Current and Future Perspective of Devices and Diagnostics for Opioid and OIRD
Abstract
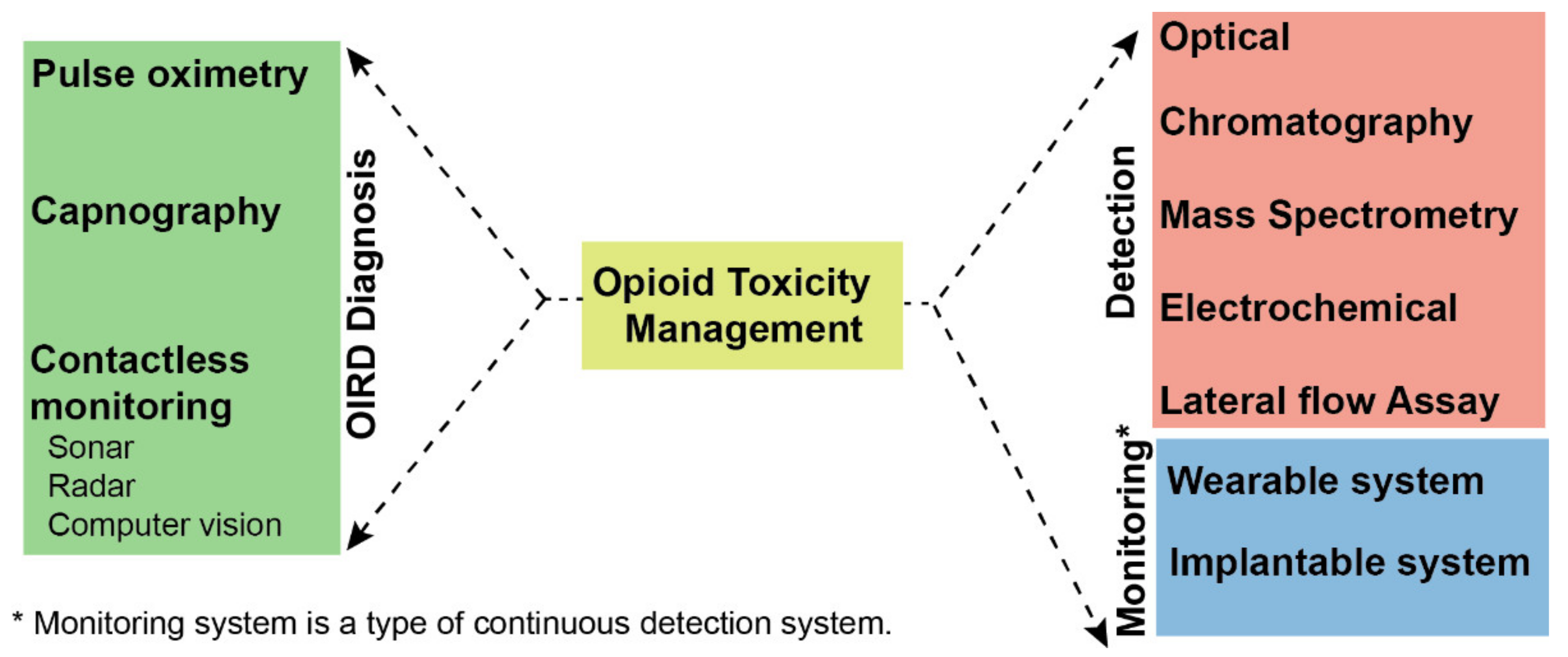
:1. Introduction
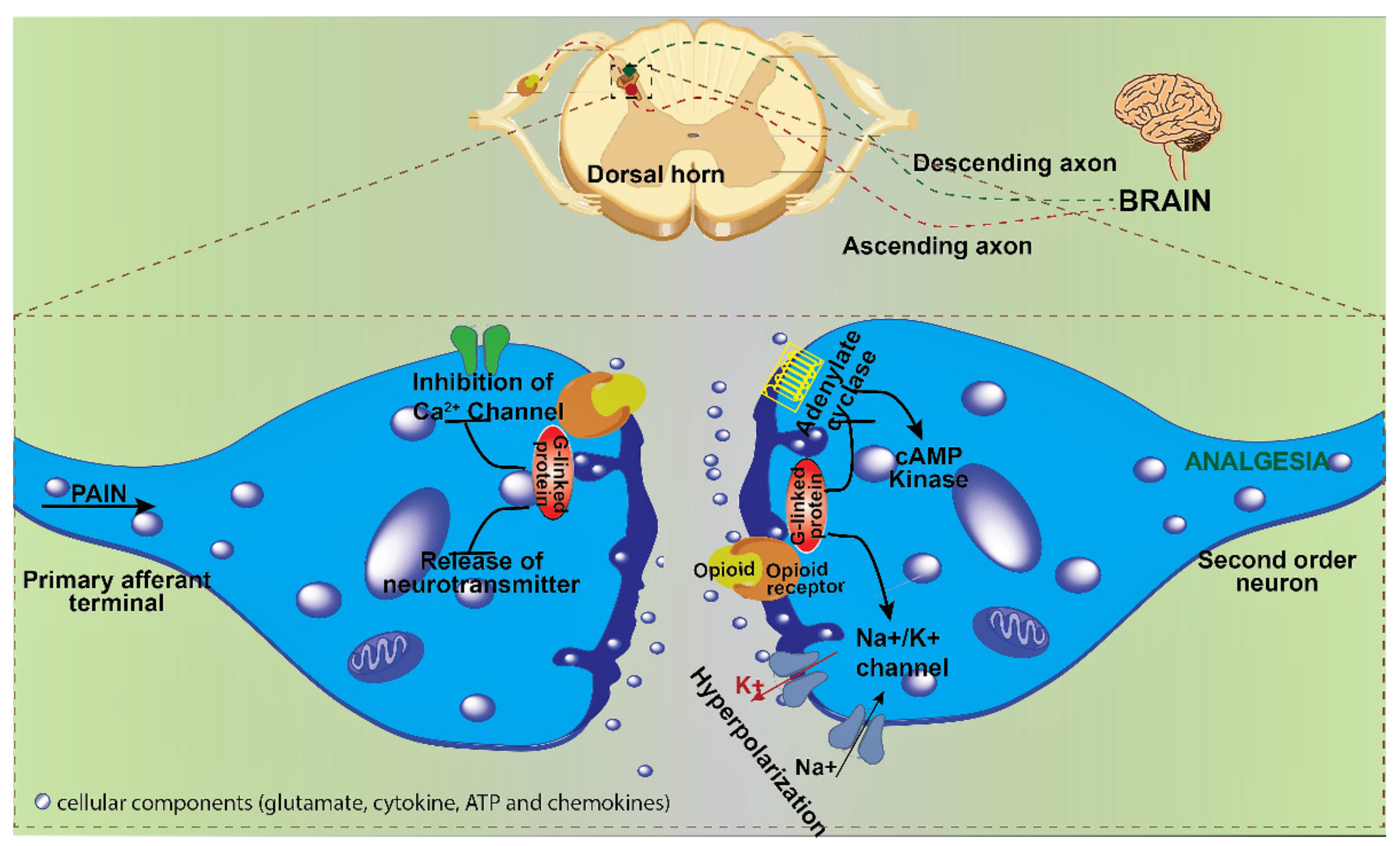
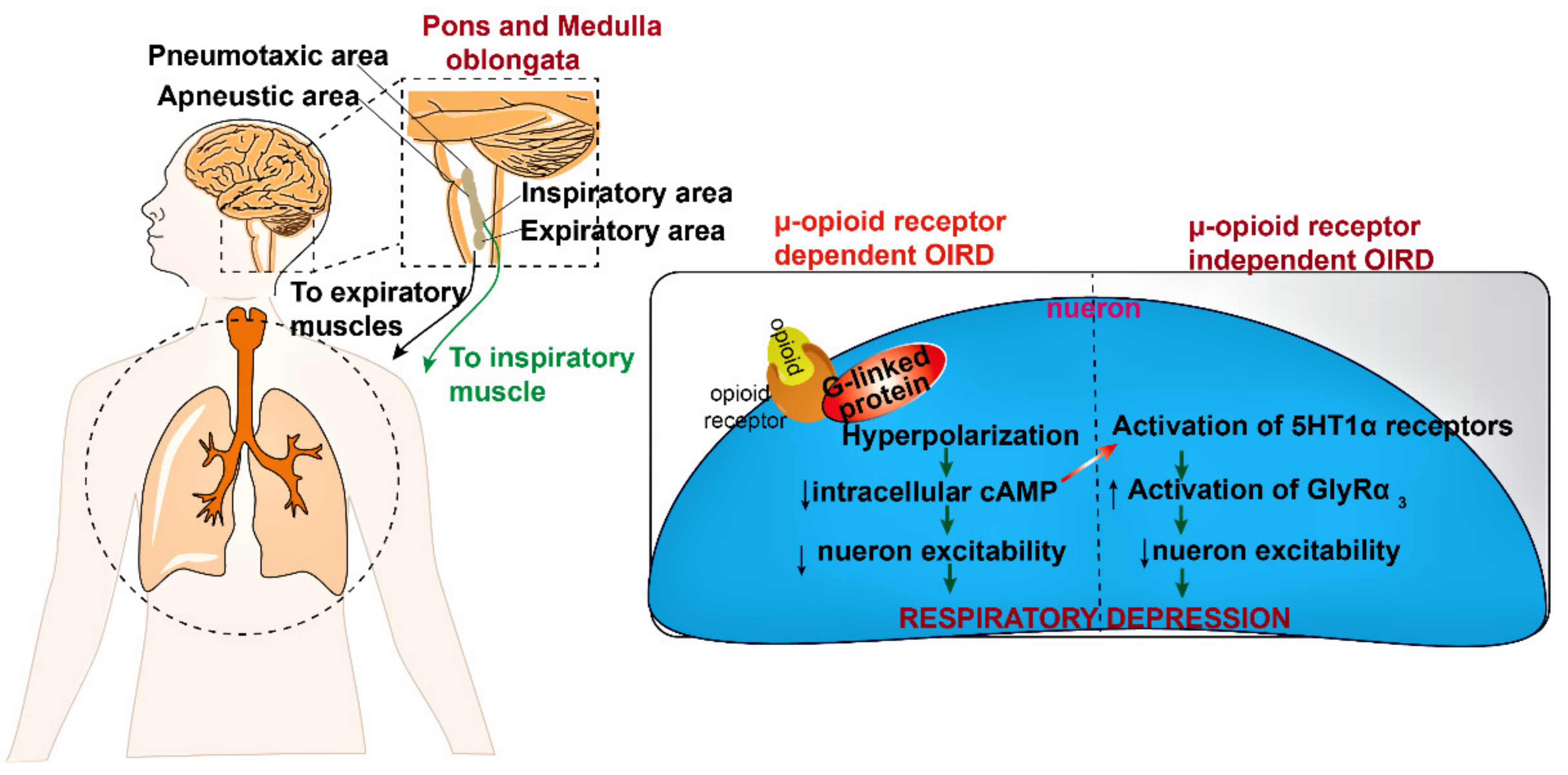
2. Mode of Action of Opioids in Pain Suppression and OIRD
3. Detection of Opioids
3.1. Clinical Testing of Opioids
3.2. Conventional Laboratories Techniques for Opioids Testing
3.2.1. Raman Spectroscopy
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.3. Absorption Spectroscopy
3.2.4. Fluorescence Spectroscopy
3.2.5. Interferometer
3.2.6. Chromatography
3.3. Advance Point of Care (POC) Techniques for Opioids Testing
4. Established Devices Used for Detection of OIRD: Requiring Modification to Impact the Public Health
4.1. Pulse Oximeter
4.2. Capnography
5. Novel, Contactless Sensing Modalities: Sonar, Radar, Computer Vision
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nandakumar, R.; Gollakota, S.; Sunshine, J.E. Opioid Overdose Detection Using Smartphones. Sci. Transl. Med. 2019, 11, eaau8914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.A.; Caplan, R.A.; Stephens, L.S.; Posner, K.L.; Terman, G.W.; Voepel-Lewis, T.; Domino, K.B. Postoperative Opioid-induced Respiratory Depression. Anesthesiology 2015, 122, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.M.; Saunders, K.W.; Rutter, C.M.; Banta-Green, C.J.; Merrill, J.O.; Sullivan, M.D.; Weisner, C.M.; Silverberg, M.J.; Campbell, C.I.; Psaty, B.M.; et al. Overdose and Prescribed Opioids: Associations among Chronic Non-Cancer Pain Patients. Ann. Intern. Med. 2010, 152, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Walley, A.Y.; Bernson, D.; Larochelle, M.R.; Green, T.C.; Young, L.; Land, T. The Contribution of Prescribed and Illicit Opioids to Fatal Overdoses in Massachusetts, 2013–2015. Public Health Rep. 2019, 134, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, D.R.; McEntire, D.M.; Hambsch, Z.J.; Kerfeld, M.J.; Smith, T.A.; Reisbig, M.D.; Youngblood, C.F.; Agrawal, D.K. Therapeutic Basis of Clinical Pain Modulation. Clin. Transl. Sci. 2015, 8, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Busch-Dienstfertig, M.; Stein, C. Opioid Receptors and Opioid Peptide-Producing Leukocytes in Inflammatory Pain–Basic and Therapeutic Aspects. Brain Behav. Immun. 2010, 24, 683–694. [Google Scholar] [CrossRef]
- O’Connor, A.B.; Dworkin, R.H. Treatment of Neuropathic Pain: An Overview of Recent Guidelines. Am. J. Med. 2009, 122, S22–S32. [Google Scholar] [CrossRef]
- Marker, C.L.; Luján, R.; Loh, H.H.; Wickman, K. Spinal G-Protein-Gated Potassium Channels Contribute in a Dose-Dependent Manner to the Analgesic Effect of Mu- and Delta- but Not Kappa-Opioids. J. Neurosci. 2005, 25, 3551–3559. [Google Scholar] [CrossRef]
- Eippert, F.; Bingel, U.; Schoell, E.D.; Yacubian, J.; Klinger, R.; Lorenz, J.; Büchel, C. Activation of the Opioidergic Descending Pain Control System Underlies Placebo Analgesia. Neuron 2009, 63, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Abramow-Newerly, M.; Roy, A.A.; Nunn, C.; Chidiac, P. RGS Proteins Have a Signalling Complex: Interactions between RGS Proteins and GPCRs, Effectors, and Auxiliary Proteins. Cell. Signal. 2006, 18, 579–591. [Google Scholar] [CrossRef]
- Baertsch, N.A.; Bush, N.E.; Burgraff, N.J.; Ramirez, J.-M. Dual Mechanisms of Opioid-Induced Respiratory Depression in the Inspiratory Rhythm-Generating Network. eLife 2021, 10, e67523. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef]
- Bailey, P.L.; Egan, T.D.; Stanley, T.H. Intravenous Opioid Anesthetics, 5th ed.; Anisthiology, Churchill Livingstone: Philadelphia, PA, USA, 2000. [Google Scholar]
- Kintz, P.; Tracqui, A.; Mangin, P.; Edel, Y. Sweat Testing in Opioid Users with a Sweat Patch. J. Anal. Toxicol. 1996, 20, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Schwilke, E.W.; Barnes, A.J.; Kacinko, S.L.; Cone, E.J.; Moolchan, E.T.; Huestis, M.A. Opioid Disposition in Human Sweat after Controlled Oral Codeine Administration. Clin. Chem. 2006, 52, 1539–1545. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P. Point-of-Care Drug of Abuse Testing in the Opioid Epidemic. Arch. Pathol. Lab. Med. 2020, 144, 1325–1334. [Google Scholar] [CrossRef]
- Angelini, D.J.; Biggs, T.D.; Maughan, M.N.; Feasel, M.G.; Sisco, E.; Sekowski, J.W. Evaluation of a Lateral Flow Immunoassay for the Detection of the Synthetic Opioid Fentanyl. Forensic. Sci. Int. 2019, 300, 75–81. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Gozdzialski, L.; Aasen, J.; Larnder, A.; Ramsay, M.; Borden, S.A.; Saatchi, A.; Gill, C.G.; Wallace, B.; Hore, D.K. Portable Gas Chromatography–Mass Spectrometry in Drug Checking: Detection of Carfentanil and Etizolam in Expected Opioid Samples. Int. J. Drug Policy 2021, 97, 103409. [Google Scholar] [CrossRef]
- Strayer, K.E.; Antonides, H.M.; Juhascik, M.P.; Daniulaityte, R.; Sizemore, I.E. LC-MS/MS-Based Method for the Multiplex Detection of 24 Fentanyl Analogues and Metabolites in Whole Blood at Sub Ng ML−1 Concentrations. ACS Omega 2018, 3, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Glasneck, F.; Bistany, L.; Burger, B.; Glasneck, F. Application Note #CBRNE-1865742 Rapid Detection and Identification of Fentanyls; ASA Publications: Atlanta, GA, USA, 2019. [Google Scholar]
- Milone, M.C. Laboratory Testing for Prescription Opioids. J. Med. Toxicol. 2012, 8, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Azadbakht, A.; Abbasi, A.R. Engineering an Aptamer-Based Recognition Sensor for Electrochemical Opium Alkaloid Biosensing. J. Mater. Sci. Mater. Electron. 2019, 30, 3432–3442. [Google Scholar] [CrossRef]
- Alharbi, O.; Xu, Y.; Goodacre, R. Detection and Quantification of the Opioid Tramadol in Urine Using Surface Enhanced Raman Scattering. Analyst 2015, 140, 5965–5970. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Cauchi, M.; Piletska, E.V.; Preston, C.; Piletsky, S.A. Rapid Qualitative and Quantitative Analysis of Opiates in Extract of Poppy Head via FTIR and Chemometrics: Towards in-Field Sensors. Biosens. Bioelectron. 2009, 24, 3322–3328. [Google Scholar] [CrossRef]
- Thermo Scientific FTIR Analyzers-Broad Spectrum, Excellent Selectivity. Available online: https://info3.thermofisher.com/FTIRAnalyzers?gclid=CjwKCAiAyPyQBhB6EiwAFUuaksol1e41_Q19F1VijP6oEaIm2MpwHkcxSzcy3Y2DffemmYEnCCDFCBoC5pcQAvD_BwE&cid=cad_SafetySecurityQ22016_adwords&leadsource=PPC&tracksrc=GoogleAdWordsFTIR&ef_id=CjwKCAiAyPyQBhB6EiwAFUuaksol1e41_Q19F1VijP6oEaIm2MpwHkcxSzcy3Y2DffemmYEnCCDFCBoC5pcQAvD_BwE:G:s&s_kwcid=AL!3652!3!248162592449!p!!g!!portable%20ftir%20spectrometer (accessed on 2 March 2022).
- DR1900 Portable Spectrophotometer|Hach USA-Overview. Available online: https://www.hach.com/dr1900-portable-spectrophotometer/product?id=18915675456&callback=pf (accessed on 2 March 2022).
- Mohseni, N.; Bahram, M. Mean Centering of Ratio Spectra for Colorimetric Determination of Morphine and Codeine in Pharmaceuticals and Biological Samples Using Melamine Modified Gold Nanoparticles. Anal. Methods 2016, 8, 6739–6747. [Google Scholar] [CrossRef]
- Kangas, M.J. A New Possible Alternative Colorimetric Drug Detection Test for Fentanyl. Org. Med. Chem. Int. J. 2017, 4, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Shcherbakova, E.G.; Zhang, B.; Gozem, S.; Minami, T.; Zavalij, P.Y.; Pushina, M.; Isaacs, L.D.; Anzenbacher, P. Supramolecular Sensors for Opiates and Their Metabolites. J. Am. Chem. Soc. 2017, 139, 14954–14960. [Google Scholar] [CrossRef] [PubMed]
- NDT | Portable Fluorescence Spectrometer and Analyzer. Available online: https://www.alliedscientificpro.com/shop/product/portable-energy-dispersive-x-ray-fluorescence-spectrometer-and-analyzer-6629 (accessed on 4 March 2022).
- Kammer, M.N.; Kussrow, A.; Gandhi, I.; Drabek, R.; Batchelor, R.H.; Jackson, G.W.; Bornhop, D.J. Quantification of Opioids in Urine Using an Aptamer-Based Free-Solution Assay. Anal. Chem. 2019, 91, 10582–10588. [Google Scholar] [CrossRef]
- Szkutnik-Fiedler, D.; Grześkowiak, E.; Gaca, M.; Borowicz, M. HPLC-UV Determination of Morphine in Human Plasma and Its Application to the Clinical Study. Acta Pol. Pharm. 2011, 68, 473–479. [Google Scholar]
- Elbardisy, H.M.; Foster, C.W.; Cumba, L.; Antonides, L.H.; Gilbert, N.; Schofield, C.J.; Belal, T.S.; Talaat, W.; Sutcliffe, O.B.; Daabees, H.G.; et al. Analytical Determination of Heroin, Fentanyl and Fentalogues Using High-Performance Liquid Chromatography with Diode Array and Amperometric Detection. Anal. Methods 2019, 11, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, R.E.; Umasankar, Y.; Manickam, P.; Nickel, J.C.; Iwasaki, L.R.; Kawamoto, B.K.; Todoki, K.C.; Scott, J.M.; Bhansali, S. Disposable Aptamer-Sensor Aided by Magnetic Nanoparticle Enrichment for Detection of Salivary Cortisol Variations in Obstructive Sleep Apnea Patients. Sci. Rep. 2017, 7, 17992. [Google Scholar] [CrossRef] [Green Version]
- Saccomanni, G.; Del Carlo, S.; Giorgi, M.; Manera, C.; Saba, A.; Macchia, M. Determination of Tramadol and Metabolites by HPLC-FL and HPLC-MS/MS in Urine of Dogs. J. Pharm. Biomed. Anal. 2010, 53, 194–199. [Google Scholar] [CrossRef]
- Yeh, G.-C.; Sheu, M.-T.; Yen, C.-L.; Wang, Y.-W.; Liu, C.-H.; Ho, H.-O. High-Performance Liquid Chromatographic Method for Determination of Tramadol in Human Plasma. J. Chromatogr. B Biomed. Sci. Appl. 1999, 723, 247–253. [Google Scholar] [CrossRef]
- Zuccaro, P.; Ricciarello, R.; Pichini, S.; Pacifici, R.; Altieri, I.; Pellegrini, M.; D’Ascenzo, G. Simultaneous Determination of Heroin 6-Monoacetylmorphine, Morphine, and Its Glucuronides by Liquid Chromatography--Atmospheric Pressure Ionspray-Mass Spectrometry. J. Anal. Toxicol. 1997, 21, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.L.; Vorce, S.P.; Holler, J.M.; Shimomura, E.; Magluilo, J.; Jacobs, A.J.; Huestis, M.A. Modern Instrumental Methods in Forensic Toxicology. J. Anal. Toxicol. 2007, 31, 237–253. [Google Scholar] [CrossRef]
- Rana, S.; Garg, R.K.; Singla, A. Rapid Analysis of Urinary Opiates Using Fast Gas Chromatography–Mass Spectrometry and Hydrogen as a Carrier Gas. Egypt. J. Forensic Sci. 2014, 4, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Rittgen, J.; Pütz, M.; Zimmermann, R. Identification of Fentanyl Derivatives at Trace Levels with Nonaqueous Capillary Electrophoresis-Electrospray-Tandem Mass Spectrometry (MS(n), n = 2, 3): Analytical Method and Forensic Applications. Electrophoresis 2012, 33, 1595–1605. [Google Scholar] [CrossRef]
- Phadnis, N.V.; Cavatur, R.K.; Suryanarayanan, R. Identification of Drugs in Pharmaceutical Dosage Forms by X-Ray Powder Diffractometry. J. Pharm. Biomed. Anal. 1997, 15, 929–943. [Google Scholar] [CrossRef]
- Da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; Giarola, J.F.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical Biosensors in Point-of-Care Devices: Recent Advances and Future Trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Heydari-Bafrooei, E.; Rezaei, B. Different Interaction of Codeine and Morphine with DNA: A Concept for Simultaneous Determination. Biosens. Bioelectron. 2013, 41, 627–633. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Sveiven, M.; Hall, D.A. Cortisol Detection in Undiluted Human Serum Using a Sensitive Electrochemical Structure-Switching Aptamer over an Antifouling Nanocomposite Layer. ACS Omega 2021, 6, 27888–27897. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, X.; Kulkarni, S.; Uranian, M.; Seenivasan, R.; Hall, D.A. A Sub-1 ΜW Multiparameter Injectable BioMote for Continuous Alcohol Monitoring. In Proceedings of the 2018 IEEE Custom Integrated Circuits Conference (CICC), San Diego, CA, USA, 8–11 April 2018; pp. 1–4. [Google Scholar]
- Mishra, R.K.; Goud, K.Y.; Li, Z.; Moonla, C.; Mohamed, M.A.; Tehrani, F.; Teymourian, H.; Wang, J. Continuous Opioid Monitoring along with Nerve Agents on a Wearable Microneedle Sensor Array. J. Am. Chem. Soc. 2020, 142, 5991–5995. [Google Scholar] [CrossRef] [PubMed]
- Barfidokht, A.; Mishra, R.K.; Seenivasan, R.; Liu, S.; Hubble, L.J.; Wang, J.; Hall, D.A. Wearable Electrochemical Glove-Based Sensor for Rapid and on-Site Detection of Fentanyl. Sens. Actuators B Chem. 2019, 296, 126422. [Google Scholar] [CrossRef] [PubMed]
- Güleken, Z.; Ünübol, B.; Toraman, S.; Bilici, R.; Gündüz, O.; Kuruca, S.E. Diagnosis of Opioid Use Disorder with High Sensitivity and Specificity by Advanced Computational Analysis of Fourier Transform Infrared Spectroscopy. Infrared Phys. Technol. 2020, 105, 103218. [Google Scholar] [CrossRef]
- FTIR Testing Services, Fourier Transform Infrared Spectroscopy Analyti–MSE Supplies LLC. Available online: https://www.msesupplies.com/products/ftir-testing-services-fourier-transform-infrared-spectroscopy-analysis?variant=23496486846522 (accessed on 4 March 2022).
- Raman Spectroscopy Analytical Services–MSE Supplies LLC. Available online: https://www.msesupplies.com/products/raman-spectroscopy-analytical-services?variant=23496530886714 (accessed on 4 March 2022).
- United Nations Office on Drugs and Crime. Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens; UNODC: Vienna, Austria, 2017; p. 72. [Google Scholar]
- Center for Environmental & Human Toxicology, College of Veterinary Medicine. Analytical Services Pricing: Analysis Performed by LC-MS/MS; University of Florida: Gainesville, FL, USA; Available online: https://toxicology.vetmed.ufl.edu/core-laboratories/analytical-services-pricing/ (accessed on 15 May 2021).
- Garby, D.M.; Cheryk, L.A. Synthetic Opioid Analysis by LC-MS/MS. LC-MS in Drug Analysis: Methods and ProtocolsLangman, L.J., Snozek, C.L.H., Eds.; Methods in Molecular BiologyHumana Press: Totowa, NJ, USA, 2012; pp. 65–73. ISBN 978-1-61779-934-1. [Google Scholar]
- Fernández, P.; Vázquez, C.; Morales, L.; Bermejo, A.M. Analysis of Opiates, Cocaine and Metabolites in Urine by High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD). J. Appl. Toxicol. 2005, 25, 200–204. [Google Scholar] [CrossRef]
- Galson Labs. Available online: http://www.galsonlabs.com/samplinganalysis/sampling-analysis-guide/ (accessed on 4 March 2022).
- Refractive Index Test. Available online: https://www.medallionlabs.com/tests/refractive-index/ (accessed on 4 March 2022).
- Verrinder, E.; Wester, N.; Leppänen, E.; Lilius, T.; Kalso, E.; Mikladal, B.; Varjos, I.; Koskinen, J.; Laurila, T. Electrochemical Detection of Morphine in Untreated Human Capillary Whole Blood. ACS Omega 2021, 6, 11563–11569. [Google Scholar] [CrossRef]
- Glasscott, M.W.; Vannoy, K.J.; Fernando, P.A.I.; Kosgei, G.K.; Moores, L.C.; Dick, J.E. Electrochemical Sensors for the Detection of Fentanyl and Its Analogs: Foundations and Recent Advances | Elsevier Enhanced Reader. TrAC Trends Anal. Chem. 2020, 132, 116037. [Google Scholar] [CrossRef]
- Kerr, T.; Tupper, K. Evidence Review Report. Drug Checking as a Harm Reduction Intervention; British Columbia Centre on Substance Use: Vancouver, BC, Canada, 2017; Available online: https://www.bccsu.ca/wp-content/uploads/2017/12/Drug-Checking-Evidence-Review-Report.pdf (accessed on 15 May 2021).
- Dahan, A.; Aarts, L.; Smith, T.W. Incidence, Reversal, and Prevention of Opioid-Induced Respiratory Depression. Anesthesiology 2010, 112, 226–238. [Google Scholar] [CrossRef] [Green Version]
- Jubran, A. Pulse Oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.; Nagappa, M.; Wong, J.; Singh, M.; Wong, D.; Chung, F. Continuous Pulse Oximetry and Capnography Monitoring for Postoperative Respiratory Depression and Adverse Events: A Systematic Review and Meta-Analysis. Anesth. Analg. 2017, 125, 2019–2029. [Google Scholar] [CrossRef]
- Ayad, S.; Khanna, A.K.; Iqbal, S.U.; Singla, N. Characterisation and Monitoring of Postoperative Respiratory Depression: Current Approaches and Future Considerations. Br. J. Anaesth. 2019, 123, 378–391. [Google Scholar] [CrossRef]
- Miller, K.M.; Kim, A.Y.; Yaster, M.; Kudchadkar, S.R.; White, E.; Fackler, J.; Monitto, C.L. Long-Term Tolerability of Capnography and Respiratory Inductance Plethysmography for Respiratory Monitoring in Pediatric Patients Treated with Patient-Controlled Analgesia. Pediatr. Anesth. 2015, 25, 1054–1059. [Google Scholar] [CrossRef] [Green Version]
- Mimoz, O.; Benard, T.; Gaucher, A.; Frasca, D.; Debaene, B. Accuracy of Respiratory Rate Monitoring Using a Non-Invasive Acoustic Method after General Anaesthesia. Br. J. Anaesth. 2012, 108, 872–875. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Sunshine, J.E.; Gollakota, S. Contactless Infant Monitoring Using White Noise. In Proceedings of the 25th Annual International Conference on Mobile Computing and Networking, ACM, Los Cabos, Mexico, 21–25 October 2019; pp. 1–16. [Google Scholar]
- Ramsay, M.A.E.; Usman, M.; Lagow, E.; Mendoza, M.; Untalan, E.; De Vol, E. The Accuracy, Precision and Reliability of Measuring Ventilatory Rate and Detecting Ventilatory Pause by Rainbow Acoustic Monitoring and Capnometry. Anesth. Analg. 2013, 117, 69–75. [Google Scholar] [CrossRef]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Khadheer Pasha, S.K. Recent Advances in Electromagnetic Interference Shielding Properties of Metal and Carbon Filler Reinforced Flexible Polymer Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Zhao, F.; Li, M.; Qian, Y.; Tsien, J.Z. Remote Measurements of Heart and Respiration Rates for Telemedicine. PLoS ONE 2013, 8, e71384. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Prathosh, A.P.; Praveena, P. Real-Time Respiration Rate Measurement from Thoracoabdominal Movement with a Consumer Grade Camera. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 2708–2711. [Google Scholar] [CrossRef]
- Hill, B.L.; Liu, X.; McDuff, D. Beat-to-Beat Cardiac Pulse Rate Measurement from Video. In Proceedings of the 2021 IEEE/CVF International Conference on Computer Vision Workshops (ICCVW), Montreal, BC, Canada, 11–17 October 2021; pp. 2739–2742. [Google Scholar]
| RDT [17] | FTIR [25,49,50] | Raman [24,51] | GC-MS [22,52,53] | LC-MS [20,54] | HPLC [37,55,56] | Interferometry [32,57] | Electrochemical [43,58,59] | |
|---|---|---|---|---|---|---|---|---|
| Detection Principle | Immuno chromatography | Identification of fingerprint spectra | Interaction of Light with molecule | Separation by vapor pressure and distribution constant and Identification by m/z | Separation of mixture based on chemical/physical properties and identification by m/z | Separation based on Distribution and Identification with UV spectroscopy | Measurement of intrinsic solution phase properties | Voltammetry or Amperometry |
| Specimen | Urine/Saliva | Blood/Powder | Urine | Urine/Saliva | Urine/Blood/saliva | Plasma/Urine | Urine | Blood/Urine/Saliva |
| Sensitivity | Moderate | Moderate | Moderate | Excellent | Excellent | Moderate | Excellent | Excellent |
| Specificity | Excellent | Moderate | Moderate | Excellent | Excellent | Poor | Good | Good |
| LOD | µM | µM | mM range | pM | pM | nM | pM | pM |
| Response Time | 5 min | 1 min | 5 min | 30 min | <30 min | 30 min | 60 min | 1 min |
| Skillset | Low | Moderate | Moderate | High | High | High | Moderate | Low |
| Use case | POC | POC/Lab | POC/Lab | Lab | Lab | Lab | Lab | POC/Lab |
| User * | LE/HC | LE/HC | LE/HC | HC | LE/HE | HC | HC | LE/HC |
| Cost/test [Instrument price] | Very low (Approximately $1–5) | Moderate, $50–100 # ($100,000 + new, advanced models Portable: $10,000–60,000) | Moderate, $80–120 # ($100,000 + new, advanced models Portable: $10,000–60,000) | High $100–200 # ($200,000 + new, advanced models) | High $100–200 # ($200,000 + new, advanced models) | Low $25–50 # ($80,000 + new advanced models) | Moderate $50–80 # ($50,000 + new advanced models) | Very low (NA) ($6000 + new, advanced models Portable: $500–5000) |
| Pulse Oximetry | Capnography | Contactless Systems | Remote Systems | |
|---|---|---|---|---|
| Detection principle | SpO2, HR | EtCO2, RR | RR | SpO2, HR |
| Sensitivity | High | High | Moderate | Moderate-High |
| Specificity | Moderate to High | High | Moderate | Moderate-High |
| Response Time | Slow | Fast | Medium | Medium-Fast |
| Skillset | Low | Low-Moderate | Low-High | Low-Moderate |
| Cost | Low | Moderate | Low-High | Low-Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.K.; Sidhu, G.K.; Gupta, K. Current and Future Perspective of Devices and Diagnostics for Opioid and OIRD. Biomedicines 2022, 10, 743. https://doi.org/10.3390/biomedicines10040743
Singh NK, Sidhu GK, Gupta K. Current and Future Perspective of Devices and Diagnostics for Opioid and OIRD. Biomedicines. 2022; 10(4):743. https://doi.org/10.3390/biomedicines10040743
Chicago/Turabian StyleSingh, Naveen K., Gurpreet K. Sidhu, and Kuldeep Gupta. 2022. "Current and Future Perspective of Devices and Diagnostics for Opioid and OIRD" Biomedicines 10, no. 4: 743. https://doi.org/10.3390/biomedicines10040743
APA StyleSingh, N. K., Sidhu, G. K., & Gupta, K. (2022). Current and Future Perspective of Devices and Diagnostics for Opioid and OIRD. Biomedicines, 10(4), 743. https://doi.org/10.3390/biomedicines10040743






