Role of Serotonin in the Maintenance of Inflammatory State in Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Tissue Treatment
2.2. Immunofluorescence
2.3. Laser Confocal Immunofluorescence
2.4. Statistical Analysis
3. Results
3.1. Light Microscopy
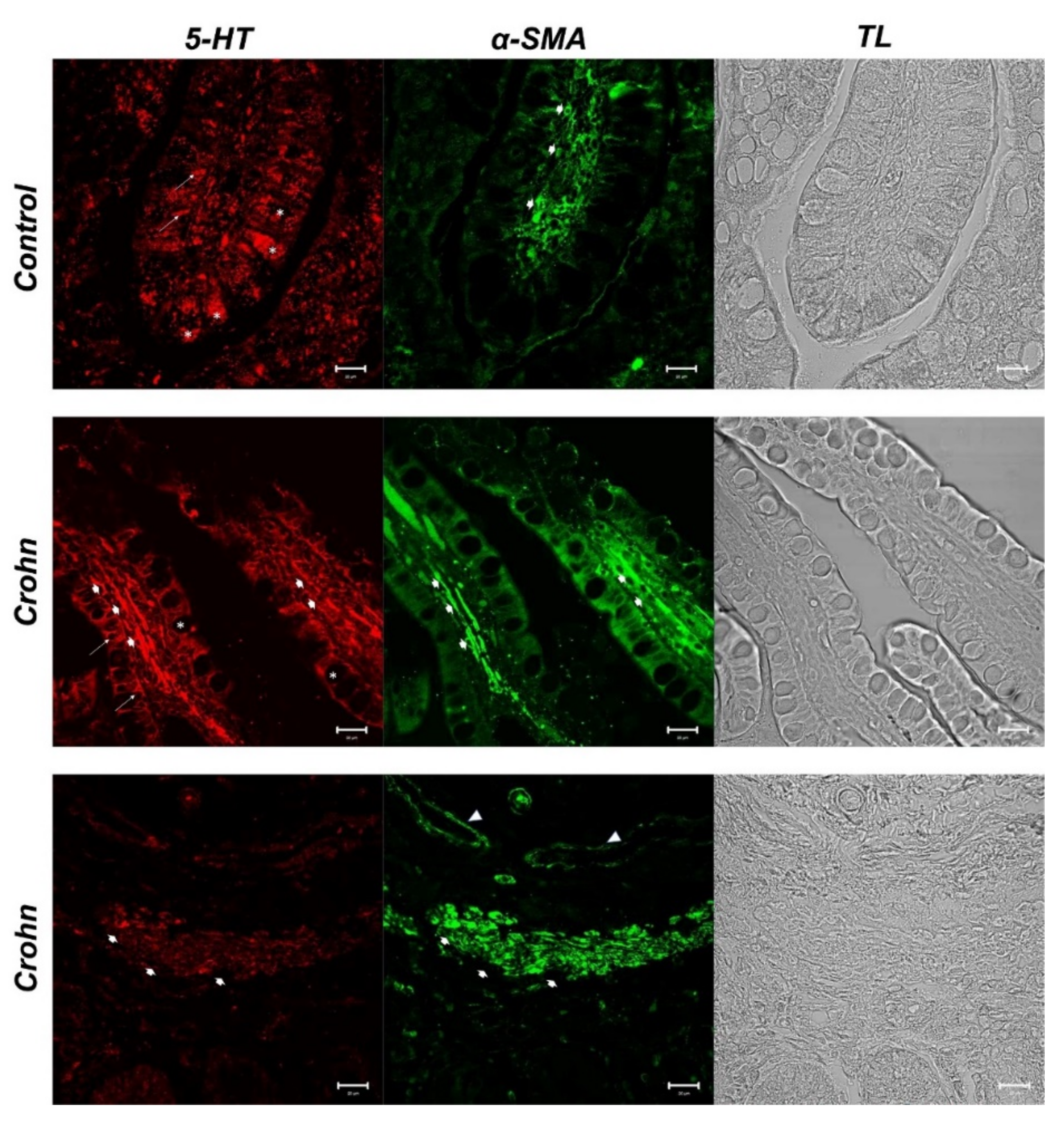
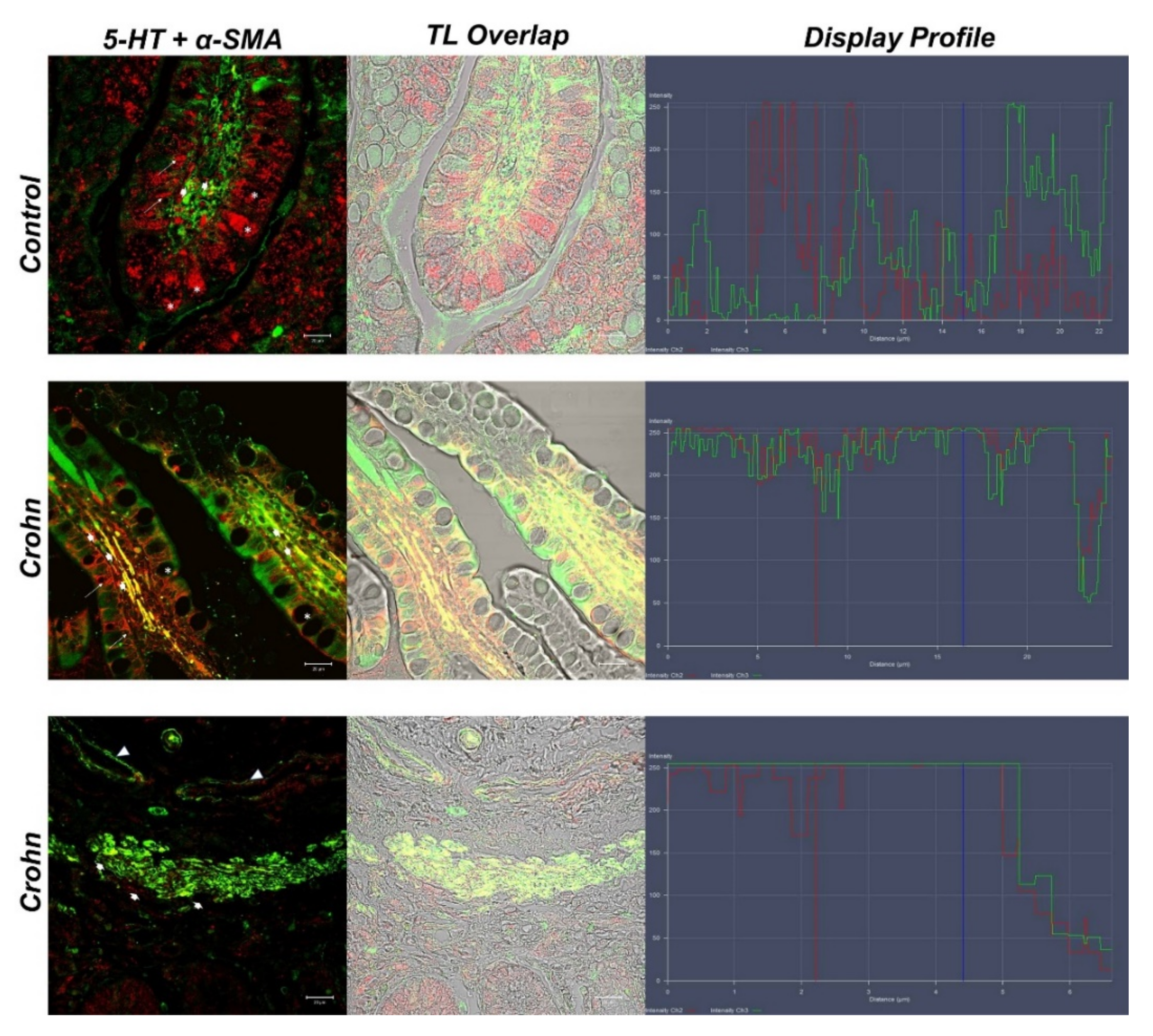
3.2. Double Labeling of 5-HT and α-SMA
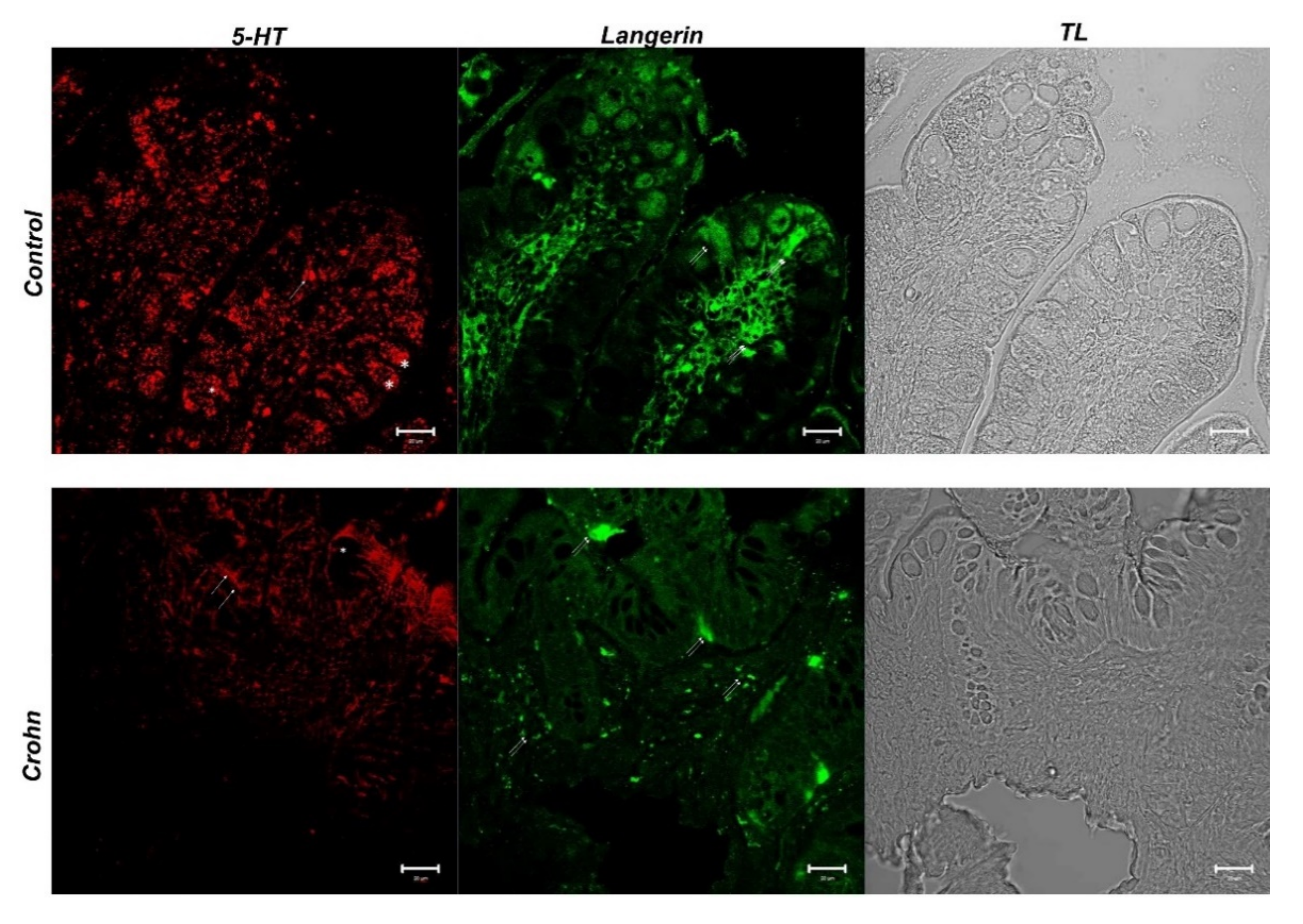
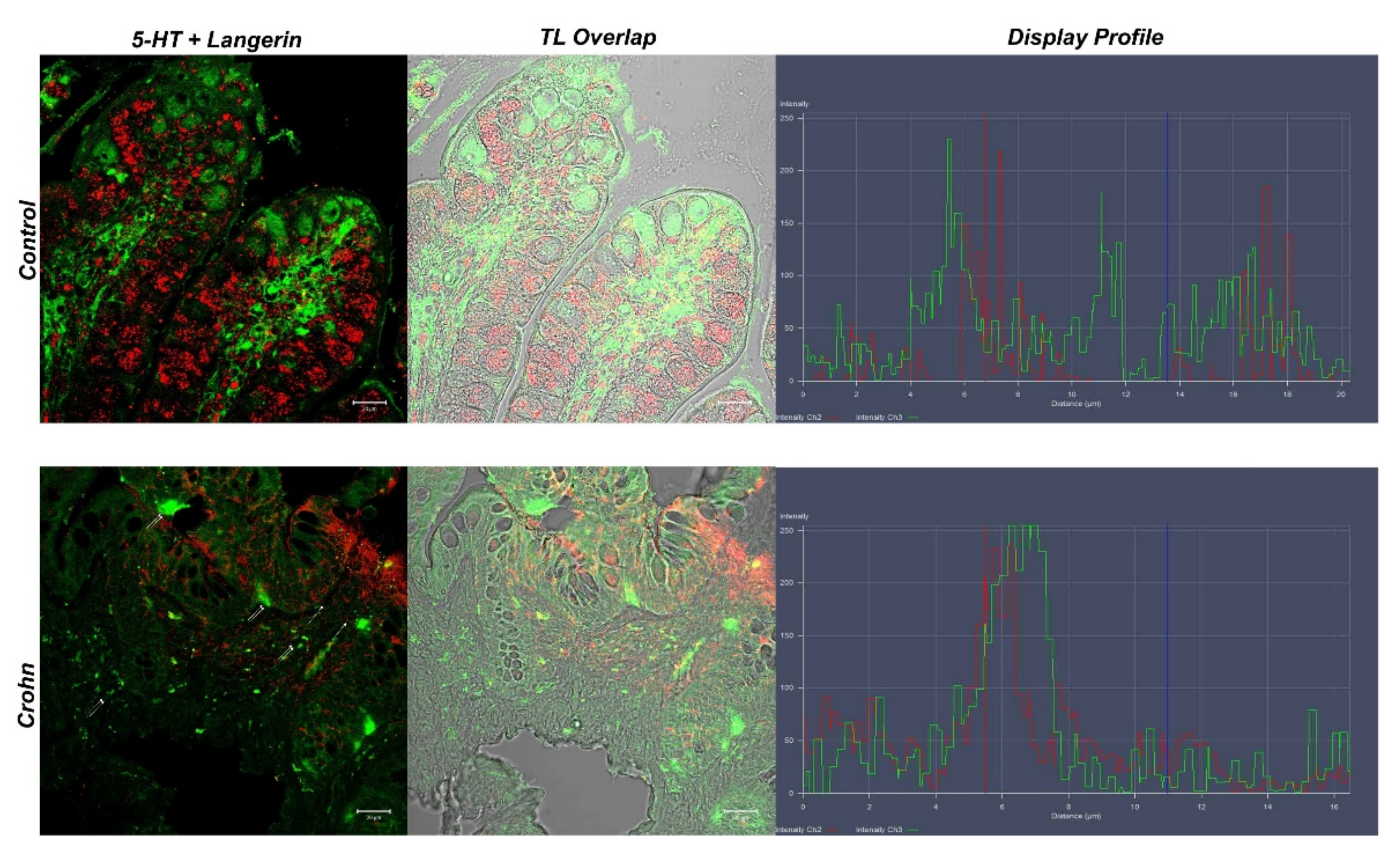
3.3. Double Labeling of 5-HT and Langerin/CD207
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mulsow, J. Prof Ronan O’Connell Festschrift: Stricture Pathogenesis in Crohn’s Disease—The Role of Intestinal Fibroblasts. Ir. J. Med. Sci. 2018, 187, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and Adaptive Immunity in Inflammatory Bowel Disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [PubMed]
- Rieder, F.; Fiocchi, C. Intestinal Fibrosis in Inflammatory Bowel Disease: Progress in Basic and Clinical Science. Curr. Opin. Gastroenterol. 2008, 24, 462–468. [Google Scholar]
- Burke, J.P.; Mulsow, J.J.; O’keane, C.; Docherty, N.G.; Watson, R.W.G.; O’connell, P.R. Fibrogenesis in Crohn’s Disease. Off. J. Am. Coll. Gastroenterol. ACG 2007, 102, 439–448. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of Fibrosis and Stricture Formation in Crohn’s Disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- Li, C.; Kuemmerle, J.F. The Fate of Myofibroblasts during the Development of Fibrosis in Crohn’s Disease. J. Dig. Dis. 2020, 21, 326–331. [Google Scholar]
- Chen, W.; Lu, C.; Hirota, C.; Iacucci, M.; Ghosh, S.; Gui, X. Smooth Muscle Hyperplasia/Hypertrophy Is the Most Prominent Histological Change in Crohn’s Fibrostenosing Bowel Strictures: A Semiquantitative Analysis by Using a Novel Histological Grading Scheme. J. Crohns Colitis 2017, 11, 92–104. [Google Scholar] [CrossRef]
- Severi, C.; Sferra, R.; Scirocco, A.; Vetuschi, A.; Pallotta, N.; Pronio, A.; Caronna, R.; Di Rocco, G.; Gaudio, E.; Corazziari, E. Contribution of Intestinal Smooth Muscle to Crohn’s Disease Fibrogenesis. Eur. J. Histochem. EJH 2014, 58, 2457. [Google Scholar]
- Zidar, N.; Langner, C.; Jerala, M.; Boštjančič, E.; Drobne, D.; Tomažič, A. Pathology of Fibrosis in Crohn’s Disease—Contribution to Understanding Its Pathogenesis. Front. Med. 2020, 7, 167. [Google Scholar]
- Leeb, S.N.; Vogl, D.; Falk, W.; Schölmerich, J.; Rogler, G.; Gelbmann, C.M. Regulation of Migration of Human Colonic Myofibroblasts. Growth Factors 2002, 20, 81–91. [Google Scholar]
- Leeb, S.N.; Vogl, D.; Grossmann, J.; Falk, W.; Schölmerich, J.; Rogler, G.; Gelbmann, C.M. Autocrine Fibronectin-Induced Migration of Human Colonic Fibroblasts. Off. J. Am. Coll. Gastroenterol. ACG 2004, 99, 335–340. [Google Scholar]
- Jørandli, J.W.; Thorsvik, S.; Skovdahl, H.K.; Kornfeld, B.; Sæterstad, S.; Gustafsson, B.I.; Sandvik, A.K.; van Beelen Granlund, A. The Serotonin Reuptake Transporter Is Reduced in the Epithelium of Active Crohn’s Disease and Ulcerative Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G761–G768. [Google Scholar] [PubMed]
- Bronzetti, E.; Artico, M.; Pompili, E.; Felici, L.M.; Stringaro, A.; Bosco, S.; Magliulo, G.; Colone, M.; Arancia, G.; Vitale, M. Neurotrophins and Neurotransmitters in Human Palatine Tonsils: An Immunohistochemical and RT-PCR Analysis. Int. J. Mol. Med. 2006, 18, 49–58. [Google Scholar]
- Li, N.; Ghia, J.-E.; Wang, H.; McClemens, J.; Cote, F.; Suehiro, Y.; Mallet, J.; Khan, W.I. Serotonin Activates Dendritic Cell Function in the Context of Gut Inflammation. Am. J. Pathol. 2011, 178, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Rizzo, G.; Favaloro, A.; Alesci, A.; Pallio, S.; Melita, G.; Cutroneo, G.; Lauriano, E.R. Expression of VAChT and 5-HT in Ulcerative Colitis Dendritic Cells. Acta Histochem. 2021, 123, 151715. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a Neurotransmitter: The Role of Serotonin in Inflammation and Immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Tarbit, E.; Singh, I.; Peart, J.N.; Bivol, S.; Rose’Meyer, R.B. Increased Release of Serotonin from Rat Primary Isolated Adult Cardiac Myofibroblasts. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Löfdahl, A.; Tornling, G.; Wigén, J.; Larsson-Callerfelt, A.-K.; Wenglén, C.; Westergren-Thorsson, G. Pathological Insight into 5-HT2B Receptor Activation in Fibrosing Interstitial Lung Diseases. Int. J. Mol. Sci. 2021, 22, 225. [Google Scholar]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar]
- Kim, D.-Y.; Camilleri, M. Serotonin: A Mediator of the Brain-Gut Connection. Am. J. Gastroenterol. 2000, 95, 2698. [Google Scholar] [PubMed]
- Granlund, A.v.B.; Flatberg, A.; Østvik, A.E.; Drozdov, I.; Gustafsson, B.I.; Kidd, M.; Beisvag, V.; Torp, S.H.; Waldum, H.L.; Martinsen, T.C.; et al. Whole Genome Gene Expression Meta-Analysis of Inflammatory Bowel Disease Colon Mucosa Demonstrates Lack of Major Differences between Crohn’s Disease and Ulcerative Colitis. PLoS ONE 2013, 8, e56818. [Google Scholar] [CrossRef]
- Tackett, J.J.; Gandotra, N.; Bamdad, M.C.; Muise, E.D.; Cowles, R.A. Enhanced Serotonin Signaling Stimulates Ordered Intestinal Mucosal Growth. J. Surg. Res. 2017, 208, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.R.; Gershon, M.D.; Margolis, K.G.; Gertsberg, Z.V.; Cowles, R.A. Neuronal Serotonin Regulates Growth of the Intestinal Mucosa in Mice. Gastroenterology 2012, 143, 408–417. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M. Bifidobacterium Dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. MBio 2019, 10, e01087-19. [Google Scholar] [CrossRef]
- Norbury, C.C. Drinking a Lot Is Good for Dendritic Cells. Immunology 2006, 117, 443–451. [Google Scholar] [CrossRef]
- Ostanin, D.V.; Pavlick, K.P.; Bharwani, S.; Furr, K.L.; Brown, C.M.; Grisham, M.B. T Cell-Induced Inflammation of the Small and Large Intestine in Immunodeficient Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G109–G119. [Google Scholar] [CrossRef]
- Savina, A.; Amigorena, S. Phagocytosis and Antigen Presentation in Dendritic Cells. Immunol. Rev. 2007, 219, 143–156. [Google Scholar]
- Alesci, A.; Lauriano, E.R.; Aragona, M.; Capillo, G.; Pergolizzi, S. Marking Vertebrates Langerhans Cells, from Fish to Mammals. Acta Histochem. 2020, 122, 151622. [Google Scholar] [CrossRef]
- Stagg, A.J.; Hart, A.L.; Knight, S.C.; Kamm, M.A. The Dendritic Cell: Its Role in Intestinal Inflammation and Relationship with Gut Bacteria. Gut 2003, 52, 1522–1529. [Google Scholar] [CrossRef]
- Pergolizzi, S.; D’Angelo, V.; Aragona, M.; Dugo, P.; Cacciola, F.; Capillo, G.; Dugo, G.; Lauriano, E.R. Evaluation of Antioxidant and Anti-Inflammatory Activity of Green Coffee Beans Methanolic Extract in Rat Skin. Nat. Prod. Res. 2020, 34, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, M.; Chieppa, M.; Salucci, V.; Avogadri, F.; Sonzogni, A.; Sampietro, G.M.; Nespoli, A.; Viale, G.; Allavena, P.; Rescigno, M. Intestinal Immune Homeostasis Is Regulated by the Crosstalk between Epithelial Cells and Dendritic Cells. Nat. Immunol. 2005, 6, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Verstege, M.I.; ten Kate, F.J.; Reinartz, S.M.; van Drunen, C.M.; Slors, F.J.; Bemelman, W.A.; Vyth-Dreese, F.A.; Velde, A.A. te Dendritic Cell Populations in Colon and Mesenteric Lymph Nodes of Patients with Crohn’s Disease. J. Histochem. Cytochem. 2008, 56, 233–241. [Google Scholar]
- Al-Hassi, H.O.; Mann, E.R.; Sanchez, B.; English, N.R.; Peake, S.T.; Landy, J.; Man, R.; Urdaci, M.; Hart, A.L.; Fernandez-Salazar, L. Altered Human Gut Dendritic Cell Properties in Ulcerative Colitis Are Reversed by Lactobacillus Plantarum Extracellular Encrypted Peptide STp. Mol. Nutr. Food Res. 2014, 58, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Marino, A.; Capillo, G.; Aragona, M.; Marconi, P.; Lauriano, E.R. Expression of Langerin/CD 207 and α-Smooth Muscle Actin in Ex Vivo Rabbit Corneal Keratitis Model. Tissue Cell 2020, 66, 101384. [Google Scholar] [PubMed]
- Lauriano, E.R.; Pergolizzi, S.; Cascio, P.L.; Kuciel, M.; Zizzo, N.; Guerrera, M.C.; Aragona, M.; Capillo, G. Expression of Langerin/CD207 in Airways, Lung and Associated Lymph Nodes of a Stranded Striped Dolphin (Stenella Coeruleoalba). Acta Histochem. 2020, 122, 151471. [Google Scholar] [PubMed]
- Despalatović, B.R.; Babić, M.; Bratanić, A.; Tonkić, A.; Vilović, K. Difference in Presence and Number of CD83+ Dendritic Cells in Patients with Ulcerative Colitis and Crohn’s Disease. Sci. Rep. 2020, 10, 1–8. [Google Scholar]
- Chang, S.-Y.; Kweon, M.-N. Langerin-Expressing Dendritic Cells in Gut-Associated Lymphoid Tissues. Immunol. Rev. 2010, 234, 233–246. [Google Scholar]
- Lauriano, E.; Silvestri, G.; Kuciel, M.; Żuwała, K.; Zaccone, D.; Palombieri, D.; Alesci, A.; Pergolizzi, S. Immunohistochemical Localization of Toll-like Receptor 2 in Skin Langerhans’ Cells of Striped Dolphin (Stenella Coeruleoalba). Tissue Cell 2014, 46, 113–121. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical Characterization of Toll-like Receptor 2 in Gut Epithelial Cells and Macrophages of Goldfish Carassius Auratus Fed with a High-Cholesterol Diet. Fish Shellfish. Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef]
- Alessio, A.; Pergolizzi, S.; Gervasi, T.; Aragona, M.; Lo Cascio, P.; Cicero, N.; Lauriano, E.R. Biological Effect of Astaxanthin on Alcohol-Induced Gut Damage in Carassius Auratus Used as Experimental Model. Nat. Prod. Res. 2021, 35, 5737–5743. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Capillo, G.; Cascio, P.L.; Lauriano, E.R. Rodlet Cells in Kidney of Goldfish (Carassius Auratus, Linnaeus 1758): A Light and Confocal Microscopy Study. Acta Histochem. 2022, 124, 151876. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Fumia, A.; Calabrò, C.; Lo Cascio, P.; Lauriano, E.R. Mast Cells in Golfish (Carassius Auratus) Gut: Immunohistochemical Characterization. Acta Zool. 2022. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Aragona, M.; Alesci, A.; Lo Cascio, P.; Pergolizzi, S. Toll-like Receptor 2 and α-Smooth Muscle Actin Expressed in the Tunica of a Urochordate, Styela Plicata. Tissue Cell 2021, 71, 101584. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Moussata, D.; Goetz, M.; Gloeckner, A.; Kerner, M.; Campbell, B.; Hoffman, A.; Biesterfeld, S.; Flourie, B.; Saurin, J.-C.; Galle, P.R.; et al. Confocal Laser Endomicroscopy Is a New Imaging Modality for Recognition of Intramucosal Bacteria in Inflammatory Bowel Disease in Vivo. Gut 2011, 60, 26–33. [Google Scholar] [CrossRef]
- Sommer, F.; Adam, N.; Johansson, M.E.V.; Xia, L.; Hansson, G.C.; Bäckhed, F. Altered Mucus Glycosylation in Core 1 O-Glycan-Deficient Mice Affects Microbiota Composition and Intestinal Architecture. PLoS ONE 2014, 9, e85254. [Google Scholar] [CrossRef]
- Yamada, T.; Hino, S.; Iijima, H.; Genda, T.; Aoki, R.; Nagata, R.; Han, K.-H.; Hirota, M.; Kinashi, Y.; Oguchi, H.; et al. Mucin O-Glycans Facilitate Symbiosynthesis to Maintain Gut Immune Homeostasis. EBioMedicine 2019, 48, 513–525. [Google Scholar] [CrossRef]
- Dorofeyev, A.E.; Vasilenko, I.V.; Rassokhina, O.A.; Kondratiuk, R.B. Mucosal Barrier in Ulcerative Colitis and Crohn’s Disease. Gastroenterol. Res. Pract. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Yu, S.; Balasubramanian, I.; Laubitz, D.; Tong, K.; Bandyopadhyay, S.; Lin, X.; Flores, J.; Singh, R.; Liu, Y.; Macazana, C. Paneth Cell-Derived Lysozyme Defines the Composition of Mucolytic Microbiota and the Inflammatory Tone of the Intestine. Immunity 2020, 53, 398–416. [Google Scholar] [CrossRef]
- Shirazi, T.; Longman, R.J.; Corfield, A.P.; Probert, C.S. Mucins and Inflammatory Bowel Disease. Postgrad. Med. J. 2000, 76, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Shajib, M.S.; Khan, W.I. The Role of Serotonin and Its Receptors in Activation of Immune Responses and Inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Chiocchetti, R.; Galiazzo, G.; Giancola, F.; Tagliavia, C.; Bernardini, C.; Forni, M.; Pietra, M. Localization of the Serotonin Transporter in the Dog Intestine and Comparison to the Rat and Human Intestines. Front. Vet. Sci. 2022, 8, 802479. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Atanasova, E.; Carlson, P.J.; Ahmad, U.; Kim, H.J.; Viramontes, B.E.; Mckinzie, S.; Urrutia, R. Serotonin-Transporter Polymorphism Pharmacogenetics in Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2002, 123, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Pant, N.; Saksena, S.; Singla, A.; Nazir, T.M.; Vohwinkel, L.; Turner, J.R.; Goldstein, J.; Alrefai, W.A.; Dudeja, P.K. Function, Expression, and Characterization of the Serotonin Transporter in the Native Human Intestine. Am. J. Physiol. -Gastrointest. Liver Physiol. 2008, 294, G254–G262. [Google Scholar] [CrossRef][Green Version]
- Wendelbo, I.; Mazzawi, T.; El-Salhy, M. Increased Serotonin Transporter Immunoreactivity Intensity in the Ileum of Patients with Irritable Bowel Disease. Mol. Med. Rep. 2014, 9, 180–184. [Google Scholar] [CrossRef][Green Version]
- Spiller, R. Recent Advances in Understanding the Role of Serotonin in Gastrointestinal Motility in Functional Bowel Disorders: Alterations in 5-HT Signalling and Metabolism in Human Disease. Neurogastroenterol. Motil. 2007, 19, 25–31. [Google Scholar] [CrossRef]
- Spiller, R.; Bennett, A. Searching for the Answer to Irritable Bowel Syndrome in the Colonic Mucosa: SERTainty and UnSERTainty. Gastroenterology 2007, 132, 437–441. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis Contributes to Chronic Constipation Development via Regulation of Serotonin Transporter in the Intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef]
- Harrison, E.; Lal, S.; McLaughlin, J.T. Enteroendocrine Cells in Gastrointestinal Pathophysiology. Curr. Opin. Pharmacol. 2013, 13, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.W.; Leslie, F.C.; Levison, S.E.; McLaughlin, J.T. Enteroendocrine Cells: Neglected Players in Gastrointestinal Disorders? Ther. Adv. Gastroenterol. 2008, 1, 51–60. [Google Scholar] [CrossRef]
- Genton, L.; Kudsk, K.A. Interactions between the Enteric Nervous System and the Immune System: Role of Neuropeptides and Nutrition. Am. J. Surg. 2003, 186, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. The Dendritic Cell System and Its Role in Immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Leithäuser, F.; Trobonjaca, Z.; Möller, P.; Reimann, J. Clustering of Colonic Lamina Propria CD4(+) T Cells to Subepithelial Dendritic Cell Aggregates Precedes the Development of Colitis in a Murine Adoptive Transfer Model. Lab. Investig. 2001, 81, 1339–1349. [Google Scholar] [CrossRef]
- Krajina, T.; Leithäuser, F.; Möller, P.; Trobonjaca, Z.; Reimann, J. Colonic Lamina Propria Dendritic Cells in Mice with CD4+ T Cell-Induced Colitis. Eur. J. Immunol. 2003, 33, 1073–1083. [Google Scholar] [CrossRef]
- Roulis, M.; Flavell, R.A. Fibroblasts and Myofibroblasts of the Intestinal Lamina Propria in Physiology and Disease. Differentiation 2016, 92, 116–131. [Google Scholar] [CrossRef]
- Filidou, E.; Valatas, V.; Drygiannakis, I.; Arvanitidis, K.; Vradelis, S.; Kouklakis, G.; Kolios, G.; Bamias, G. Cytokine Receptor Profiling in Human Colonic Subepithelial Myofibroblasts: A Differential Effect of Th Polarization-Associated Cytokines in Intestinal Fibrosis. Inflamm. Bowel Dis. 2018, 24, 2224–2241. [Google Scholar] [CrossRef]
- Valatas, V. Stromal and Immune Cells in Gut Fibrosis: The Myofibroblast and the Scarface. Ann. Gastroenterol. 2017, 30, 393–404. [Google Scholar] [CrossRef]
- Hinz, B. Masters and Servants of the Force: The Role of Matrix Adhesions in Myofibroblast Force Perception and Transmission. Eur. J. Cell Biol. 2006, 85, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Pergolizzi, S.; Cimino, F.; Lauriano, E.; Speciale, A.; D’Angelo, V.; Sicurella, M.; Argnani, R.; Manservigi, R.; Marconi, P. Role of Herpes Simplex Envelope Glycoprotein B and Toll-Like Receptor 2 in Ocular Inflammation: An Ex Vivo Organotypic Rabbit Corneal Model. Viruses 2019, 11, 819. [Google Scholar] [CrossRef]
- Giuffrida, P.; Pinzani, M.; Corazza, G.R.; Di Sabatino, A. Biomarkers of Intestinal Fibrosis—One Step towards Clinical Trials for Stricturing Inflammatory Bowel Disease. United Eur. Gastroenterol. J. 2016, 4, 523–530. [Google Scholar] [CrossRef]
- Latella, G.; Di Gregorio, J.; Flati, V.; Rieder, F.; Lawrance, I.C. Mechanisms of Initiation and Progression of Intestinal Fibrosis in IBD. Scand. J. Gastroenterol. 2015, 50, 53–65. [Google Scholar] [CrossRef]
- Jacob, N.; Targan, S.R.; Shih, D.Q. Cytokine and Anti-Cytokine Therapies in Prevention or Treatment of Fibrosis in IBD. United Eur. Gastroenterol. J. 2016, 4, 531–540. [Google Scholar] [CrossRef]
- Walton, K.L.W.; Holt, L.; Sartor, R.B. Lipopolysaccharide Activates Innate Immune Responses in Murine Intestinal Myofibroblasts through Multiple Signaling Pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G601–G611. [Google Scholar] [CrossRef]
- Brown, M.; Hughes, K.R.; Moossavi, S.; Robins, A.; Mahida, Y.R. Toll-like Receptor Expression in Crypt Epithelial Cells, Putative Stem Cells and Intestinal Myofibroblasts Isolated from Controls and Patients with Inflammatory Bowel Disease. Clin. Exp. Immunol. 2014, 178, 28–39. [Google Scholar] [CrossRef]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Alesci, A.; Fumia, A.; Lo Cascio, P.; Miller, A.; Cicero, N. Immunostimulant and Antidepressant Effect of Natural Compounds in the Management of Covid-19 Symptoms. J. Am. Coll. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can Nutraceuticals Assist Treatment and Improve Covid-19 Symptoms? Nat. Prod. Res. 2021, 1–20. [Google Scholar] [CrossRef]
- Fumia, A.; Cicero, N.; Gitto, M.; Nicosia, N.; Alesci, A. Role of Nutraceuticals on Neurodegenerative Diseases: Neuroprotective and Immunomodulant Activity. Nat. Prod. Res. 2021, 1–18. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Fumia, A.; Lauriano, E.R. Neuronal Regeneration: Vertebrates Comparative Overview and New Perspectives for Neurodegenerative Diseases. Acta Zool. 2021, 103, 129–140. [Google Scholar] [CrossRef]
- Alesci, A.; Lauriano, E.R.; Fumia, A.; Irrera, N.; Mastrantonio, E.; Vaccaro, M.; Gangemi, S.; Santini, A.; Cicero, N.; Pergolizzi, S. Relationship between Immune Cells, Depression, Stress, and Psoriasis: Could the Use of Natural Products Be Helpful? Molecules 2022, 27, 1953. [Google Scholar] [CrossRef]
- Gardiner, K.R.; Maxwell, R.J.; Rowlands, B.J.; Barclay, G.R. Intestinal Permeability. Gut 1995, 37, 589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and Its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed]

| Laboratory studies | Electrolytes, creatinine, erythrocyte sedimentation rate, complete blood count with differential, blood urea nitrogen, liver function tests, transferrin, bilirubin, ferritin, folic acid, vitamin B12, urine strip, C-reactive protein, faecal calprotectin. |
| Microbial studies | Stool cultures |
| Endoscopy | Ileocolonoscopy (spared rectum, ileal inflammation, skip lesions, cobble stoning, fissural and longitudinal ulcers, strictures, fistulas). Oesophagogastroduodenoscopy with biopsies when symptoms occur in the upper gastrointestinal tract.The SES-CD criteria (The Simple Endoscopic Index for Crohn’s Disease) for patients with unoperated colon and ileus, and The Rutgeert’s Score for patients with surgery (with particular reference to anastomotic recurrence) were used in the endoscopic assessment of the severity of patients with Crohn’s disease. |
| Primary Antibodies | Supplier | Catalogue Number | Source | Dilution | Antibody ID |
| Anti-serotonin (5-HT) | Sigma-Aldrich | S5545 | rabbit | 1:500 | AB_477522 |
| Langerin (H-4) | Santa Cruz biotechnology, inc | sc-271272 | mouse | 1:500 | AB_10611518 |
| α-Smooth Muscle Actin | Sigma-Aldrich | A5228 | mouse | 1:200 | AB_262054 |
| Secondary Antibodies | Supplier | Catalogue Number | Source | Dilution | Antibody ID |
| Alexa Fluor 488 anti-mouse IgG FITC conjugated | Invitrogen | A-21202 | donkey | 1:300 | AB_141607 |
| Alexa Fluor 594 anti-rabbit IgG TRITC conjugated | Invitrogen | A32754 | donkey | 1:300 | AB_2762827 |
| Intestine Sections of Patients with Crohn’s Disease | Intestine Sections of Control Subjects | |
|---|---|---|
| Myofibroblasts α-SMA+ | 542.38 ± 85.82 ** | 103.86 ± 9.48 * |
| 5-HT+ cells in lamina propria | 369.71 ± 7.76 * | 97.13 ± 9.33 * |
| Merge α-SMA/5-HT | 306.75 ± 12.40 * | 93.17 ± 9.52 * |
| LCs Langerin+ | 816.53 ± 118.91 * | 121.63 ± 11.22 ** |
| Goblet Cells 5-HT+ | 102.38 ± 11.05 ** | 1056.67 ± 37.14 * |
| Merge Lan/5-HT | 327.52 ± 6.01 ** | 102.69 ± 11.53 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergolizzi, S.; Alesci, A.; Centofanti, A.; Aragona, M.; Pallio, S.; Magaudda, L.; Cutroneo, G.; Lauriano, E.R. Role of Serotonin in the Maintenance of Inflammatory State in Crohn’s Disease. Biomedicines 2022, 10, 765. https://doi.org/10.3390/biomedicines10040765
Pergolizzi S, Alesci A, Centofanti A, Aragona M, Pallio S, Magaudda L, Cutroneo G, Lauriano ER. Role of Serotonin in the Maintenance of Inflammatory State in Crohn’s Disease. Biomedicines. 2022; 10(4):765. https://doi.org/10.3390/biomedicines10040765
Chicago/Turabian StylePergolizzi, Simona, Alessio Alesci, Antonio Centofanti, Marialuisa Aragona, Socrate Pallio, Ludovico Magaudda, Giuseppina Cutroneo, and Eugenia Rita Lauriano. 2022. "Role of Serotonin in the Maintenance of Inflammatory State in Crohn’s Disease" Biomedicines 10, no. 4: 765. https://doi.org/10.3390/biomedicines10040765
APA StylePergolizzi, S., Alesci, A., Centofanti, A., Aragona, M., Pallio, S., Magaudda, L., Cutroneo, G., & Lauriano, E. R. (2022). Role of Serotonin in the Maintenance of Inflammatory State in Crohn’s Disease. Biomedicines, 10(4), 765. https://doi.org/10.3390/biomedicines10040765










