (227.6) | 38.7 (16.8) |
| γ-GT, U/L | 335.5 (304.2) | 231.9 (204.0) | 349.2 (252.4) | 18.6 (4.8) |
| Albumin (g/dL) | 3.6 (1.2) | 3.4 (2.4) | 2.9 (1.1) | 4.5 (2.3) |
| γ-globulin (g/dL) | 1.8 (1.1) | 1.7 (1.6) | 1.5 (1.8) | 1.1 (0.2) |
| AMA M2 | 113 (82%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anti-gp210 antibody | 65 (47%) | 0 (0%) | 1 (2.5%) | 0 (0%) |
| Anti-p62 antibody | 39 (28%) | 1 (5%) | 1 (2.5%) | 0 (0%) |
| Anti-LBR antibody | 21 (15%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anti-KLHL12 antibodies | 49 (36%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Early histological stage (I/II) | 82 (59%) | 6 (30%) | 11 (28%) | 0 (0%) |
| Advanced histological stage (III/IV) | 52 (37%) | 3 (15%) | 5 (13%) | 0 (0%) |
| Ambiguous histological stage | 4 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anti-gp210 | Anti-p62 | Anti-LBR | Anti-NE | |
|---|---|---|---|---|
| Sensitivity [%, 95% CI] | 47.1 [38.6–55.8] | 28.3 [20.9–36.6] | 15.2 [9.7–22.3] | 55.1 [46.4–63.5] |
| Specificity [%, 95% CI] | 98.9 [93.9–99.9] | 97.8 [92.2–99.7] | 100.0 [96.0–100.0] | 96.7 [90.6–99.3] |
| PPV [%, 95% CI] | 98.5 [90.1–98.9] | 95.1 [82.8–98.8] | 100.0 | 96.2 [89.2–98.7] |
| NPV [%, 95% CI] | 54.9 [51.0–58.8] | 47.1 [44.4–49.8] | 43.5 [41.8–45.2] | 58.4 [53.8–62.9] |
| Positive Likelihood Ratio (LR+) | 42.4 [6.0–300.1] | 12.7 [3.2–51.4] | ND | 16.5 [5.4–50.8] |
| Negative Likelihood Ratio (LR−) | 0.5 [0.4–0.6] | 0.7 [0.6–0.8] | 0.9 [0.8–0.9] | 0.5 [0.4–0.6] |
| Disease prevalence [%, 95% CI] | 60.5 [53.4–65.9] | 60.5 [53.9–65.9] | 60.5 [53.9–66.9] | 60.5 [53.9–66.9] |
| Accuracy [%, 95% CI] | 67.5 [61.1–73.4] | 55.7 [49.0–62.3] | 48.7 [42.0–55.4] | 71.5 [65.1–77.3] |
| Number of Patients | Anti-KLHL12 Antibodies | |
|---|---|---|
| PBC | 138 | 49 (36%) |
| Controls (total) | 90 | 1 (1.1%) |
| PSC | 40 | 0 (0%) |
| AIH | 20 | 1 (5%) |
| Healthy | 30 | 0 (0%) |
| Anti-NE Antibodies | Anti-KLHL12 Antibodies | |||||
|---|---|---|---|---|---|---|
| Positive n = 25 | Negative n = 68 | p-Value | Positive n = 37 | Negative n = 56 | p-Value | |
| Bilirubin (total), mg/dL | 2.4 (2.2) | 1.7 (1.6) | 0.016 | 2.6 (2.6) | 1.5 (1.1) | 0.037 |
| AST, U/L | 108.8 (95.0) | 72.5 (35.0) | 0.008 | 86.9 (55.4) | 68.8 (52.8) | ns |
| ALT, U/L | 98.0 (95.4) | 88.1 (75.1) | ns | 95.3 (94.5) | 89.1 (69.8) | ns |
| AP, U/L | 586.7 (530.9) | 398.9 (304.1) | 0.036 | 557.3 (466.6) | 429.8 (362.1) | ns |
| γ-GT, U/L | 367.2 (345.8) | 329.9 (327.7) | ns | 343.4 (317.5) | 310.8 (291.0) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, A.; Habior, A.; Gawel, D. Diagnostic and Clinical Value of Specific Autoantibodies against Kelch-like 12 Peptide and Nuclear Envelope Proteins in Patients with Primary Biliary Cholangitis. Biomedicines 2022, 10, 801. https://doi.org/10.3390/biomedicines10040801
Bauer A, Habior A, Gawel D. Diagnostic and Clinical Value of Specific Autoantibodies against Kelch-like 12 Peptide and Nuclear Envelope Proteins in Patients with Primary Biliary Cholangitis. Biomedicines. 2022; 10(4):801. https://doi.org/10.3390/biomedicines10040801
Chicago/Turabian StyleBauer, Alicja, Andrzej Habior, and Damian Gawel. 2022. "Diagnostic and Clinical Value of Specific Autoantibodies against Kelch-like 12 Peptide and Nuclear Envelope Proteins in Patients with Primary Biliary Cholangitis" Biomedicines 10, no. 4: 801. https://doi.org/10.3390/biomedicines10040801
APA StyleBauer, A., Habior, A., & Gawel, D. (2022). Diagnostic and Clinical Value of Specific Autoantibodies against Kelch-like 12 Peptide and Nuclear Envelope Proteins in Patients with Primary Biliary Cholangitis. Biomedicines, 10(4), 801. https://doi.org/10.3390/biomedicines10040801






