Modulation of Macrophage Response by Copper and Magnesium Ions in Combination with Low Concentrations of Dexamethasone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Macrophage Metabolic Activity Assay
2.3. Macrophage Morphology Analysis
2.4. Macrophage Gene Expression Analysis
2.5. Mitigating the Effect of a Pro-Inflammatory Stimulus with Ions and Dexamethasone
2.6. Statistical Analysis
3. Results
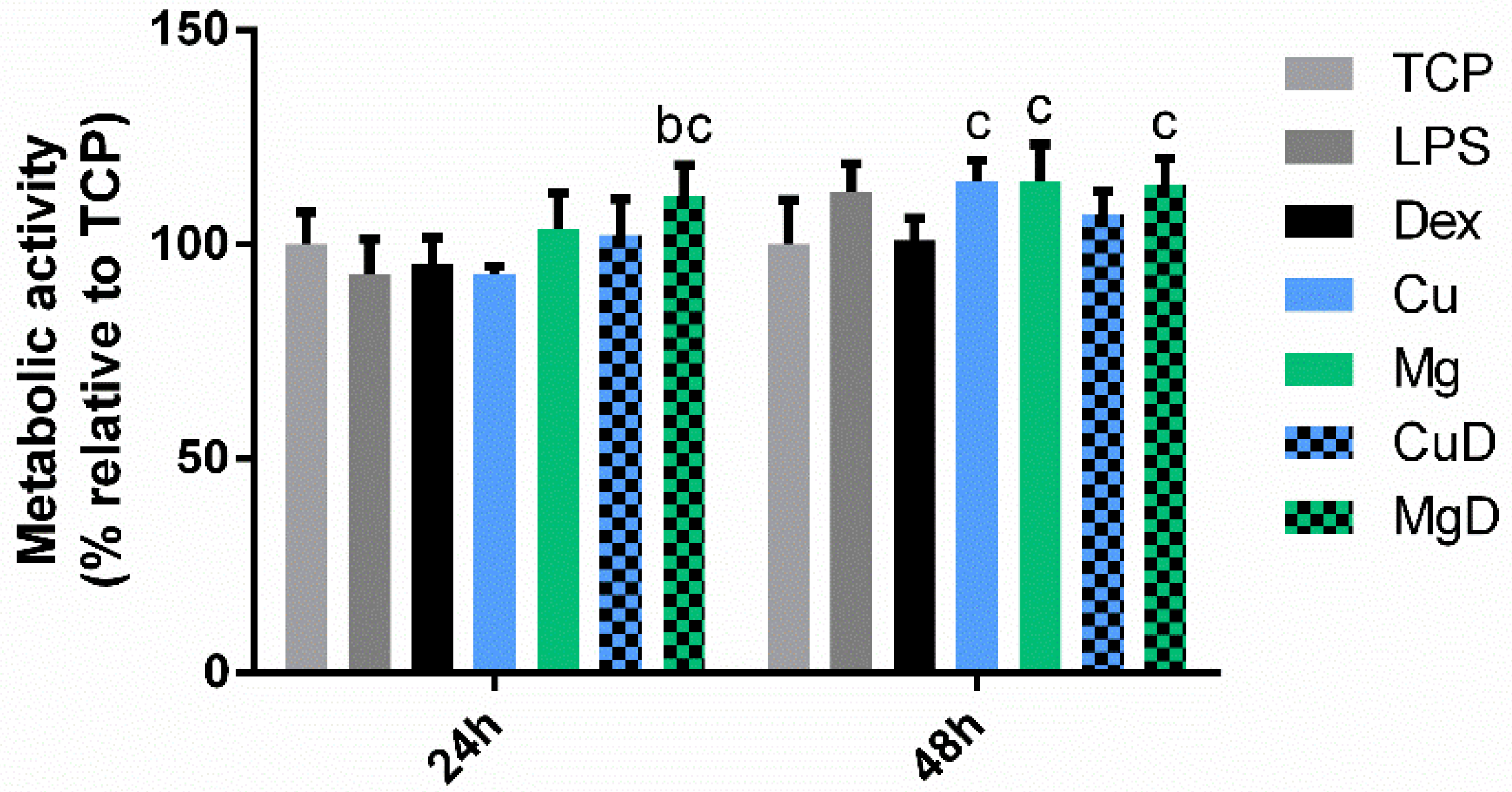
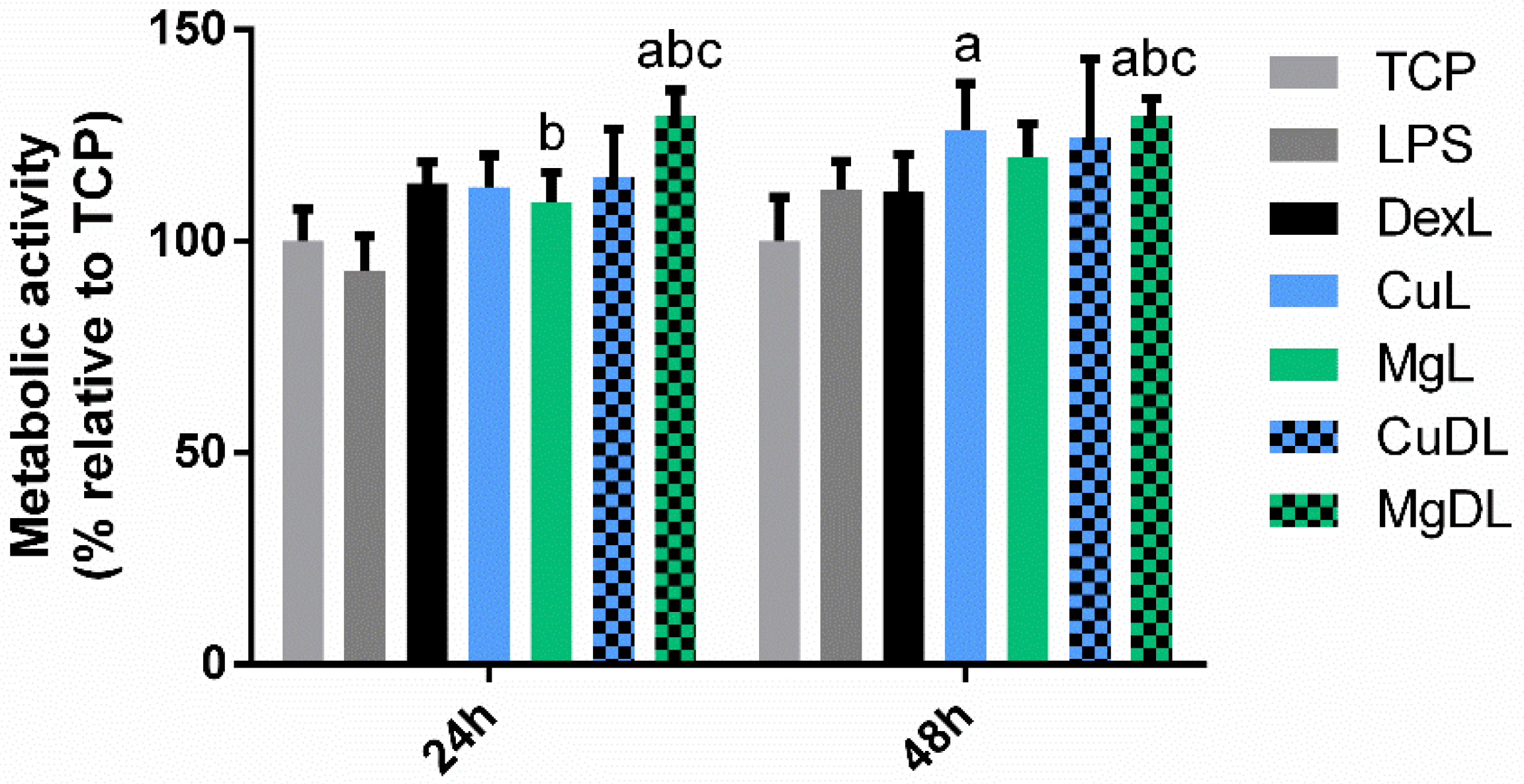
3.1. Macrophage Metabolic Activity Assay
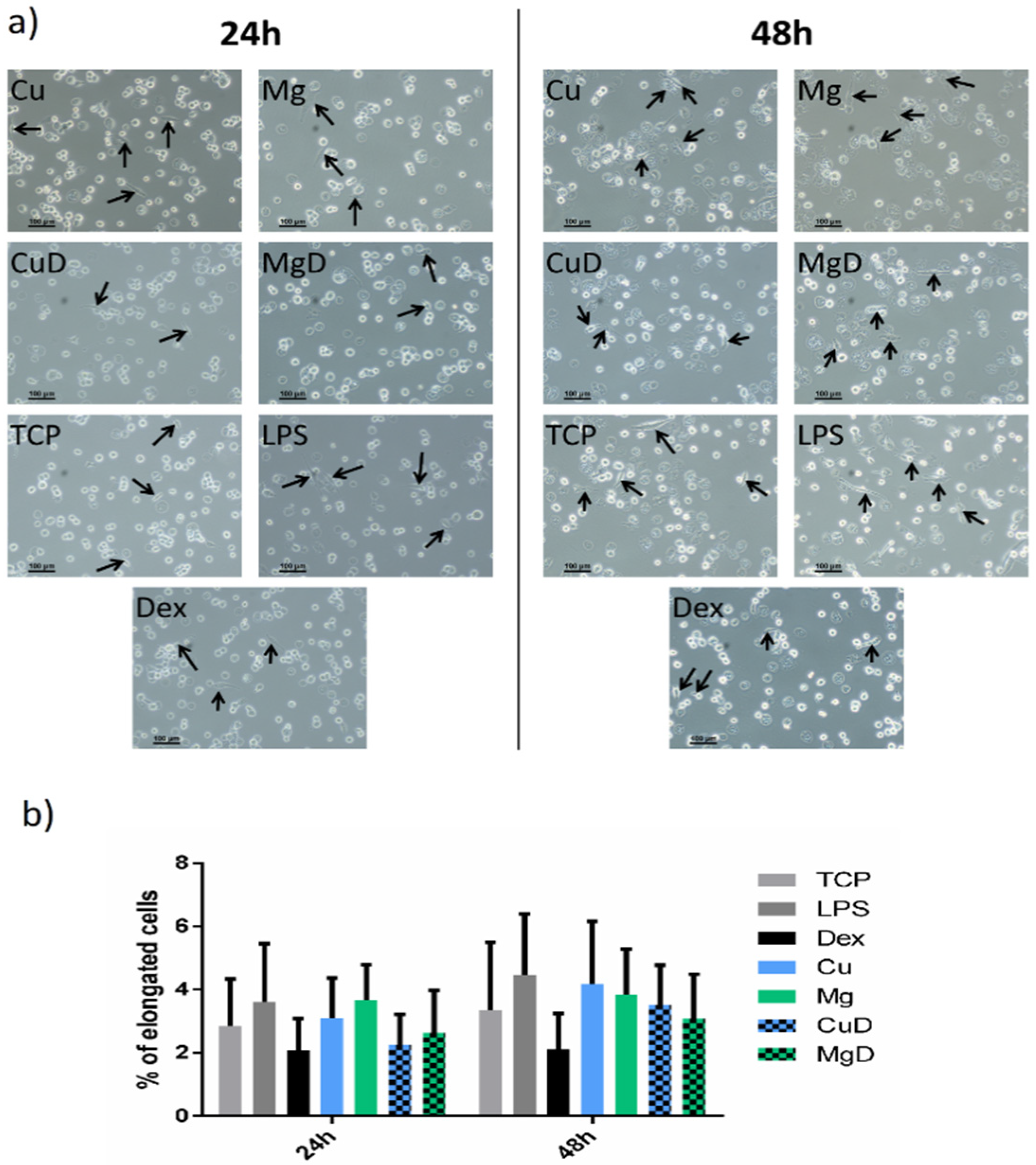
3.2. Macrophage Morphology Analysis
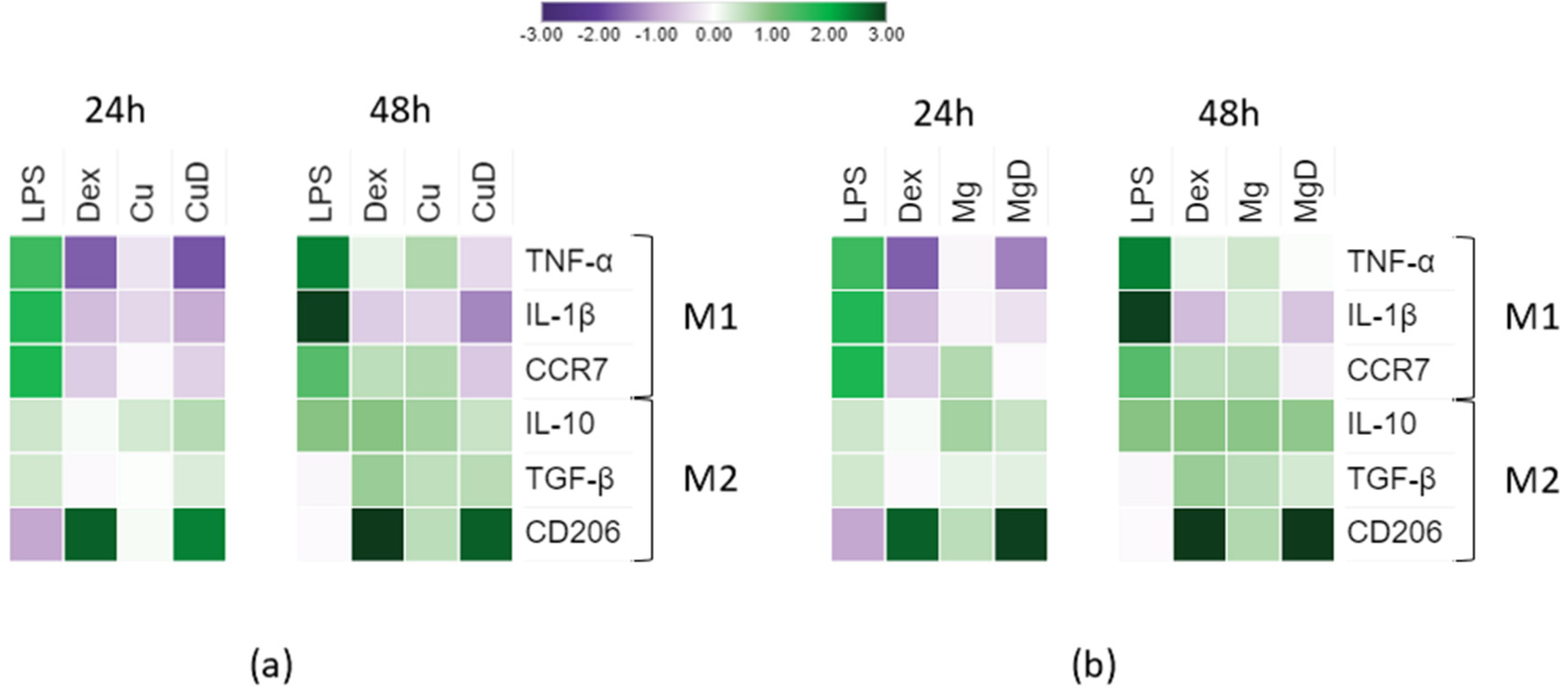
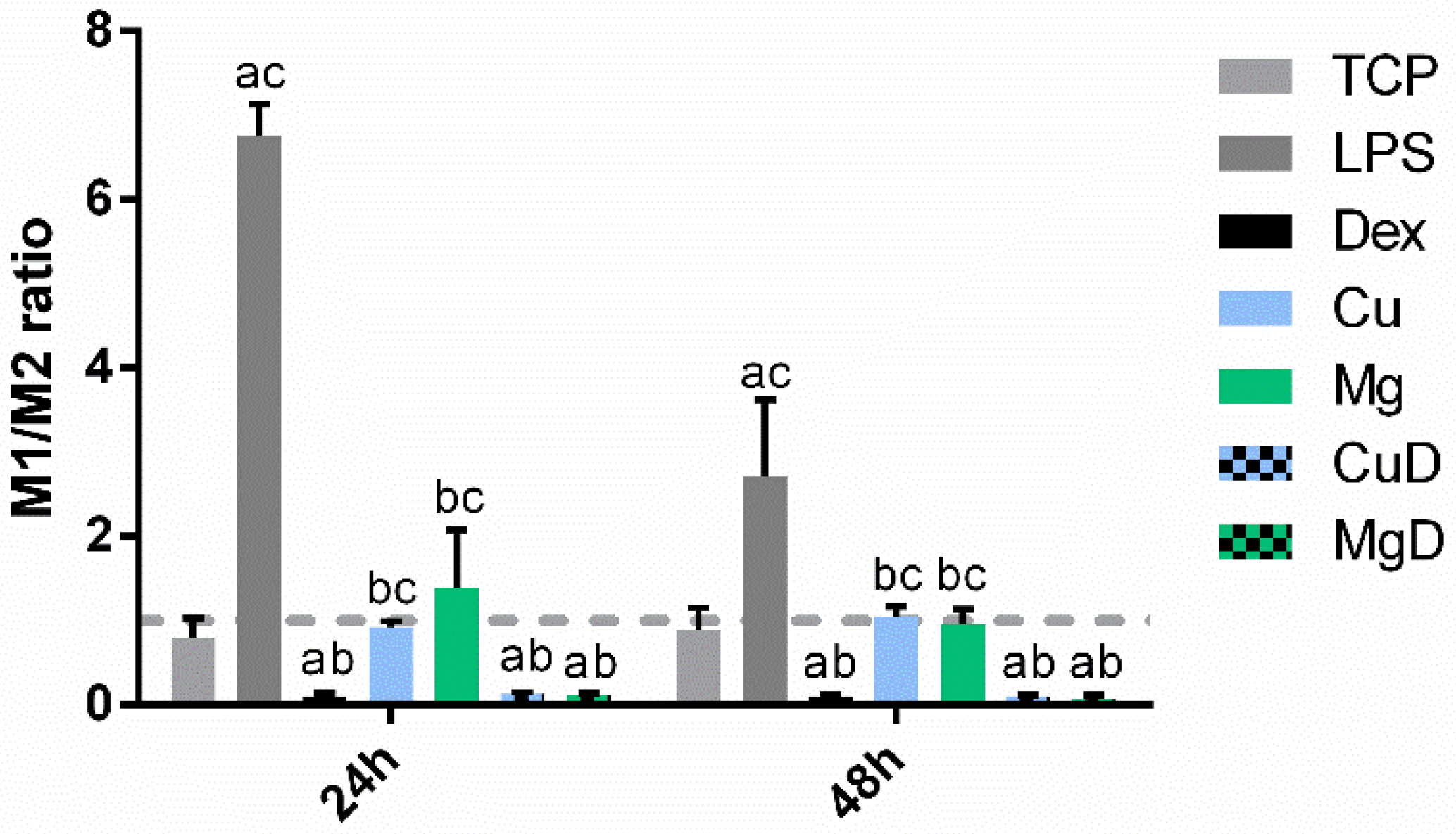
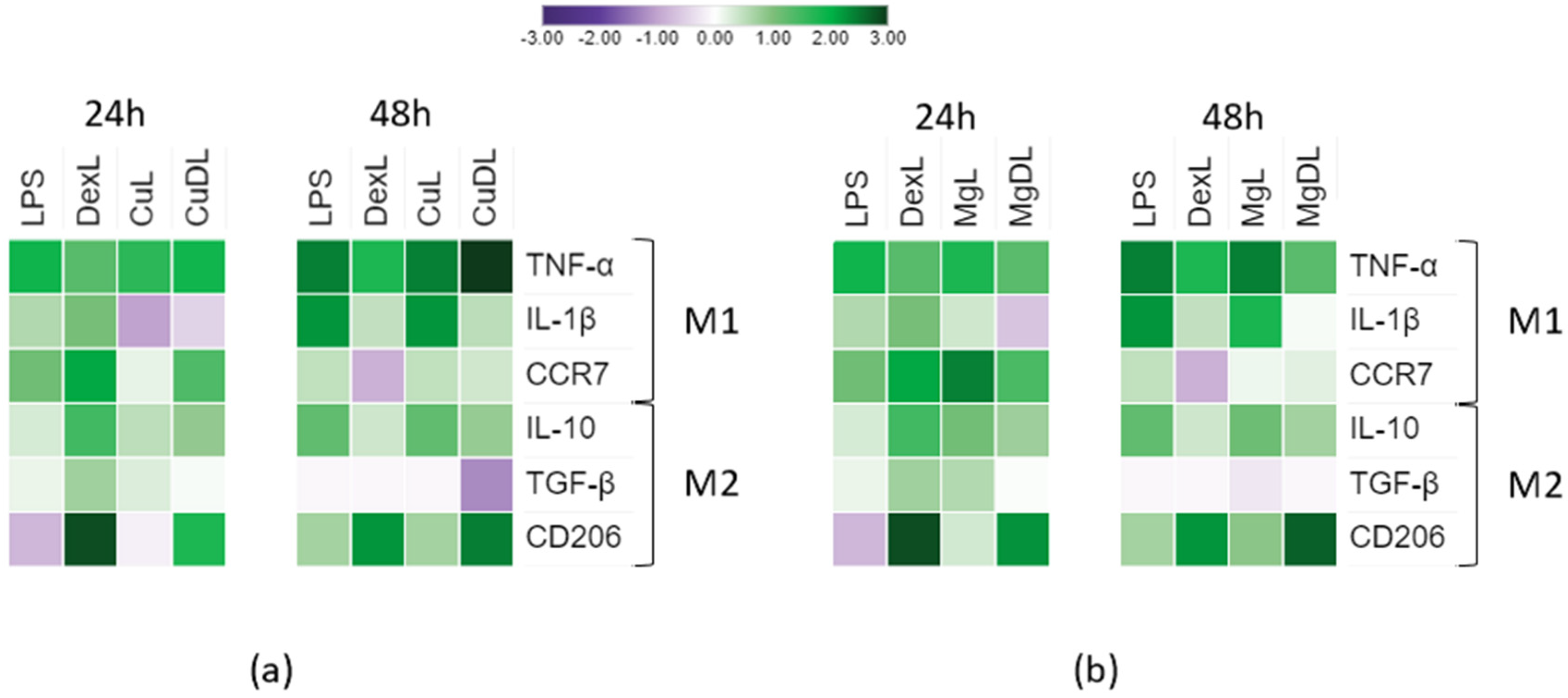
3.3. Macrophage Gene Expression Analysis
3.4. Mitigating the Effect of a Pro-inflammatory Stimulus with Ions and Dexamethasone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Perez, R.; Seo, S.-J.; Won, J.-E.; Lee, E.-J.; Jang, J.-H.; Knowles, J.C.; Kim, H.-W. Therapeutically relevant aspects in bone repair and regeneration. Mater. Today 2015, 18, 573–589. [Google Scholar] [CrossRef]
- Baker-LePain, J.C.; Nakamura, M.C.; Lane, N.E. Effects of inflammation on bone: An update. Curr. Opin. Rheumatol. 2011, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Chen, M.H.; Chung, J.J.; Mealy, J.E.; Zaman, S.; Li, E.C.; Arisi, M.F.; Atluri, P.; Burdick, J.A. Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery. Macromol. Biosci. 2019, 19, 117–138. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Park, J.-S.; Lee, S.J.; Jang, J.; Park, J.S.; Back, S.H.; Bahn, G.; Park, J.H.; Kang, Y.M.; Kim, S.H.; et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J. Control. Release 2015, 216, 140–148. [Google Scholar] [CrossRef]
- Chen, S.-H.; Zhaori, G. Potential clinical applications of siRNA technique: Benefits and limitations. Eur. J. Clin. Investig. 2011, 41, 221–232. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Tabata, Y. Dual-controlled release system of drugs for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 28–40. [Google Scholar] [CrossRef]
- Mathew, A.; Vaquette, C.; Hashimi, S.; Rathnayake, I.; Huygens, F.; Hutmacher, D.W.; Ivanovski, S. Antimicrobial and Immunomodulatory Surface-Functionalized Electrospun Membranes for Bone Regeneration. Adv. Healthc. Mater. 2017, 6, 1601345. [Google Scholar] [CrossRef]
- Feng, S.; Nie, L.; Zou, P.; Suo, J. Effects of drug and polymer molecular weight on drug release from PLGA-mPEG microspheres. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Rué, E.; Diez-Tercero, L.; Giordano-Kelhoffer, B.; Delgado, L.M.; Bosch, B.M.; Hoyos-Nogués, M.; Mateos-Timoneda, M.A.; Tran, P.A.; Gil, F.J.; Perez, R.A. Biological Roles and Delivery Strategies for Ions to Promote Osteogenic Induction. Front. Cell Dev. Biol. 2021, 8, 1809. [Google Scholar] [CrossRef]
- Díez-Tercero, L.; Delgado, L.M.; Bosch-Rué, E.; Perez, R.A. Evaluation of the immunomodulatory effects of cobalt, copper and magnesium ions in a pro inflammatory environment. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Xu, M.; Yang, F.; Wang, W.; Niu, X. In vitro immunomodulation of magnesium on monocytic cell toward anti-inflammatory macrophages. Regen. Biomater. 2020, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Keeler, G.D.; Durdik, J.; Stenken, J.A. Localized delivery of dexamethasone-21-phosphate via microdialysis implants in rat induces M(GC) macrophage polarization and alters CCL2 concentrations. Acta Biomater. 2015, 12, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.J.; Han, S.H.; Lee, Y.W.; Lee, M.; Yang, K.H.; Kim, H.M. Dexamethasone inhibits IL-1β gene expression in LPS-stimulated RAW 264.7 cells by blocking NF-κB/Rel and AP-1 activation. Immunopharmacology 2000, 48, 173–183. [Google Scholar] [CrossRef]
- Chuang, T.-Y.; Cheng, A.-J.; Chen, I.-T.; Lan, T.-Y.; Huang, I.-H.; Shiau, C.-W.; Hsu, C.-L.; Liu, Y.-W.; Chang, Z.-F.; Tseng, P.-H.; et al. Suppression of LPS-induced inflammatory responses by the hydroxyl groups of dexamethasone. Oncotarget 2017, 8, 49735–49748. [Google Scholar] [CrossRef]
- Gewert, K.; Hiller, G.; Sundler, R. Effects of dexamethasone on mitogen-activated protein kinases in mouse macrophages. Biochem. Pharmacol. 2000, 60, 545–551. [Google Scholar] [CrossRef]
- Su, N.-Y.; Peng, T.-C.; Tsai, P.-S.; Huang, C.-J. Phosphoinositide 3-kinase/Akt pathway is involved in mediating the anti-inflammation effects of magnesium sulfate. J. Surg. Res. 2013, 185, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, L.; Ren, L.; Tang, T.; Dai, K.; Yang, K. A novel nano-copper-bearing stainless steel with reduced Cu2+ release only inducing transient foreign body reaction via affecting the activity of NF-κB and Caspase 3. Int. J. Nanomed. 2015, 10, 6725–6739. [Google Scholar] [CrossRef] [Green Version]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.-Y.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.; Badylak, S. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Gewert, K.; Svensson, U.; Andersson, K.; Holst, E.; Sundler, R. Dexamethasone Differentially Regulates Cytokine Transcription and Translation in Macrophages Responding to Bacteria or Okadaic Acid. Cell. Signal. 1999, 11, 665–670. [Google Scholar] [CrossRef]
- Delgado, L.M.; Shologu, N.; Fuller, K.; Zeugolis, D.I. Acetic acid and pepsin result in high yield, high purity and low macrophage response collagen for biomedical applications. Biomed. Mater. 2017, 12, 065009. [Google Scholar] [CrossRef] [Green Version]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.-C.; Léone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Roth, J.; Barczyk-Kahlert, K. More Than Suppression: Glucocorticoid Action on Monocytes and Macrophages. Front. Immunol. 2019, 10, 2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, R.H.; Kumar, M.N.; Kumar, K.K.; Nagesh, R.; Kavya, K.; Babu, R.; Ramesh, G.T.; Sharma, S.C. Dexamethasone inhibits inflammatory response via down regulation of AP-1 transcription factor in human lung epithelial cells. Gene 2017, 645, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013, 34, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Liu, H.; Sidiropoulos, P.; Song, G.; Pagliari, L.J.; Birrer, M.J.; Stein, B.; Anrather, J.; Pope, R.M. TNF-α Gene Expression in Macrophages: Regulation by NF-κB Is Independent of c-Jun or C/EBPβ. J. Immunol. 2000, 164, 4277–4285. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Verma, R.; Balakrishnan, L.; Sharma, K.; Khan, A.A.; Advani, J.; Gowda, H.; Tripathy, S.P.; Suar, M.; Pandey, A.; Gandotra, S.; et al. A network map of Interleukin-10 signaling pathway. J. Cell Commun. Signal. 2016, 10, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Labonte, A.C.; Tosello-Trampont, A.-C.; Hahn, Y.S. The Role of Macrophage Polarization in Infectious and Inflammatory Diseases. Mol. Cells 2014, 37, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Hudson, W.; de Vera, I.M.; Nwachukwu, J.C.; Weikum, E.R.; Herbst, A.; Yang, Q.; Bain, D.L.; Nettles, K.W.; Kojetin, D.J.; Ortlund, E.A. Cryptic glucocorticoid receptor-binding sites pervade genomic NF-κB response elements. Nat. Commun. 2018, 9, 1337. [Google Scholar] [CrossRef]
- Jin, L.; Chen, C.; Li, Y.; Yuan, F.; Gong, R.; Wu, J.; Zhang, H.; Kang, B.; Yuan, G.; Zeng, H.; et al. A Biodegradable Mg-Based Alloy Inhibited the Inflammatory Response of THP-1 Cell-Derived Macrophages Through the TRPM7–PI3K–AKT1 Signaling Axis. Front. Immunol. 2019, 10, 2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessa-Gonçalves, M.; Silva, A.M.; Brás, J.; Helmholz, H.; Luthringer-Feyerabend, B.J.; Willumeit-Römer, R.; Barbosa, M.A.; Santos, S.G. Fibrinogen and magnesium combination biomaterials modulate macrophage phenotype, NF-kB signaling and crosstalk with mesenchymal stem/stromal cells. Acta Biomater. 2020, 114, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, S.; Li, W.; Tao, W.; Shi, T.; Zhao, Y.L. Theoretical Investigation of the Structural Characteristics in the Active State of Akt1 Kinase. J. Chem. Inf. Model. 2019, 59, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, Z.; Farnaghi, S.; Friis, T.; Mao, X.; Xiao, Y.; Wu, C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016, 30, 334–344. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Zhao, Q.; Xie, Y.; Luo, R.; Yang, P.; Weng, Y. Immobilization of nano Cu-MOFs with polydopamine coating for adaptable gasotransmitter generation and copper ion delivery on cardiovascular stents. Biomaterials 2019, 204, 36–45. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Yang, X.; Du, Z.; Zhou, L.; Li, S.; Chen, C.; Luo, K.; Lin, J. Copper-Modified Ti6Al4 V Suppresses Inflammatory Response and Osteoclastogenesis while Enhancing Extracellular Matrix Formation for Osteoporotic Bone Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3364–3373. [Google Scholar] [CrossRef]
- Bartuzi, P.; Hofker, M.H.; van de Sluis, B. Tuning NF-κB activity: A touch of COMMD proteins. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 2315–2321. [Google Scholar] [CrossRef] [Green Version]
- Balsano, C.; Porcu, C.; Sideri, S. Is copper a new target to counteract the progression of chronic diseases? Metallomics 2018, 10, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Muller, P.; Van Bakel, H.; Van De Sluis, B.; Holstege, F.; Wijmenga, C.; Klomp, L.W.J. Gene expression profiling of liver cells after copper overload in vivo and in vitro reveals new copper-regulated genes. J. Biol. Inorg. Chem. 2007, 12, 495–507. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Zhou, L.; He, M.; Zhuo, J.; Zhong, Q.; Luo, K.; Lin, J. Tuning osteoporotic macrophage responses to favour regeneration by Cu-bearing titanium alloy in Porphyromonas gingivalis lipopolysaccharide-induced microenvironments. Regen. Biomater. 2021, 8, 1–10. [Google Scholar] [CrossRef]

| Macrophage Phenotype | Gene | Forward (Sequence 5′–3′) | Reverse (Sequence 5′–3′) |
|---|---|---|---|
| M1 | TNF-α | TTCCAGACTTCCTTGAGACACG | AAACATGTCTGAGCCAAGGC |
| IL-1β | GACACATGGGATAACGAGGC | ACGCAGGACAGGTACAGATT | |
| CCR7 | GGCTGGTCGTGTTGACCTAT | ACGTAGCGGTCAATGCTGAT | |
| M2 | IL-10 | AAGCCTGACCACGCTTTCTA | ATGAAGTGGTTGGGGAATGA |
| TGF-β | TTGATGTCACCGGAGTTGTG | TGATGTCCACTTGCAGTGTG | |
| CD206 | CCTGGAAAAAGCTGTGTGTCAC | AGTGGTGTTGCCCTTTTTGC | |
| Housekeeping | β-actin | AGAGCTACGAGCTGCCTGAC | AGCACTGTGTTGGCGTACAG |
| Molecules Incorporated Into the Cell Culture Medium | ||||
|---|---|---|---|---|
| LPS (10 ng/mL) | Dex (0.1 nM) | Cu2+ Ions (10 µM) | Mg2+ Ions (3.2 mM) | |
| TCP | − | − | − | − |
| LPS | + | − | − | − |
| DexL | + | + | − | − |
| CuL | + | − | + | − |
| MgL | + | − | − | + |
| CuDL | + | + | + | − |
| MgDL | + | + | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Tercero, L.; Delgado, L.M.; Perez, R.A. Modulation of Macrophage Response by Copper and Magnesium Ions in Combination with Low Concentrations of Dexamethasone. Biomedicines 2022, 10, 764. https://doi.org/10.3390/biomedicines10040764
Díez-Tercero L, Delgado LM, Perez RA. Modulation of Macrophage Response by Copper and Magnesium Ions in Combination with Low Concentrations of Dexamethasone. Biomedicines. 2022; 10(4):764. https://doi.org/10.3390/biomedicines10040764
Chicago/Turabian StyleDíez-Tercero, Leire, Luis M. Delgado, and Roman A. Perez. 2022. "Modulation of Macrophage Response by Copper and Magnesium Ions in Combination with Low Concentrations of Dexamethasone" Biomedicines 10, no. 4: 764. https://doi.org/10.3390/biomedicines10040764
APA StyleDíez-Tercero, L., Delgado, L. M., & Perez, R. A. (2022). Modulation of Macrophage Response by Copper and Magnesium Ions in Combination with Low Concentrations of Dexamethasone. Biomedicines, 10(4), 764. https://doi.org/10.3390/biomedicines10040764







