Time-Specific Factors Influencing the Development of Asthma in Children
Abstract
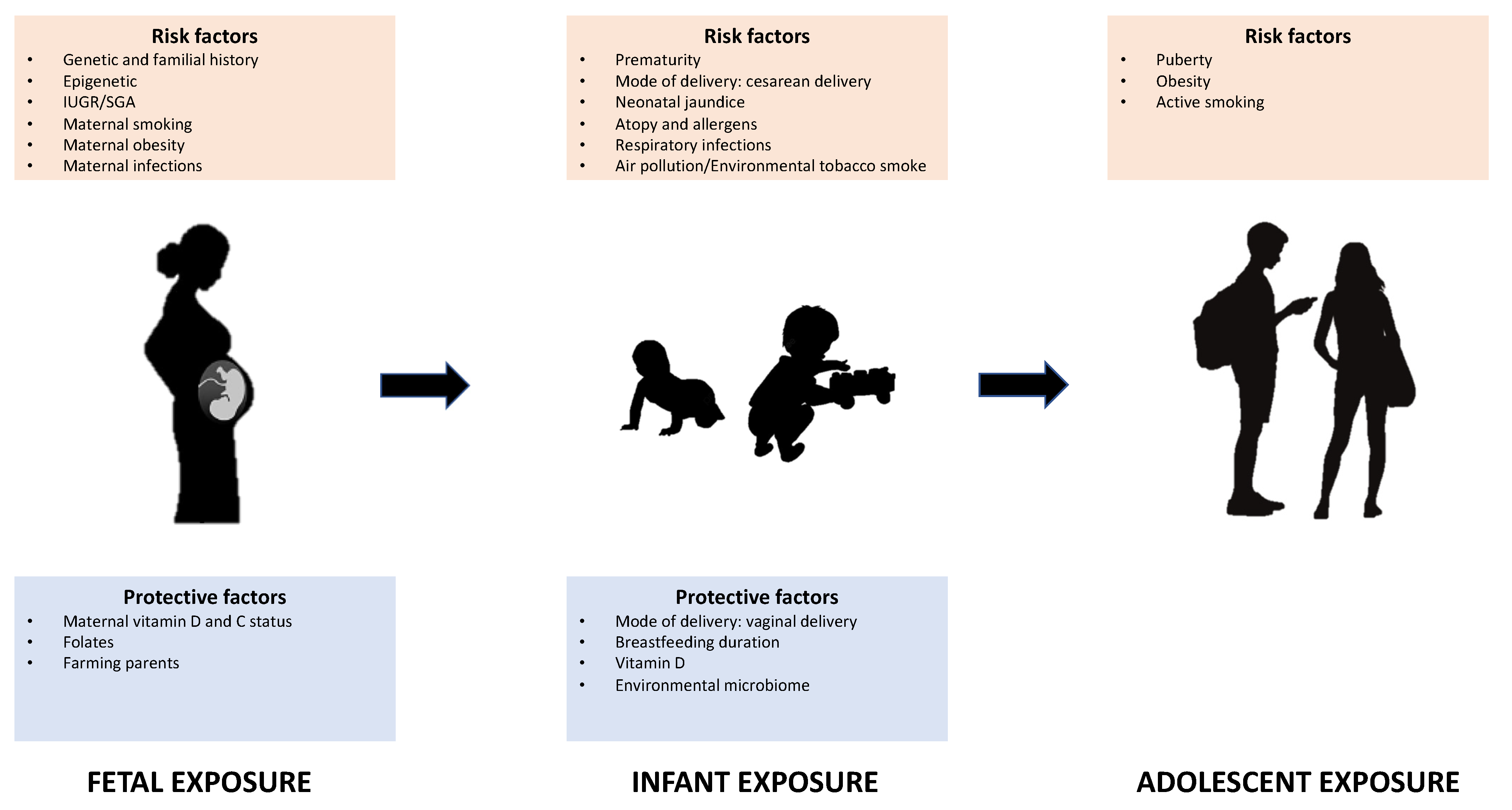
1. Introduction
2. Materials and Methods
3. Fetal and Perinatal Exposure
3.1. Genetic Factors
3.2. Maternal Infections and Drugs in Pregnancy
3.3. Allergen Exposure
3.4. Maternal Smoking Exposure and Oxidative Stress
3.5. Intrauterine Growth Restriction
3.6. Environmental Exposures
3.7. Endocrine Disruptors
3.8. Epigenetics
3.9. Prenatal Farming Exposure
3.10. Vitamins
3.11. Prematurity
3.12. Birth Characteristics
3.13. Breastfeeding
3.14. Neonatal Jaundice
4. Infant Exposure
4.1. Antibiotics and Paracetamol
4.2. Vitamin D
4.3. Viral Infections
4.4. Day-Care Attendance and Parental Socioeconominc Status
4.5. Atopy
4.6. Infant Farming Exposure
4.7. Air Pollution
4.8. Gastroesophageal Reflux Disease
5. Adolescent Exposure
5.1. Obesity
5.2. Tobacco Smoke
5.3. Gender and Puberty
5.4. Nutrition
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Asher, I.; Pearce, N. Global burden of asthma among children. Int. J. Tuberc. Lung Dis. 2014, 18, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2021. Available online: www.ginasthma.org (accessed on 26 January 2022).
- NICE Guideline [NG80]. Asthma: Diagnosis, Monitoring and Chronic Asthma Management. 2017. Available online: https://www.nice.org.uk/guidance/ng80 (accessed on 22 January 2022).
- de Jong, C.C.M.; Pedersen, E.S.L.; Mozun, R.; Goutaki, M.; Trachsel, D.; Barben, J.; Kuehni, C.E. Diagnosis of asthma in children: The contribution of a detailed history and test results. Eur. Respir. J. 2019, 54, 1901326. [Google Scholar] [CrossRef]
- Duijts, L. Fetal and infant origins of asthma. Eur. J. Epidemiol. 2012, 27, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Duijts, L.; Reiss, I.K.; Brusselle, G.; de Jongste, J.C. Early origins of chronic obstructive lung diseases across the life course. Eur. J. Epidemiol. 2014, 29, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Balbus, J.; Birnbaum, L.; Brune-Drisse, M.N.; Grandjean, P.; Gray, K.; Landrigan, P.J.; Sly, P.D.; Suk, W.; Slechta, D.C.; et al. Developmental origins of health and disease: Integrating environmental influences. Endocrinology 2015, 156, 3416–3421. [Google Scholar] [CrossRef]
- Calkins, K.; Devaskar, S.U. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care 2011, 41, 158–176. [Google Scholar] [CrossRef]
- Barker, D.J.P. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- Brew, B.K.; Marks, G.B. Perinatal factors and respiratory health in children. Clin. Exp. Allergy 2012, 42, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.R.; Burge, H.A.; Carey, V.; Milton, D.K.; Platts-Mills, T.; Weiss, S.T. Predictors of repeated wheeze in the first year of life. Am. J. Respir. Crit. Care Med. 1999, 160, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, P.K. The development of large and small airways. Am. J. Respir. Crit. Care Med. 1998, 157, S174–S180. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Beardsmore, C.S.; Owers-Bradley, J.; Dogaru, C.M.; Mada, M.; Ball, I.; Garipov, R.R.; Kuehni, C.E.; Spycher, B.D.; Silverman, M. Catch-up alveolarization in ex-preterm children. Evidence from 3 he magnetic resonance. Am. J. Respir. Crit. Care Med. 2013, 187, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Joss-Moore, L.A.; Lane, R.H.; Albertine, K.H. Epigenetic contributions to the developmental origins of adult lung disease. Biochem. Cell Biol. 2015, 93, 119–127. [Google Scholar] [CrossRef]
- Kahr, N.; Naeser, V.; Stensballe, L.G.; Kyvik, K.O.; Skytthe, A.; Backer, V.; Bønnelykke, K.; Thomsen, S.F. Gene-environment interaction in atopic diseases: A population-based twin study of early-life exposures. Clin. Respir. J. 2015, 9, 79–86. [Google Scholar] [CrossRef]
- Morales, E.; Duffy, D. Genetics and gene-environment interactions in childhood and adult onset asthma. Front. Pediatr. 2019, 7, 499. [Google Scholar] [CrossRef]
- D’Elios, M.; Del Prete, G. Th1/Th2 balance in human disease. Transplant. Proc. 1998, 30, 2373–2377. [Google Scholar] [CrossRef]
- Dietert, R.R. Developmental immunotoxicology (DIT): Windows of vulnerability, immune dysfunction and safety assessment. J. Immunotoxicol. 2008, 5, 401–412. [Google Scholar] [CrossRef]
- Kamali, A.N.; Noorbakhsh, S.M.; Hamedifar, H.; Jadidi-Niaragh, F.; Yazdani, R.; Bautista, J.M.; Azizi, G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2019, 105, 107–115. [Google Scholar] [CrossRef]
- García-Serna, A.M.; Martín-Orozco, E.; Hernández-Caselles, T.; Morales, E. Prenatal and perinatal environmental influences shaping the neonatal immune system: A focus on asthma and allergy origins. Int. J. Environ. Res. Public Health 2021, 18, 3962. [Google Scholar] [CrossRef]
- de Kleer, I.M.; Kool, M.; de Bruijn, M.J.W.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef]
- Saluzzo, S.; Gorki, A.-D.; Rana, B.M.J.; Martins, R.; Scanlon, S.; Starkl, P.; Lakovits, K.; Hladik, A.; Korosec, A.; Sharif, O.; et al. First-breath-induced type 2 pathways shape the lung immune environment. Cell Rep. 2017, 18, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Pattaroni, C.; Watzenboeck, M.L.; Schneidegger, S.; Kieser, S.; Wong, N.C.; Bernasconi, E.; Pernot, J.; Mercier, L.; Knapp, S.; Nicod, L.P.; et al. Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe 2018, 24, 857–865.e4. [Google Scholar] [CrossRef]
- Thomsen, S.F.; Van Der Sluis, S.; Kyvik, K.O.; Skytthe, A.; Backer, V. Estimates of asthma heritability in a large twin sample. Clin. Exp. Allergy 2010, 40, 1054–1061. [Google Scholar] [CrossRef]
- Bobolea, I.; Arismendi, E.; Valero, A.; Agustí, A. Early life origins of asthma: A review of potential effectors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O.C.M. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.M.; Thompson, E.E.; Schoettler, N.; Helling, B.A.; Magnaye, K.M.; Stanhope, C.; Igartua, C.; Morin, A.; Washington, C.; Nicolae, D.; et al. A decade of research on the 17q12-21 asthma locus: Piecing together the puzzle. J. Allergy Clin. Immunol. 2018, 142, 749–764.e3. [Google Scholar] [CrossRef]
- Dijk, F.N.; Xu, C.; Melén, E.; Carsin, A.-E.; Kumar, A.; Nolte, I.M.; Gruzieva, O.; Pershagen, G.; Grotenboer, N.S.; Savenije, O.E.M.; et al. Genetic regulation of IL1RL1 methylation and IL1RL1-a protein levels in asthma. Eur. Respir. J. 2018, 51, 1701377. [Google Scholar] [CrossRef]
- Wang, Q.-P.; Wu, K.-M.; Li, Z.-Q.; Xue, F.; Chen, W.; Ji, H.; Wang, B.-L. Association between maternal allergic rhinitis and asthma on the prevalence of atopic disease in offspring. Int. Arch. Allergy Immunol. 2012, 157, 379–386. [Google Scholar] [CrossRef]
- Lim, R.H.; Kobzik, L.; Dahl, M. Risk for asthma in offspring of asthmatic mothers versus fathers: A meta-analysis. PLoS ONE 2010, 5, e10134. [Google Scholar] [CrossRef] [PubMed]
- van Meel, E.R.; Attanasi, M.; Jaddoe, V.W.V.; Reiss, I.K.M.; Moll, H.A.; de Jongste, J.C.; Duijts, L. Chlamydia trachomatis during pregnancy and childhood asthma-related morbidity: A population-based prospective cohort. Eur. Respir. J. 2020, 56, 1901829. [Google Scholar] [CrossRef]
- Gray, L.E.K.; O’Hely, M.; Ranganathan, S.; Sly, P.D.; Vuillermin, P. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front. Immunol. 2017, 8, 365. [Google Scholar] [CrossRef]
- Gaffin, J.M.; Kanchongkittiphon, W.; Phipatanakul, W. Perinatal and early childhood environmental factors influencing allergic asthma immunopathogenesis. Int. Immunopharmacol. 2014, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Lu, C.; Wang, J.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Qian, H.; Sun, Y.; et al. Asthma and rhinitis among Chinese children—Indoor and outdoor air pollution and indicators of socioeconomic status (SES). Environ. Int. 2018, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-J.; Chiang, T.-L.; Lin, S.-J.; Guo, Y.L. Predicting risk for childhood asthma by pre-pregnancy, perinatal, and postnatal factors. Pediatr. Allergy Immunol. 2015, 26, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Yoshida, K.; Adachi, Y.; Furukawa, M.; Itazawa, T.; Odajima, H.; Saito, H.; Akasawa, A. Factors associated with asthma control in children: Findings from a national web-based survey. Pediatr. Allergy Immunol. 2014, 25, 804–809. [Google Scholar] [CrossRef]
- Kang, X.; Tu, H.; Tian, T.; Huang, Z.; Luo, L.; Shen, L.; Ye, J. Home environment and diseases in early life are associated with allergic rhinitis. Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Lu, C.; Ou, C.; Chen, L.; Yuan, H. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere 2016, 152, 459–467. [Google Scholar] [CrossRef] [PubMed]
- North, M.L.; Brook, J.R.; Lee, E.Y.; Omana, V.; Daniel, N.M.; Steacy, L.M.; Evans, G.J.; Diamond, M.L.; Ellis, A.K. The Kingston allergy birth cohort. Ann. Allergy Asthma Immunol. 2017, 118, 465–473. [Google Scholar] [CrossRef]
- Goudarzi, H.; Konno, S.; Kimura, H.; Araki, A.; Miyashita, C.; Itoh, S.; Ait Bamai, Y.; Kimura, H.; Shimizu, K.; Suzuki, M.; et al. Contrasting associations of maternal smoking and pre-pregnancy BMI with wheeze and eczema in children. Sci. Total Environ. 2018, 639, 1601–1609. [Google Scholar] [CrossRef]
- De Queiroz Andrade, E.; Da Silva Sena, C.R.; Collison, A.; Murphy, V.E.; Gould, G.S.; Bonevski, B.; Mattes, J. Association between active tobacco use during pregnancy and infant respiratory health: A systematic review and meta-analysis. BMJ Open 2020, 10, e037819. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Hohmann, C.; Patelarou, E.; Martinez, D.; Henderson, A.J.; Granell, R.; Sunyer, J.; Torrent, M.; Fantini, M.P.; Gori, D.; et al. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. Eur. Respir. J. 2016, 48, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Montgomery, S.; Treglown, L.; Furnham, A. Associations between childhood biomedical factors, maternal smoking, personality traits, Body and Mass Index and the prevalence of asthma in adulthood. Psychol. Health 2018, 33, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Thacher, J.D.; Gehring, U.; Gruzieva, O.; Standl, M.; Pershagen, G.; Bauer, C.-P.; Berdel, D.; Keller, T.; Koletzko, S.; Koppelman, G.H.; et al. Maternal smoking during pregnancy and early childhood and development of asthma and rhinoconjunctivitis—A MeDALL project. Environ. Health Perspect. 2018, 126, 047005. [Google Scholar] [CrossRef] [PubMed]
- Lanari, M.; Vandini, S.; Adorni, F.; Prinelli, F.; Di Santo, S.; Silvestri, M.; Musicco, M. Prenatal tobacco smoke exposure increases hospitalizations for bronchiolitis in infants. Respir. Res. 2015, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Thacher, J.D.; Schultz, E.S.; Hallberg, J.; Hellberg, U.; Kull, I.; Thunqvist, P.; Pershagen, G.; Gustafsson, P.M.; Melén, E.; Bergström, A. Tobacco smoke exposure in early life and adolescence in relation to lung function. Eur. Respir. J. 2018, 51, 1702111. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-W.; Yang, M.; Chen, S.; Shah, K.; Hailegiorgis, Y.; Burgens, R.; Vaughn, M.; Huang, J.; Xaverius, P.; Paul, G.; et al. Effects of in utero and postnatal exposure to secondhand smoke on lung function by gender and asthma status: The seven northeastern cities (SNEC) study. Respiration 2017, 93, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Harju, M.; Keski-Nisula, L.; Georgiadis, L.; Heinonen, S. Parental smoking and cessation during pregnancy and the risk of childhood asthma. BMC Public Health 2016, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; He, T.; Huang, H.; Feng, F.; Liu, X.; Li, Z.; Zhang, Y.; Ba, Y. Prenatal ambient air pollution exposure and SOD2 promoter methylation in maternal and cord blood. Ecotoxicol. Environ. Saf. 2019, 181, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Yucesoy, B.; Johnson, V.J.; Lummus, Z.L.; Kissling, G.E.; Fluharty, K.; Gautrin, D.; Malo, J.-L.; Cartier, A.; Boulet, L.-P.; Sastre, J.; et al. Genetic variants in antioxidant genes are associated with diisocyanate-induced asthma. Toxicol. Sci. 2012, 129, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-J.; Karmaus, W. Oxidative stress-related genetic variants may modify associations of phthalate exposures with asthma. Int. J. Environ. Res. Public Health 2017, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, T.; Arger, C.A.; Heil, S.H.; Higgins, S.T.; Tyndale, R.F. Longitudinal influence of pregnancy on nicotine metabolic pathways. J. Pharmacol. Exp. Ther. 2018, 364, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sabra, S.; Gratacós, E.; Gómez Roig, M.D. Smoking-induced changes in the maternal immune, endocrine, and metabolic pathways and their impact on fetal growth: A topical review. Fetal Diagn. Ther. 2017, 41, 241–250. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Spindel, E.R. Pulmonary effects of maternal smoking on the fetus and child: Effects on lung development, respiratory morbidities, and life long lung health. Paediatr. Respir. Rev. 2017, 21, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, J.M.; Gómez-López, J.; Medina-Bravo, P.; Juárez-Sánchez, F.; Contreras-Ramos, A.; Galicia-Esquivel, M.; Sánchez-Urbina, R.; Klünder-Klünder, M. Maternal obesity increases oxidative stress in the newborn. Obesity 2015, 23, 1650–1654. [Google Scholar] [CrossRef]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G.; Le Grand, B.; Hsu, H.-H.L.; Chiu, Y.-H.M.; Brennan, K.J.; Bose, S.; Rosa, M.J.; Brunst, K.J.; Kloog, I.; Wilson, A.; et al. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia GSTP1 hypermethylation: Sex-specific effects. Respir. Res. 2018, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A. The IUGR Newborn. Semin. Perinatol. 2008, 32, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Malamitsi-Puchner, A. Perinatal biomarkers implying ‘Developmental origins of health and disease’ consequences in intrauterine growth restriction. Acta Paediatr. 2020, 109, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- den Dekker, H.T.; Jaddoe, V.W.V.; Reiss, I.K.; de Jongste, J.C.; Duijts, L. Fetal and infant growth patterns and risk of lower lung function and asthma. The generation R study. Am. J. Respir. Crit. Care Med. 2018, 197, 183–192. [Google Scholar] [CrossRef]
- Dai, H.; Jing, S.; Wang, H.; Ma, Y.; Li, L.; Song, W.; Kan, H. VOC characteristics and inhalation health risks in newly renovated residences in Shanghai, China. Sci. Total Environ. 2017, 577, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Franck, U.; Weller, A.; Röder, S.W.; Herberth, G.; Junge, K.M.; Kohajda, T.; von Bergen, M.; Rolle-Kampczyk, U.; Diez, U.; Borte, M.; et al. Prenatal VOC exposure and redecoration are related to wheezing in early infancy. Environ. Int. 2014, 73, 393–401. [Google Scholar] [CrossRef]
- Hansen, S.; Strøm, M.; Olsen, S.F.; Maslova, E.; Rantakokko, P.; Kiviranta, H.; Rytter, D.; Bech, B.H.; Hansen, L.V.; Halldorsson, T.I. Maternal concentrations of persistent organochlorine pollutants and the risk of asthma in offspring: Results from a prospective cohort with 20 years of follow-up. Environ. Health Perspect. 2014, 122, 93–99. [Google Scholar] [CrossRef]
- Mamane, A.; Raherison, C.; Tessier, J.-F.; Baldi, I.; Bouvier, G. Environmental exposure to pesticides and respiratory health. Eur. Respir. Rev. 2015, 24, 462–473. [Google Scholar] [CrossRef]
- Parker-Lalomio, M.; McCann, K.; Piorkowski, J.; Freels, S.; Persky, V.W. Prenatal exposure to polychlorinated biphenyls and asthma, eczema/hay fever, and frequent ear infections. J. Asthma 2018, 55, 1105–1115. [Google Scholar] [CrossRef]
- Impinen, A.; Longnecker, M.P.; Nygaard, U.C.; London, S.J.; Ferguson, K.K.; Haug, L.S.; Granum, B. Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian Mother and Child (MoBa) cohort. Environ. Int. 2019, 124, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Salgado, C.B.; Granum, B.; Lopez-Espinosa, M.-J.; Ballester, F.; Iñiguez, C.; Gascón, M.; Martínez, D.; Guxens, M.; Basterretxea, M.; Zabaleta, C.; et al. Prenatal exposure to perfluoroalkyl substances, immune-related outcomes, and lung function in children from a Spanish birth cohort study. Int. J. Hyg. Environ. Health 2019, 222, 945–954. [Google Scholar] [CrossRef]
- Vernet, C.; Pin, I.; Giorgis-Allemand, L.; Philippat, C.; Benmerad, M.; Quentin, J.; Calafat, A.M.; Ye, X.; Annesi-Maesano, I.; Siroux, V.; et al. In utero exposure to select phenols and phthalates and respiratory health in five-year-old boys: A prospective study. Environ. Health Perspect. 2017, 125, 097006. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Balmes, J.; Kogut, K.; Holland, N.; Calafat, A.M.; Harley, K.G. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr. Allergy Immunol. 2019, 30, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.C.; Li, Z.; Palys, T.; Jackson, B.; Subbiah, M.; Malipatlolla, M.; Sampath, V.; Maecker, H.; Karagas, M.R.; Nadeau, K.C. Cord blood T cell subpopulations and associations with maternal cadmium and arsenic exposures. PLoS ONE 2017, 12, e0179606. [Google Scholar] [CrossRef]
- Varvarigou, A.A.; Liatsis, S.G.; Vassilakos, P.; Decavalas, G.; Beratis, N.G. Effect of maternal smoking on cord blood estriol, placental lactogen, chorionic gonadotropin, FSH, LH, and cortisol. J. Perinat. Med. 2009, 37, 364–369. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Chen, H.; Chan, Y.L.; Wang, G.; Oliver, B.G. Why do intrauterine exposure to air pollution and cigarette smoke increase the risk of asthma? Front. Cell Dev. Biol. 2020, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine disrupting chemicals: Effects on endocrine glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Street, M.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current knowledge on endocrine disrupting chemicals (edcs) from animal biology to humans, from pregnancy to adulthood: Highlights from a national italian meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef]
- Benincasa, G.; DeMeo, D.L.; Glass, K.; Silverman, E.K.; Napoli, C. Epigenetics and pulmonary diseases in the horizon of precision medicine: A review. Eur. Respir. J. 2021, 57, 2003406. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Benincasa, G.; Donatelli, F.; Ambrosio, G. Precision medicine in distinct heart failure phenotypes: Focus on clinical epigenetics. Am. Heart J. 2020, 224, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Benincasa, G.; Schiano, C.; Salvatore, M. Differential epigenetic factors in the prediction of cardiovascular risk in diabetic patients. Eur. Hear. J. Cardiovasc. Pharmacother. 2020, 6, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Sugden, K.; Arseneault, L.; Corcoran, D.L.; Danese, A.; Fisher, H.L.; Moffitt, T.E.; Newbury, J.B.; Odgers, C.; Prinz, J.; et al. Association of neighborhood disadvantage in childhood with dna methylation in young adulthood. JAMA Netw. Open 2020, 3, e206095. [Google Scholar] [CrossRef]
- Dwi Putra, S.E.; Reichetzeder, C.; Hasan, A.A.; Slowinski, T.; Chu, C.; Krämer, B.K.; Kleuser, B.; Hocher, B. Being born large for gestational age is associated with increased global placental DNA methylation. Sci. Rep. 2020, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Czamara, D.; Eraslan, G.; Page, C.M.; Lahti, J.; Lahti-Pulkkinen, M.; Hämäläinen, E.; Kajantie, E.; Laivuori, H.; Villa, P.M.; Reynolds, R.M.; et al. Integrated analysis of environmental and genetic influences on cord blood DNA methylation in new-borns. Nat. Commun. 2019, 10, 2548. [Google Scholar] [CrossRef]
- Wiklund, P.; Karhunen, V.; Richmond, R.C.; Parmar, P.; Rodriguez, A.; De Silva, M.; Wielscher, M.; Rezwan, F.I.; Richardson, T.G.; Veijola, J.; et al. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin. Epigenetics 2019, 11, 97. [Google Scholar] [CrossRef]
- de Nigris, F.; Cacciatore, F.; Mancini, F.P.; Vitale, D.F.; Mansueto, G.; D’Armiento, F.P.; Schiano, C.; Soricelli, A.; Napoli, C. Epigenetic hallmarks of fetal early atherosclerotic lesions in humans. JAMA Cardiol. 2018, 3, 1184. [Google Scholar] [CrossRef]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Carsin, A.-E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Meiners, S.; Eickelberg, O.; Königshoff, M. Hallmarks of the ageing lung. Eur. Respir. J. 2015, 45, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.T.; Sikdar, S.; Xu, C.-J.; Lee, M.K.; Cardwell, J.; Forno, E.; Imboden, M.; Jeong, A.; Madore, A.-M.; Qi, C.; et al. Epigenome-wide association study of DNA methylation and adult asthma in the agricultural lung health study. Eur. Respir. J. 2020, 56, 2000217. [Google Scholar] [CrossRef]
- Napoli, C.; Benincasa, G.; Loscalzo, J. Epigenetic inheritance underlying pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 653–664. [Google Scholar] [CrossRef]
- Regan, E.A.; Hersh, C.P.; Castaldi, P.J.; DeMeo, D.L.; Silverman, E.K.; Crapo, J.D.; Bowler, R.P. Omics and the search for blood biomarkers in chronic obstructive pulmonary disease. Insights from COPDGene. Am. J. Respir. Cell Mol. Biol. 2019, 61, 143–149. [Google Scholar] [CrossRef]
- Lodge, C.J.; Allen, K.J.; Lowe, A.J.; Hill, D.J.; Hosking, C.S.; Abramson, M.J.; Dharmage, S.C. Perinatal Cat and dog exposure and the risk of asthma and allergy in the urban environment: A systematic review of longitudinal studies. Clin. Dev. Immunol. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Schaub, B.; Liu, J.; Höppler, S.; Schleich, I.; Huehn, J.; Olek, S.; Wieczorek, G.; Illi, S.; von Mutius, E. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J. Allergy Clin. Immunol. 2009, 123, 774–782.e5. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, P.I.; Büchele, G.; Blümer, N.; Roponen, M.; Ege, M.J.; Krauss-Etschmann, S.; Genuneit, J.; Hyvärinen, A.; Hirvonen, M.-R.; Lauener, R.; et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: The PASTURE Study. J. Allergy Clin. Immunol. 2010, 125, 108–115.e3. [Google Scholar] [CrossRef]
- von Mutius, E.; Vercelli, D. Farm living: Effects on childhood asthma and allergy. Nat. Rev. Immunol. 2010, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Schilling, D.; Clay, N.; Jackson, K.; Go, M.D.; Spitale, P.; Bunten, C.; Leiva, M.; Gonzales, D.; Hollister-Smith, J.; et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants. JAMA 2014, 311, 2074. [Google Scholar] [CrossRef]
- Preston, A.M. Cigarette smoking-nutritional implications. Prog. Food Nutr. Sci. 1991, 15, 183–217. [Google Scholar]
- Chan, Y.L.; Saad, S.; Al-Odat, I.; Oliver, B.G.; Pollock, C.; Jones, N.M.; Chen, H. Maternal L-carnitine supplementation improves brain health in offspring from cigarette smoke exposed mothers. Front. Mol. Neurosci. 2017, 10, 33. [Google Scholar] [CrossRef]
- Sukjamnong, S.; Chan, Y.L.; Zakarya, R.; Nguyen, L.T.; Anwer, A.G.; Zaky, A.A.; Santiyanont, R.; Oliver, B.G.; Goldys, E.; Pollock, C.A.; et al. MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci. Rep. 2018, 8, 6631. [Google Scholar] [CrossRef]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedón, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef]
- Yang, H.; Xun, P.; He, K. Fish and fish oil intake in relation to risk of asthma: A systematic review and meta-analysis. PLoS ONE 2013, 8, e80048. [Google Scholar] [CrossRef]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdóttir S, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N. Engl. J. Med. 2016, 29, 2530–2539. [Google Scholar] [CrossRef]
- Turner, S.W.; Campbell, D.; Smith, N.; Craig, L.C.A.; McNeill, G.; Forbes, S.H.; Harbour, P.J.; Seaton, A.; Helms, P.J.; Devereux, G.S. Associations between fetal size, maternal α-tocopherol and childhood asthma. Thorax 2010, 65, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.; Ahmed, P.; Ieromnimon, A.; Goepfert, P.; Laiou, E.; Quansah, R.; Jaakkola, M. Preterm delivery and asthma: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2006, 118, 823–830. [Google Scholar] [CrossRef]
- Been, J.V.; Lugtenberg, M.J.; Smets, E.; van Schayck, C.P.; Kramer, B.W.; Mommers, M.; Sheikh, A. Preterm birth and childhood wheezing disorders: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001596. [Google Scholar] [CrossRef]
- Harju, M.; Keski-Nisula, L.; Georgiadis, L.; Räisänen, S.; Gissler, M.; Heinonen, S. The burden of childhood asthma and late preterm and early term births. J. Pediatr. 2014, 164, 295–299.e1. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, M.L.; Gonzalez-Carrasco, E.; Bracamonte, T.; Molinero, M.; Pozo, F.; Casas, I.; Calvo, C. Impact of prematurity and severe viral bronchiolitis on asthma development at 6–9 years. J. Asthma Allergy 2020, 13, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; McKay, K.O.; van Asperen, P.P.; Selvadurai, H.; Fitzgerald, D.A. Normal development of the lung and premature birth. Paediatr. Respir. Rev. 2010, 11, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, P.; Giannini, C.; Attanasi, M.; Dodi, G.; Scaparrotta, A.; Petrosino, M.I.; Di Pillo, S.; Chiarelli, F. Pulmonary outcomes in children born extremely and very preterm at 11 years of age. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Leps, C.; Carson, C.; Quigley, M.A. Gestational age at birth and wheezing trajectories at 3–11 years. Arch. Dis. Child. 2018, 103, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- den Dekker, H.T.; Sonnenschein-van der Voort, A.M.M.; de Jongste, J.C.; Anessi-Maesano, I.; Arshad, S.H.; Barros, H.; Beardsmore, C.S.; Bisgaard, H.; Phar, S.C.; Craig, L.; et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J. Allergy Clin. Immunol. 2016, 137, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein-van der Voort, A.M.M.; Arends, L.R.; de Jongste, J.C.; Annesi-Maesano, I.; Arshad, S.H.; Barros, H.; Basterrechea, M.; Bisgaard, H.; Chatzi, L.; Corpeleijn, E.; et al. Preterm birth, infant weight gain, and childhood asthma risk: A meta-analysis of 147,000 European children. J. Allergy Clin. Immunol. 2014, 133, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Winkleby, M.A.; Sundquist, J.; Sundquist, K. Risk of asthma in young adults who were born preterm: A Swedish national cohort study. Pediatrics 2011, 127, e913–e920. [Google Scholar] [CrossRef]
- Vogt, H.; Lindström, K.; Bråbäck, L.; Hjern, A. Preterm birth and inhaled corticosteroid use in 6- to 19-year-olds: A Swedish national cohort study. Pediatrics 2011, 127, 1052–1059. [Google Scholar] [CrossRef]
- Davidson, L.; Berkelhamer, S. Bronchopulmonary dysplasia: Chronic lung disease of infancy and long-term pulmonary outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Källén, B.; Finnström, O.; Nygren, K.-G.; Otterblad Olausson, P. Association between preterm birth and intrauterine growth retardation and child asthma. Eur. Respir. J. 2013, 41, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Filippone, M. Chronic lung disease after premature birth. N. Engl. J. Med. 2007, 357, 1946–1955. [Google Scholar] [CrossRef]
- Hwang, J.S.; Rehan, V.K. Recent advances in bronchopulmonary dysplasia: Pathophysiology, prevention, and treatment. Lung 2018, 196, 129–138. [Google Scholar] [CrossRef]
- Narang, I.; Baraldi, E.; Silverman, M.; Bush, A. Airway function measurements and the long-term follow-up of survivors of preterm birth with and without chronic lung disease. Pediatr. Pulmonol. 2006, 41, 497–508. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Edwards, M.O.; Watkins, W.J.; Henderson, A.J.; Paranjothy, S.; Dunstan, F.D.; Kotecha, S. Effect of preterm birth on later FEV 1: A systematic review and meta-analysis. Thorax 2013, 68, 760–766. [Google Scholar] [CrossRef]
- Boerma, T.; Ronsmans, C.; Melesse, D.Y.; Barros, A.J.D.; Barros, F.C.; Juan, L.; Moller, A.-B.; Say, L.; Hosseinpoor, A.R.; Yi, M.; et al. Global epidemiology of use of and disparities in caesarean sections. Lancet 2018, 392, 1341–1348. [Google Scholar] [CrossRef]
- Liao, S.-L.; Tsai, M.-H.; Yao, T.-C.; Hua, M.-C.; Yeh, K.-W.; Chiu, C.-Y.; Su, K.-W.; Huang, S.-Y.; Kao, C.-C.; Lai, S.-H.; et al. Caesarean section is associated with reduced perinatal cytokine response, increased risk of bacterial colonization in the airway, and infantile wheezing. Sci. Rep. 2017, 7, 9053. [Google Scholar] [CrossRef]
- Sevelsted, A.; Stokholm, J.; Bisgaard, H. Risk of asthma from cesarean delivery depends on membrane rupture. J. Pediatr. 2016, 171, 38–42.e4. [Google Scholar] [CrossRef]
- van Berkel, A.C.; den Dekker, H.T.; Jaddoe, V.W.V.; Reiss, I.K.; Gaillard, R.; Hofman, A.; de Jongste, J.C.; Duijts, L. Mode of delivery and childhood fractional exhaled nitric oxide, interrupter resistance and asthma: The generation R study. Pediatr. Allergy Immunol. 2015, 26, 330–336. [Google Scholar] [CrossRef]
- Keag, O.E.; Norman, J.E.; Stock, S.J. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS Med. 2018, 15, e1002494. [Google Scholar] [CrossRef]
- Wypych-Ślusarska, A.; Niewiadomska, E.; Oleksiuk, K.; Krupa-Kotara, K.; Głogowska-Ligus, J.; Słowiński, J. Caesarean delivery and risk of childhood asthma development: Meta-analysis. Adv. Dermatology Allergol. 2021, 38, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, F.; Zugna, D.; Annesi-Maesano, I.; Baïz, N.; Barros, H.; Correia, S.; Duijts, L.; Forastiere, F.; Inskip, H.; Kelleher, C.C.; et al. Mode of delivery and asthma at school age in 9 european birth cohorts. Am. J. Epidemiol. 2017, 185, 465–473. [Google Scholar] [CrossRef]
- Darabi, B.; Rahmati, S.; HafeziAhmadi, M.R.; Badfar, G.; Azami, M. The association between caesarean section and childhood asthma: An updated systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2019, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.C.; Leventer-Roberts, M.; Levinkron, O.; Wilson, K.M. Elective caesarean section and bronchiolitis hospitalization: A retrospective cohort study. Pediatr. Allergy Immunol. 2021, 32, 280–287. [Google Scholar] [CrossRef]
- Thysen, A.H.; Larsen, J.M.; Rasmussen, M.A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Brix, S. Prelabor cesarean section bypasses natural immune cell maturation. J. Allergy Clin. Immunol. 2015, 136, 1123–1125.e6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thysen, A.H.; Rasmussen, M.A.; Kreiner-Møller, E.; Larsen, J.M.; Følsgaard, N.V.; Bønnelykke, K.; Stokholm, J.; Bisgaard, H.; Brix, S. Season of birth shapes neonatal immune function. J. Allergy Clin. Immunol. 2016, 137, 1238–1246.e13. [Google Scholar] [CrossRef]
- Francino, M.P. Birth mode-related differences in gut microbiota colonization and immune system development. Ann. Nutr. Metab. 2018, 73, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, aax9929. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, aab2271. [Google Scholar] [CrossRef] [PubMed]
- Gürdeniz, G.; Ernst, M.; Rago, D.; Kim, M.; Courraud, J.; Stokholm, J.; Bønnelykke, K.; Björkbom, A.; Trivedi, U.; Sørensen, S.J.; et al. Neonatal metabolome of cesarean section and risk of childhood asthma. Eur. Respir. J. 2021, 2102406. [Google Scholar] [CrossRef]
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Field, A.E.; Chavarro, J.E. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016, e162385. [Google Scholar] [CrossRef]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.M.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 2015, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Visness, C.M.; London, S.J.; Daniels, J.L.; Kaufman, J.S.; Yeatts, K.B.; Siega-Riz, A.-M.; Calatroni, A.; Zeldin, D.C. Association of childhood obesity with atopic and nonatopic asthma: Results from the national health and nutrition examination survey 1999–2006. J. Asthma 2010, 47, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.J.; Mannion, C.A.; McDonald, S.W.; Brockway, M.; Tough, S.C. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, S.; Song, Y.; Feng, Y.; Lv, N.; Xue, Y.; Liu, F.; Wang, S.; Zhu, B.; Ma, J.; et al. The perturbation of infant gut microbiota caused by cesarean delivery is partially restored by exclusive breastfeeding. Front. Microbiol. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Thavagnanam, S.; Fleming, J.; Bromley, A.; Shields, M.D.; Cardwell, C.R. A meta-analysis of the association between caesarean section and childhood asthma. Clin. Exp. Allergy 2008, 38, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Miliku, K.; Azad, M. Breastfeeding and the Developmental origins of asthma: Current evidence, possible mechanisms, and future research priorities. Nutrients 2018, 10, 995. [Google Scholar] [CrossRef]
- Lodge, C.; Tan, D.; Lau, M.; Dai, X.; Tham, R.; Lowe, A.; Bowatte, G.; Allen, K.; Dharmage, S. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and childhood asthma: Systematic review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- de Benedictis, F.M.; Bush, A. Infantile wheeze: Rethinking dogma. Arch. Dis. Child. 2017, 102, 371–375. [Google Scholar] [CrossRef]
- Frank, N.M.; Lynch, K.F.; Uusitalo, U.; Yang, J.; Lönnrot, M.; Virtanen, S.M.; Hyöty, H.; Norris, J.M. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.W.; Denlinger, L.C. Role of infection in the development and exacerbation of asthma. Expert Rev. Respir. Med. 2010, 4, 71–83. [Google Scholar] [CrossRef]
- Xue, M.; Dehaas, E.; Chaudhary, N.; O’Byrne, P.; Satia, I.; Kurmi, O.P. Breastfeeding and risk of childhood asthma: A systematic review and meta-analysis. ERJ Open Res. 2021, 7, 00504–02021. [Google Scholar] [CrossRef] [PubMed]
- Bigman, G. Exclusive breastfeeding for the first 3 months of life may reduce the risk of respiratory allergies and some asthma in children at the age of 6 years. Acta Paediatr. 2020, 109, 1627–1633. [Google Scholar] [CrossRef]
- Kuniyoshi, Y.; Tsujimoto, Y.; Banno, M.; Taito, S.; Ariie, T. Neonatal jaundice, phototherapy and childhood allergic diseases: An updated systematic review and meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Procianoy, R.; Silveira, R.; Fonseca, L.; Heidemann, L.; Neto, E. The Influence of phototherapy on serum cytokine concentrations in newborn infants. Am. J. Perinatol. 2010, 27, 375–379. [Google Scholar] [CrossRef]
- Han, X.; Krempski, J.W.; Nadeau, K. Advances and novel developments in mechanisms of allergic inflammation. Allergy 2020, 75, 3100–3111. [Google Scholar] [CrossRef]
- Ren, J.; Xu, J.; Zhang, P.; Bao, Y. Prevalence and risk factors of asthma in preschool children in Shanghai, China: A cross-sectional study. Front. Pediatr. 2022, 9, 793495. [Google Scholar] [CrossRef]
- Patrick, D.M.; Sbihi, H.; Dai, D.L.Y.; Al Mamun, A.; Rasali, D.; Rose, C.; Marra, F.; Boutin, R.C.T.; Petersen, C.; Stiemsma, L.T.; et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: Evidence from population-based and prospective cohort studies. Lancet Respir. Med. 2020, 8, 1094–1105. [Google Scholar] [CrossRef]
- Gonzalez-Barcala, F.J.; Pertega, S.; Castro, T.P.; Sampedro, M.; Lastres, J.S.; Gonzalez, M.A.S.J.; Bamonde, L.; Garnelo, L.; Valdes, L.; Carreira, J.-M.; et al. Exposure to paracetamol and asthma symptoms. Eur. J. Public Health 2013, 23, 706–710. [Google Scholar] [CrossRef]
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Al-Garawi, A.; Weiss, S.T.; Litonjua, A.A. Vitamin D and the development of allergic disease: How important is it? Clin. Exp. Allergy 2015, 45, 114–125. [Google Scholar] [CrossRef]
- Lipińska-Opałka, A.; Tomaszewska, A.; Kubiak, J.Z.; Kalicki, B. Vitamin D and immunological patterns of allergic diseases in children. Nutrients 2021, 13, 177. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.H.; Chambers, E.S.; Pfeffer, P.E.; Hawrylowicz, C.M. Immunoregulatory mechanisms of vitamin D relevant to respiratory health and asthma. Ann. N. Y. Acad. Sci. 2014, 1317, 57–69. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Reinehr, T.; Schnabel, D.; Wabitsch, M.; Bechtold-Dalla Pozza, S.; Bührer, C.; Heidtmann, B.; Jochum, F.; Kauth, T.; Körner, A.; Mihatsch, W.; et al. Vitamin D supplementation after the second year of life: Joint position of the Committee on Nutrition, German Society for Pediatric and Adolescent Medicine (DGKJ e.V.), and the German Society for Pediatric Endocrinology and Diabetology (DGKED e.V.). Mol. Cell. Pediatr. 2019, 6, 3. [Google Scholar] [CrossRef]
- Forno, E.; Bacharier, L.B.; Phipatanakul, W.; Guilbert, T.W.; Cabana, M.D.; Ross, K.; Covar, R.; Gern, J.E.; Rosser, F.J.; Blatter, J.; et al. Effect of Vitamin D 3 supplementation on severe asthma exacerbations in children with asthma and low vitamin d levels. JAMA 2020, 324, 752. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of prenatal supplementation with vitamin d on asthma or recurrent wheezing in offspring by age 3 years. JAMA 2016, 315, 362. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Eliasen, A.U.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. High-dose vitamin d supplementation during pregnancy and asthma in offspring at the age of 6 years. JAMA 2019, 321, 1003. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-year follow-up of a trial of antenatal vitamin d for asthma reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- Martinez, F.D. Early-life origins of chronic obstructive pulmonary disease. N. Engl. J. Med. 2016, 375, 871–878. [Google Scholar] [CrossRef]
- Budden, K.F.; Shukla, S.D.; Rehman, S.F.; Bowerman, K.L.; Keely, S.; Hugenholtz, P.; Armstrong-James, D.P.H.; Adcock, I.M.; Chotirmall, S.H.; Chung, K.F.; et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir. Med. 2019, 7, 907–920. [Google Scholar] [CrossRef]
- Robinson, P.F.M.; Pattaroni, C.; Cook, J.; Gregory, L.; Alonso, A.M.; Fleming, L.J.; Lloyd, C.M.; Bush, A.; Marsland, B.J.; Saglani, S. Lower airway microbiota associates with inflammatory phenotype in severe preschool wheeze. J. Allergy Clin. Immunol. 2019, 143, 1607–1610.e3. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Meissner, H.C. Viral Bronchiolitis in children. N. Engl. J. Med. 2016, 374, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Sly, P.D.; Kusel, M.; Holt, P.G. Do early-life viral infections cause asthma? J. Allergy Clin. Immunol. 2010, 125, 1202–1205. [Google Scholar] [CrossRef]
- Rantala, A.K.; Jaakkola, M.S.; Mäkikyrö, E.M.S.; Hugg, T.T.; Jaakkola, J.J.K. Early respiratory infections and the development of asthma in the first 27 years of life. Am. J. Epidemiol. 2015, 182, 615–623. [Google Scholar] [CrossRef]
- Belgrave, D.C.M.; Granell, R.; Turner, S.W.; Curtin, J.A.; Buchan, I.E.; Le Souëf, P.N.; Simpson, A.; Henderson, A.J.; Custovic, A. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: A retrospective analysis of three population-based birth cohort studies. Lancet Respir. Med. 2018, 6, 526–534. [Google Scholar] [CrossRef]
- Shi, T.; Ooi, Y.; Zaw, E.M.; Utjesanovic, N.; Campbell, H.; Cunningham, S.; Bont, L.; Nair, H.; Nair, H.; Campbell, H.; et al. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J. Infect. Dis. 2020, 222, S628–S633. [Google Scholar] [CrossRef]
- Lu, S.; Hartert, T.V.; Everard, M.L.; Giezek, H.; Nelsen, L.; Mehta, A.; Patel, H.; Knorr, B.; Reiss, T.F. Predictors of asthma following severe respiratory syncytial virus (RSV) bronchiolitis in early childhood. Pediatr. Pulmonol. 2016, 51, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, N.M.; Nibbelke, E.E.; Pouw, J.; Blanken, M.O.; Rovers, M.M.; Naaktgeboren, C.A.; Mazur, N.I.; Wildenbeest, J.G.; van der Ent, C.K.; Bont, L.J. Respiratory syncytial virus prevention and asthma in healthy preterm infants: A randomised controlled trial. Lancet Respir. Med. 2018, 6, 257–264. [Google Scholar] [CrossRef]
- Jartti, T.; Bønnelykke, K.; Elenius, V.; Feleszko, W. Role of viruses in asthma. Semin. Immunopathol. 2020, 42, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Rubner, F.J.; Jackson, D.J.; Evans, M.D.; Gangnon, R.E.; Tisler, C.J.; Pappas, T.E.; Gern, J.E.; Lemanske, R.F. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J. Allergy Clin. Immunol. 2017, 139, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Magnier, J.; Julian, V.; Mulliez, A.; Usclade, A.; Rochette, E.; Evrard, B.; Amat, F.; Egron, C. Rhinovirus infection and familial atopy predict persistent asthma and sensitisation 7 years after a first episode of acute bronchiolitis in infancy. Children 2021, 8, 850. [Google Scholar] [CrossRef] [PubMed]
- Dumas, O.; Erkkola, R.; Bergroth, E.; Hasegawa, K.; Mansbach, J.M.; Piedra, P.A.; Jartti, T.; Camargo, C.A. Severe bronchiolitis profiles and risk of asthma development in Finnish children. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bønnelykke, K.; Coleman, A.T.; Evans, M.D.; Thorsen, J.; Waage, J.; Vissing, N.H.; Carlsson, C.J.; Stokholm, J.; Chawes, B.L.; Jessen, L.E.; et al. Cadherin-related family member 3 genetics and rhinovirus C respiratory illnesses. Am. J. Respir. Crit. Care Med. 2018, 197, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Gans, M.D.; Gavrilova, T. Understanding the immunology of asthma: Pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lemanske, R.F. The Childhood Origins of Asthma (COAST) study. Pediatr. Allergy Immunol. 2002, 13, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ochoa Sangrador, C.; Vázquez Blanco, A. Day-care center attendance and risk of asthma—a systematic review. Allergol. Immunopathol. 2018, 46, 578–584. [Google Scholar] [CrossRef]
- Caffrey Osvald, E.; Gong, T.; Lundholm, C.; Larsson, H.; BK, B.; Almqvist, C. Parental socioeconomic status and asthma in children: Using a population-based cohort and family design. Clin. Exp. Allergy 2022, 52, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Sly, P.D.; Boner, A.L.; Björksten, B.; Bush, A.; Custovic, A.; Eigenmann, P.A.; Gern, J.E.; Gerritsen, J.; Hamelmann, E.; Helms, P.J.; et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet 2008, 372, 1100–1106. [Google Scholar] [CrossRef]
- Oksel, C.; Custovic, A. Development of allergic sensitization and its relevance to paediatric asthma. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Havstad, S.; Johnson, C.C.; Kim, H.; Levin, A.M.; Zoratti, E.M.; Joseph, C.L.M.; Ownby, D.R.; Wegienka, G. Atopic phenotypes identified with latent class analyses at age 2 years. J. Allergy Clin. Immunol. 2014, 134, 722–727.e2. [Google Scholar] [CrossRef]
- Schoos, A.M.; Jelding-Dannemand, E.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Single and multiple time-point allergic sensitization during childhood and risk of asthma by age 13. Pediatr. Allergy Immunol. 2019, 30, 716–723. [Google Scholar] [CrossRef]
- Simpson, A.; Tan, V.Y.F.; Winn, J.; Svensén, M.; Bishop, C.M.; Heckerman, D.E.; Buchan, I.; Custovic, A. Beyond atopy. Am. J. Respir. Crit. Care Med. 2010, 181, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Obaseki, D.; Potts, J.; Joos, G.; Baelum, J.; Haahtela, T.; Ahlström, M.; Matricardi, P.; Kramer, U.; Gjomarkaj, M.; Fokkens, W.; et al. The relation of airway obstruction to asthma, chronic rhinosinusitis and age: Results from a population survey of adults. Allergy 2014, 69, 1205–1214. [Google Scholar] [CrossRef]
- Warm, K.; Hedman, L.; Lindberg, A.; Lötvall, J.; Lundbäck, B.; Rönmark, E. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J. Allergy Clin. Immunol. 2015, 136, 1559–1565.e2. [Google Scholar] [CrossRef] [PubMed]
- Dhami, S.; Kakourou, A.; Asamoah, F.; Agache, I.; Lau, S.; Jutel, M.; Muraro, A.; Roberts, G.; Akdis, C.A.; Bonini, M.; et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy 2017, 72, 1825–1848. [Google Scholar] [CrossRef]
- Illi, S.; Depner, M.; Genuneit, J.; Horak, E.; Loss, G.; Strunz-Lehner, C.; Büchele, G.; Boznanski, A.; Danielewicz, H.; Cullinan, P.; et al. Protection from childhood asthma and allergy in Alpine farm environments—The GABRIEL Advanced Studies. J. Allergy Clin. Immunol. 2012, 129, 1470–1477.e6. [Google Scholar] [CrossRef] [PubMed]
- von Mutius, E. The microbial environment and its influence on asthma prevention in early life. J. Allergy Clin. Immunol. 2016, 137, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Genuneit, J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: A systematic review with meta-analysis. Pediatr. Allergy Immunol. 2012, 23, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Büchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773.e4. [Google Scholar] [CrossRef]
- Gasana, J.; Dillikar, D.; Mendy, A.; Forno, E.; Ramos Vieira, E. Motor vehicle air pollution and asthma in children: A meta-analysis. Environ. Res. 2012, 117, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Muñoz, X.; Barreiro, E.; Bustamante, V.; Lopez-Campos, J.L.; González-Barcala, F.J.; Cruz, M.J. Diesel exhausts particles: Their role in increasing the incidence of asthma. Reviewing the evidence of a causal link. Sci. Total Environ. 2019, 652, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Gauderman, W.J.; Urman, R.; Avol, E.; Berhane, K.; McConnell, R.; Rappaport, E.; Chang, R.; Lurmann, F.; Gilliland, F. Association of improved air quality with lung development in children. N. Engl. J. Med. 2015, 372, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.; Brunekreef, B.; Ellwood, P.; Anderson, H.R.; Asher, M.I.; Crane, J.; Lai, C.K. Cooking fuels and prevalence of asthma: A global analysis of phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Lancet Respir. Med. 2013, 1, 386–394. [Google Scholar] [CrossRef]
- Cantarutti, A.; Barbiellini Amidei, C.; Valsecchi, C.; Scamarcia, A.; Corrao, G.; Gregori, D.; Giaquinto, C.; Ludvigsson, J.; Canova, C. Association of treated and untreated gastroesophageal reflux disease in the first year of life with the subsequent development of asthma. Int. J. Environ. Res. Public Health 2021, 18, 9633. [Google Scholar] [CrossRef]
- Griffiths, T.L.; Nassar, M.; Soubani, A.O. Pulmonary manifestations of gastroesophageal reflux disease. Expert Rev. Respir. Med. 2020, 14, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Cohen, R.T.; Rhein, L.M.; McCormick, M.C. Preterm infant growth and asthma at age 8 years. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F230–F234. [Google Scholar] [CrossRef] [PubMed]
- Weinmayr, G.; Forastiere, F.; Büchele, G.; Jaensch, A.; Strachan, D.P.; Nagel, G. Overweight/obesity and respiratory and allergic disease in children: International study of asthma and allergies in childhood (ISAAC) phase two. PLoS ONE 2014, 9, e113996. [Google Scholar] [CrossRef]
- Egan, K.B.; Ettinger, A.S.; Bracken, M.B. Childhood body mass index and subsequent physician-diagnosed asthma: A systematic review and meta-analysis of prospective cohort studies. BMC Pediatr. 2013, 13, 121. [Google Scholar] [CrossRef]
- Forno, E.; Weiner, D.J.; Mullen, J.; Sawicki, G.; Kurland, G.; Han, Y.Y.; Cloutier, M.M.; Canino, G.; Weiss, S.T.; Litonjua, A.A.; et al. Obesity and airway dysanapsis in children with and without asthma. Am. J. Respir. Crit. Care Med. 2017, 195, 314–323. [Google Scholar] [CrossRef]
- Kattan, M.; Kumar, R.; Bloomberg, G.R.; Mitchell, H.E.; Calatroni, A.; Gergen, P.J.; Kercsmar, C.M.; Visness, C.M.; Matsui, E.C.; Steinbach, S.F.; et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J. Allergy Clin. Immunol. 2010, 125, 584–592. [Google Scholar] [CrossRef]
- Chen, Y.; Su, M.; Brumpton, B.M.; Lee, Y.L. Investigating obesity-related risk factors for childhood asthma. Pediatr. Allergy Immunol. 2022, 33, e13710. [Google Scholar] [CrossRef]
- Burke, H.; Leonardi-Bee, J.; Hashim, A.; Pine-Abata, H.; Chen, Y.; Cook, D.G.; Britton, J.R.; McKeever, T.M. Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 2012, 129, 735–744. [Google Scholar] [CrossRef]
- Ayuk, A.C.; Ramjith, J.; Zar, H.J. Environmental risk factors for asthma in 13-14-year-old African children. Pediatr. Pulmonol. 2018, 53, 1475–1484. [Google Scholar] [CrossRef]
- Gonzalez-Barcala, F.-J.; Pertega, S.; Sampedro, M.; Lastres, J.S.; Gonzalez, M.A.S.J.; Bamonde, L.; Garnelo, L.; Castro, T.P.; Valdés-Cuadrado, L.; Carreira, J.-M.; et al. Impact of parental smoking on childhood asthma. J. Pediatr. 2013, 89, 294–299. [Google Scholar] [CrossRef]
- Vork, K.L.; Broadwin, R.L.; Blaisdell, R.J. Developing asthma in childhood from exposure to secondhand tobacco smoke: Insights from a meta-regression. Environ. Health Perspect. 2007, 115, 1394–1400. [Google Scholar] [CrossRef]
- Silvestri, M.; Franchi, S.; Pistorio, A.; Petecchia, L.; Rusconi, F. Smoke exposure, wheezing, and asthma development: A systematic review and meta-analysis in unselected birth cohorts. Pediatr. Pulmonol. 2015, 50, 353–362. [Google Scholar] [CrossRef]
- Thacher, J.D.; Gruzieva, O.; Pershagen, G.; Neuman, Å.; Wickman, M.; Kull, I.; Melén, E.; Bergström, A. Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics 2014, 134, 428–434. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wu, H.; Zhang, S.; Lin, Y.; Li, R.; Xie, L.; Li, Z.; Sun, W.; Huang, X.; Zhang, C.J.P.; et al. The association between secondhand smoke and childhood asthma: A systematic review and meta-analysis. Pediatr. Pulmonol. 2020, 55, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Lajunen, K.; Kalliola, S.; Kotaniemi-Syrjänen, A.; Malmberg, L.P.; Pelkonen, A.S.; Mäkelä, M.J. Environmental tobacco smoke affects lung function of preschoolers with asthma even after a decade. Am. J. Respir. Crit. Care Med. 2019, 199, 534–536. [Google Scholar] [CrossRef]
- Mackay, D.; Haw, S.; Ayres, J.G.; Fischbacher, C.; Pell, J.P. Smoke-free legislation and hospitalizations for childhood asthma. N. Engl. J. Med. 2010, 363, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Burki, T.K. Asthma control: Learning from Finland’s success. Lancet Respir. Med. 2019, 7, 207–208. [Google Scholar] [CrossRef]
- Gilliland, F.D.; Islam, T.; Berhane, K.; Gauderman, W.J.; McConnell, R.; Avol, E.; Peters, J.M. Regular Smoking and asthma incidence in adolescents. Am. J. Respir. Crit. Care Med. 2006, 174, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Hancox, R.J.; Gray, A.R.; Poulton, R.; Sears, M.R. The effect of cigarette smoking on lung function in young adults with asthma. Am. J. Respir. Crit. Care Med. 2016, 194, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Stern, D.A.; Zhou, M.; Sherrill, D.L.; Wright, A.L.; Morgan, W.J.; Martinez, F.D. Combined effects of parental and active smoking on early lung function deficits: A prospective study from birth to age 26 years. Thorax 2013, 68, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, R.J.; Wills, T.A.; Tam, E.; Pagano, I.; Choi, K. E-cigarette use and asthma in a multiethnic sample of adolescents. Prev. Med. 2017, 105, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Gawron, M.; Smith, D.M.; Peng, M.; Jacob, P.; Benowitz, N.L. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: A longitudinal within-subjects observational study. Nicotine Tob. Res. 2017, 19, 160–167. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lyes, M.; Sladewski, K.; Enany, S.; McEachern, E.; Mathew, D.P.; Das, S.; Moshensky, A.; Bapat, S.; Pride, D.T.; et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. 2016, 94, 667–679. [Google Scholar] [CrossRef] [PubMed]
- McConnell, R.; Barrington-Trimis, J.L.; Wang, K.; Urman, R.; Hong, H.; Unger, J.; Samet, J.; Leventhal, A.; Berhane, K. Electronic cigarette use and respiratory symptoms in adolescents. Am. J. Respir. Crit. Care Med. 2017, 195, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Newcomb, D.C. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of asthma in children and adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Wijga, A.; Tabak, C.; Postma, D.S.; Kerkhof, M.; Wieringa, M.H.; Hoekstra, M.O.; Brunekreef, B.; de Jongste, J.C.; Smit, H.A. Sex differences in asthma during the first 8 years of life: The prevention and incidence of asthma and mite allergy (PIAMA) birth cohort study. J. Allergy Clin. Immunol. 2011, 127, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, H.; Yang, C.; Lee, Y.L. Early pubertal maturation and risk of childhood asthma: A Mendelian randomization and longitudinal study. Allergy 2020, 75, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Becklake, M.R.; Kauffmann, F. Gender differences in airway behaviour over the human life span. Thorax 1999, 54, 1119–1138. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Newcomb, D.C. Sex bias in asthma prevalence and pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef]
- van den Berge, M.; Heijink, H.I.; van Oosterhout, A.J.M.; Postma, D.S. The role of female sex hormones in the development and severity of allergic and non-allergic asthma. Clin. Exp. Allergy 2009, 39, 1477–1481. [Google Scholar] [CrossRef]
- McCleary, N.; Nwaru, B.I.; Nurmatov, U.B.; Critchley, H.; Sheikh, A. Endogenous and exogenous sex steroid hormones in asthma and allergy in females: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2018, 141, 1510–1513.e8. [Google Scholar] [CrossRef] [PubMed]
- Minelli, C.; van der Plaat, D.A.; Leynaert, B.; Granell, R.; Amaral, A.F.S.; Pereira, M.; Mahmoud, O.; Potts, J.; Sheehan, N.A.; Bowden, J.; et al. Age at puberty and risk of asthma: A Mendelian randomisation study. PLoS Med. 2018, 15, e1002634. [Google Scholar] [CrossRef] [PubMed]
- Juber, N.F.; Waits, A.; Dlamini, L.P.; Nguyen, T.; Masango, B.Z. Associations between pediatric asthma and age at menarche: Evidence from the Indonesian Family Life Survey. J. Asthma 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Barcala, F.J.; Pertega, S.; Bamonde, L.; Garnelo, L.; Perez Castro, T.; Sampedro, M.; Sanchez Lastres, J.; San Jose Gonzalez, M.A.; Lopez Silvarrey, A. Mediterranean diet and asthma in Spanish schoolchildren. Pediatr. Allergy Immunol. 2010, 21, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Cilluffo, G.; Han, Y.; Ferrante, G.; Dello Russo, M.; Lauria, F.; Fasola, S.; Montalbano, L.; Malizia, V.; Forno, E.; La Grutta, S. The Dietary Inflammatory Index and asthma burden in children: A latent class analysis. Pediatr. Allergy Immunol. 2022, 33, e13667. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, D.; Lizzi, M.; Di Filippo, P.; Di Pillo, S.; Chiarelli, F.; Attanasi, M. Time-Specific Factors Influencing the Development of Asthma in Children. Biomedicines 2022, 10, 758. https://doi.org/10.3390/biomedicines10040758
Russo D, Lizzi M, Di Filippo P, Di Pillo S, Chiarelli F, Attanasi M. Time-Specific Factors Influencing the Development of Asthma in Children. Biomedicines. 2022; 10(4):758. https://doi.org/10.3390/biomedicines10040758
Chicago/Turabian StyleRusso, Daniele, Mauro Lizzi, Paola Di Filippo, Sabrina Di Pillo, Francesco Chiarelli, and Marina Attanasi. 2022. "Time-Specific Factors Influencing the Development of Asthma in Children" Biomedicines 10, no. 4: 758. https://doi.org/10.3390/biomedicines10040758
APA StyleRusso, D., Lizzi, M., Di Filippo, P., Di Pillo, S., Chiarelli, F., & Attanasi, M. (2022). Time-Specific Factors Influencing the Development of Asthma in Children. Biomedicines, 10(4), 758. https://doi.org/10.3390/biomedicines10040758







