Targeted Accumulation of Macrophages Induced by Microbeam Irradiation in a Tissue-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Cell Lines and Irradiations
2.1.1. MRT Irradiation of Lung Carcinoma and Collateral Irradiation of the Liver
2.1.2. MRT Irradiation of Normal Mouse Ear Pinnae and Melanoma
2.2. Immunohistochemistry
3. Results
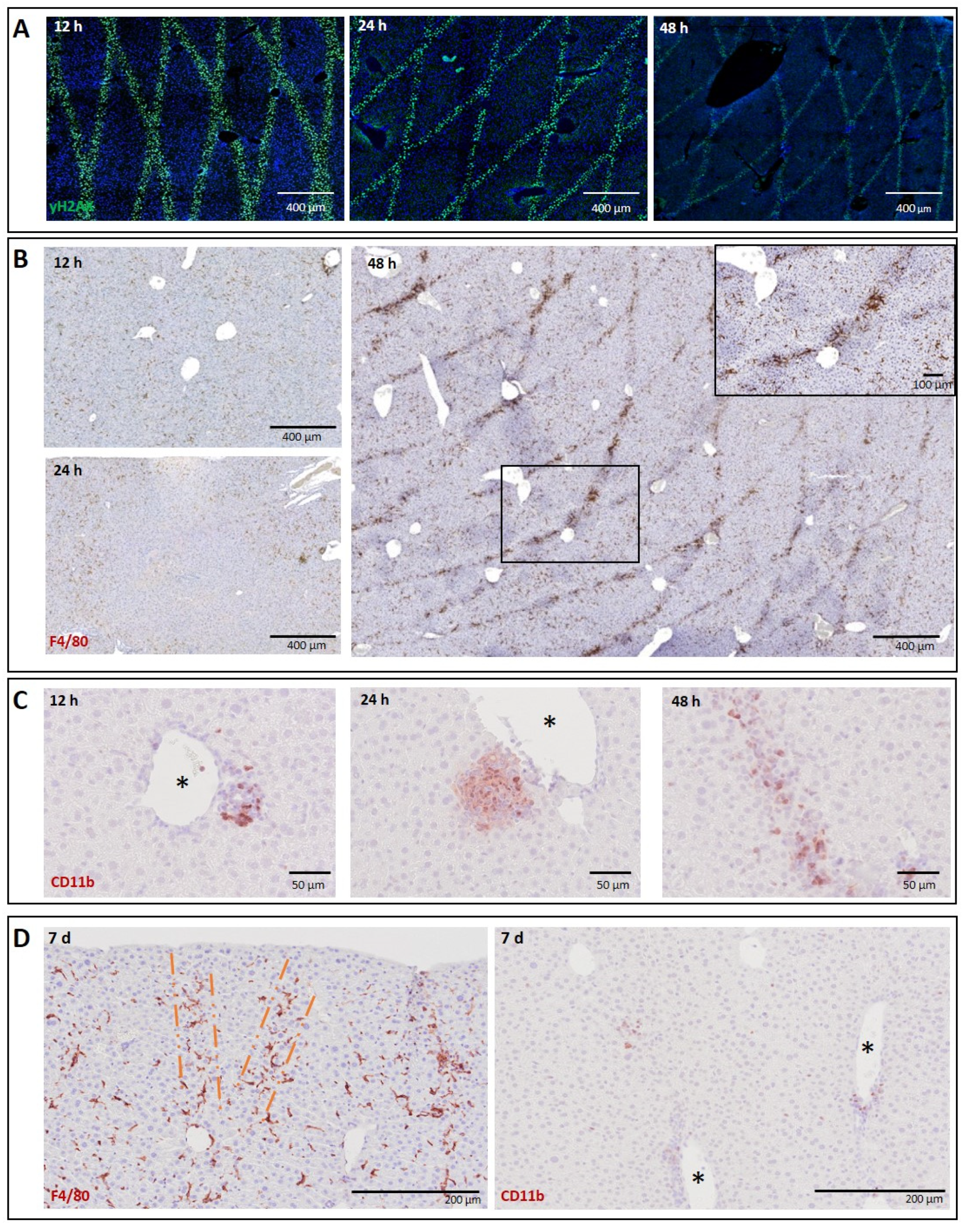
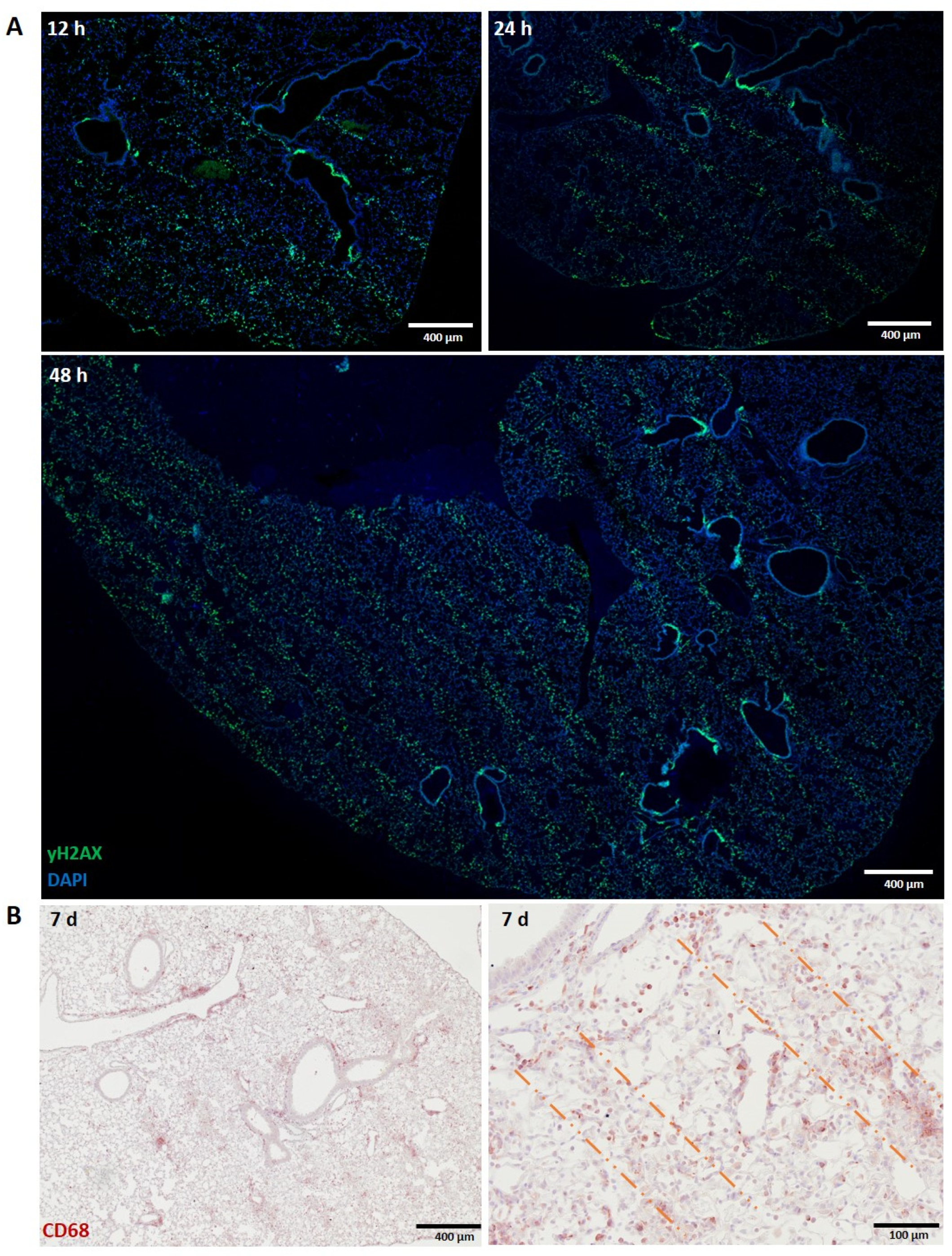
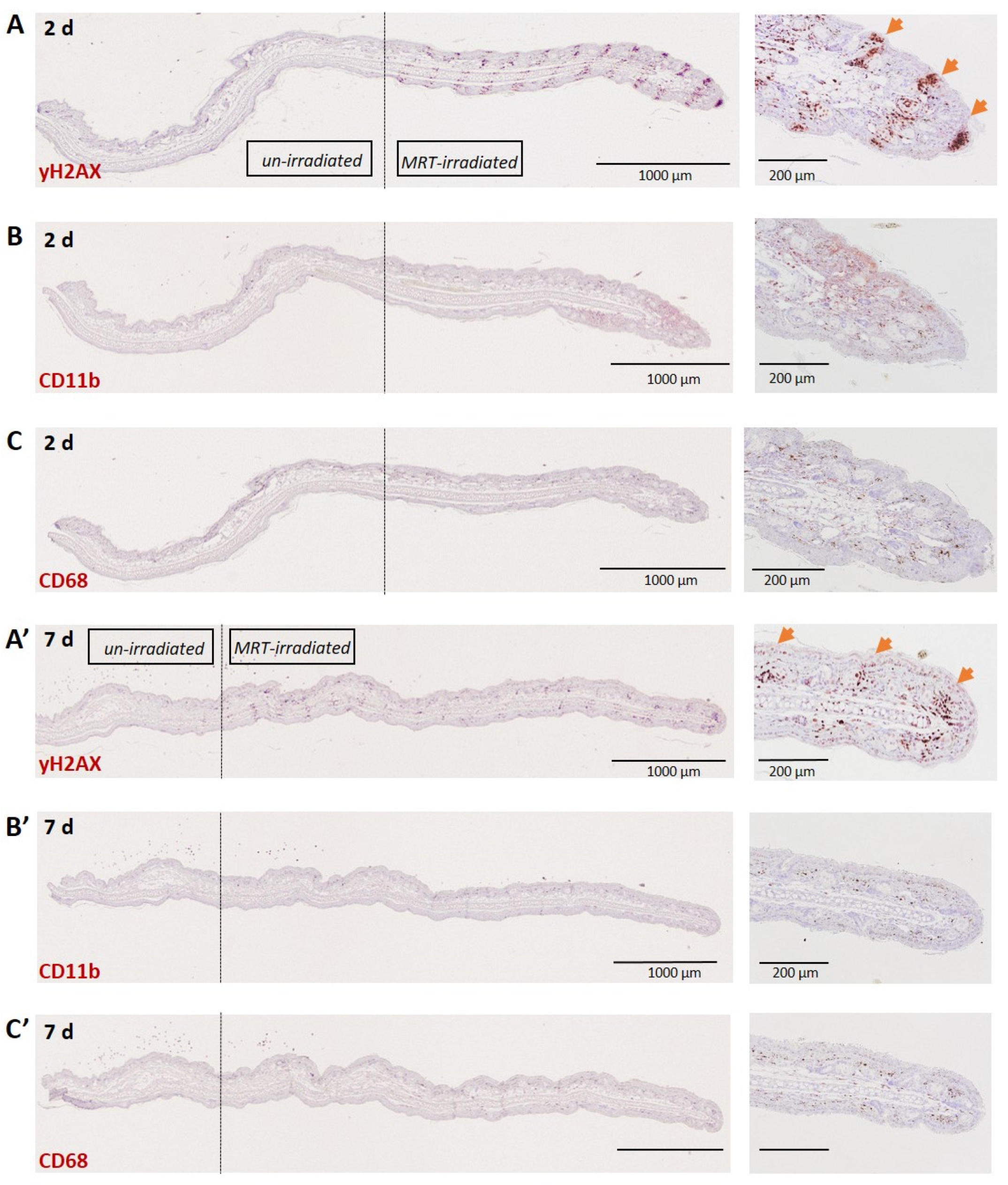
3.1. Normal Tissues
3.1.1. Liver
3.1.2. Lung
3.1.3. Skin (Ear)
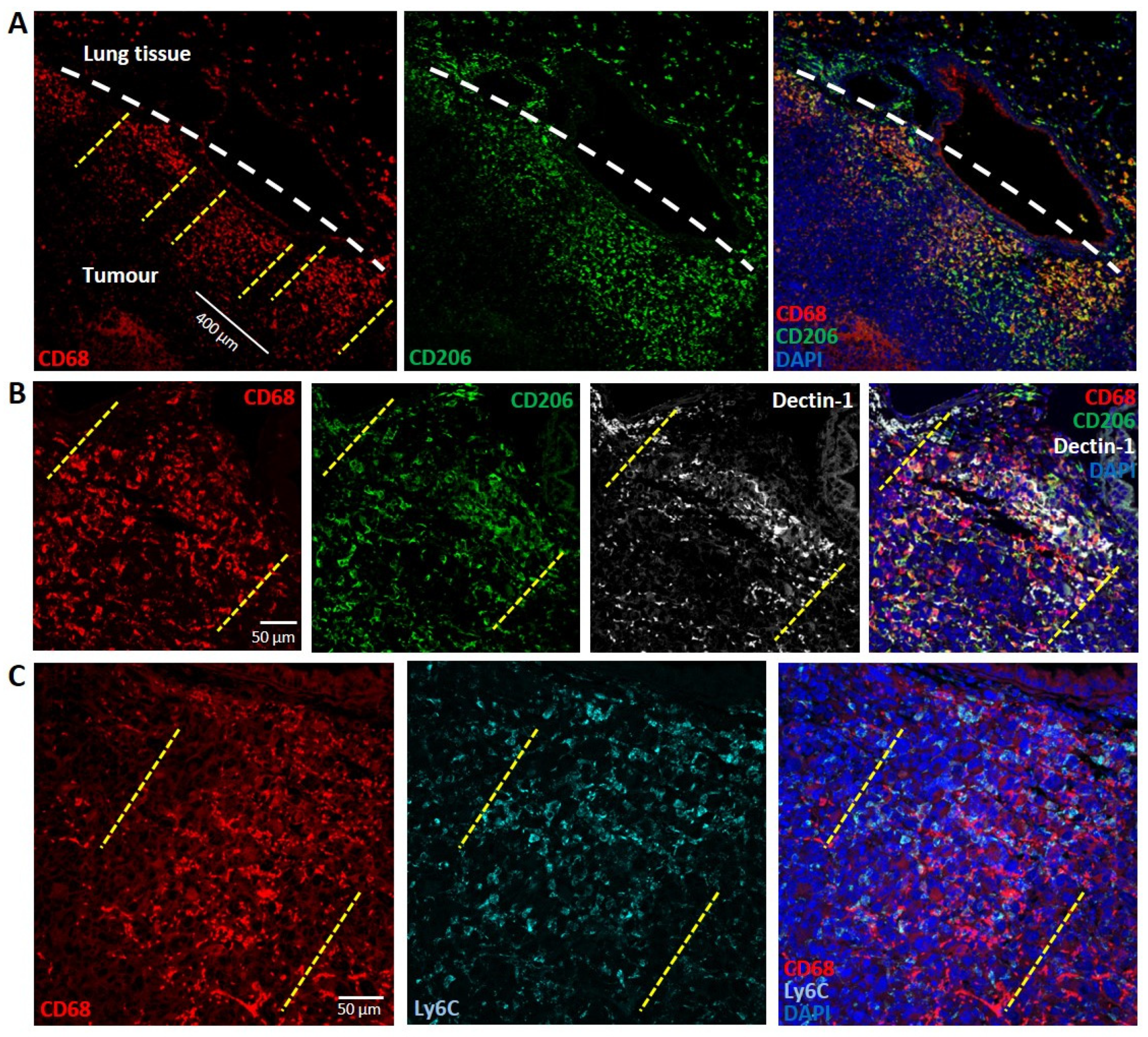
3.2. Tumours
3.2.1. Lung Carcinoma
3.2.2. Melanoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH Radiotherapy: History and Future. Front. Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palomo, C.; Fazzari, J.; Trappetti, V.; Smyth, L.; Janka, H.; Laissue, J.; Djonov, V. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers 2020, 12, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukunaga, H.; Butterworth, K.T.; McMahon, S.J.; Prise, K.M. A Brief Overview of the Preclinical and Clinical Radiobiology of Microbeam Radiotherapy. Clin. Oncol. 2021, 33, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Palomo, C.; Trappetti, V.; Potez, M.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers 2020, 12, 2656. [Google Scholar] [CrossRef]
- Yang, Y.; Swierczak, A.; Ibahim, M.; Paiva, P.; Cann, L.; Stevenson, A.W.; Crosbie, J.C.; Anderson, R.L.; Rogers, P.A. Synchrotron microbeam radiotherapy evokes a different early tumor immunomodulatory response to conventional radiotherapy in EMT6.5 mammary tumors. Radiother. Oncol. 2019, 133, 93–99. [Google Scholar] [CrossRef]
- Forrester, H.B.; Lobachevsky, P.N.; Stevenson, A.W.; Hall, C.J.; Martin, O.A.; Sprung, C.N. Abscopal Gene Expression in Response to Synchrotron Radiation Indicates a Role for Immunological and DNA Damage Response Genes. Radiat. Res. 2020, 194, 678–687. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. OncoImmunology 2014, 3, e28518. [Google Scholar] [CrossRef] [Green Version]
- Haikerwal, S.J.; Hagekyriakou, J.; MacManus, M.; Martin, O.A.; Haynes, N.M. Building immunity to cancer with radiation therapy. Cancer Lett. 2015, 368, 198–208. [Google Scholar] [CrossRef]
- Rastogi, S.; Boylan, M.; Wright, E.G.; Coates, P. Interactions of Apoptotic Cells with Macrophages in Radiation-Induced Bystander Signaling. Radiat. Res. 2012, 179, 135–145. [Google Scholar] [CrossRef]
- Mukherjee, D.; Coates, P.J.; Lorimore, S.A.; Wright, E.G. Responses to ionizing radiation mediated by inflammatory mechanisms. J. Pathol. 2014, 232, 289–299. [Google Scholar] [CrossRef]
- Lauber, K.; Ernst, A.; Orth, M.; Herrmann, M.J.; Belka, C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front. Oncol. 2012, 2, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meziani, L.; Mondini, M.; Petit, B.; Boissonnas, A.; De Montpreville, V.T.; Mercier, O.; Vozenin, M.-C.; Deutsch, E. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J. 2018, 51, 1702120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, E.L. The sequence of histological changes in mouse lungs after single doses of X-rays. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 345–347. [Google Scholar] [CrossRef]
- Shi, X.; Shiao, S.L. The role of macrophage phenotype in regulating the response to radiation therapy. Transl. Res. 2018, 191, 64–80. [Google Scholar] [CrossRef]
- Gough, M.J.; Young, K.; Crittenden, M. The Impact of the Myeloid Response to Radiation Therapy. Clin. Dev. Immunol. 2013, 2013, e281958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrador, E.; Salvador, R.; Villaescusa, J.; Soriano, J.; Estrela, J.; Montoro, A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.; Lobachevsky, P.N.; Palazzolo, J.S.; Forrester, H.; Haynes, N.M.; Ivashkevich, A.; Stevenson, A.W.; Hall, C.J.; Ntargaras, A.; Kotsaris, V.; et al. Localized Synchrotron Irradiation of Mouse Skin Induces Persistent Systemic Genotoxic and Immune Responses. Cancer Res. 2017, 77, 6389–6399. [Google Scholar] [CrossRef] [Green Version]
- Trappetti, V.; Fazzari, J.; Fernandez-Palomo, C.; Scheidegger, M.; Volarevic, V.; Martin, O.; Djonov, V. Microbeam Radiotherapy—A Novel Therapeutic Approach to Overcome Radioresistance and Enhance Anti-Tumour Response in Melanoma. Int. J. Mol. Sci. 2021, 22, 7755. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.-X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Coates, P.J.; Rundle, J.K.; Lorimore, S.A.; Wright, E.G. Indirect Macrophage Responses to Ionizing Radiation: Implications for Genotype-Dependent Bystander Signaling. Cancer Res. 2008, 68, 450–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Ishihara, H.; Tanaka, I.; Suzuki, G.; Tsuneoka, K.; Yoshida, K.; Ohtsu, H. Expression of IL-1.BETA. mRNA in Mice after Whole Body X-Irradiation. J. Radiat. Res. 1995, 36, 125–133. [Google Scholar] [CrossRef]
- Willett, C.G.; Boucher, Y.; Di Tomaso, E.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Chung, D.C.; Sahani, D.V.; Kalva, S.P.; Kozin, S.V.; et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004, 10, 145–147. [Google Scholar] [CrossRef]
- Xu, F.; Wei, Y.; Tang, Z.; Liu, B.; Dong, J. Tumor-associated macrophages in lung cancer: Friend or foe? Mol. Med. Rep. 2020, 22, 4107–4115. [Google Scholar]
- Gordon, S.; Plüddemann, A.; Estrada, F.M. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [Green Version]
- Trappetti, V.; Fernandez-Palomo, C.; Smyth, L.; Klein, M.; Haberthür, D.; Butler, D.; Barnes, M.; Shintani, N.; de Veer, M.; Laissue, J.A.; et al. Synchrotron Microbeam Radiation Therapy for the Treatment of Lung Carcinoma: A Preclinical Study. Int. J. Radiat. Oncol. 2021, 111, 1276–1288. [Google Scholar] [CrossRef]
- Potez, M.; Bouchet, A.; Wagner, J.; Donzelli, M.; Bräuer-Krisch, E.; Hopewell, J.W.; Laissue, J.; Djonov, V. Effects of Synchrotron X-Ray Micro-beam Irradiation on Normal Mouse Ear Pinnae. Int. J. Radiat. Oncol. 2018, 101, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. GammaH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef] [PubMed]
- MacParland, S.A.; Liu, J.C.; Ma, X.-Z.; Innes, B.T.; Bartczak, A.M.; Gage, B.K.; Manuel, J.; Khuu, N.; Echeverri, J.; Linares, I.; et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 2018, 9, 4383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Kubes, P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell 2016, 165, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, M.; Ni, B.; Carlson, T.; Schlesinger, L. Mannose receptor (CD206)-mediated signaling in human macrophages in the context of tuberculosis (INC7P.418). J. Immunol. 2014, 192, 186.19. [Google Scholar]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L709–L725. [Google Scholar] [CrossRef]
- Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; Van den Bossche, J.; Mack, M.; Pipeleers, D.; Veld, P.I.; De Baetselier, P.; et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived from Ly6C (high) Monocytes. Cancer Res. 2010, 70, 5728–5739. [Google Scholar] [CrossRef] [Green Version]
- Eling, L.; Bouchet, A.; Ocadiz, A.; Adam, J.-F.; Kershmiri, S.; Elleaume, H.; Krisch, M.; Verry, C.; Laissue, J.; Balosso, J.; et al. Unexpected Benefits of Multiport Synchrotron Microbeam Radiation Therapy for Brain Tumors. Cancers 2021, 13, 936. [Google Scholar] [CrossRef]
- Bouchet, A.; Bräuer-Krisch, E.; Prezado, Y.; El Atifi, M.; Rogalev, L.; Le Clec’H, C.; Laissue, J.A.; Pelletier, L.; Le Duc, G. Better Efficacy of Synchrotron Spatially Microfractionated Radiation Therapy Than Uniform Radiation Therapy on Glioma. Int. J. Radiat. Oncol. 2016, 95, 1485–1494. [Google Scholar] [CrossRef]
- Meziani, L.; Deutsch, E.; Mondini, M. Macrophages in radiation injury: A new therapeutic target. OncoImmunology 2018, 7, e1494488. [Google Scholar] [CrossRef]
- Sabatasso, S.; Fernandez-Palomo, C.; Hlushchuk, R.; Fazzari, J.; Tschanz, S.; Pellicioli, P.; Krisch, M.; Laissue, J.A.; Djonov, V. Transient and Efficient Vascular Permeability Window for Adjuvant Drug Delivery Triggered by Microbeam Radiation. Cancers 2021, 13, 2103. [Google Scholar] [CrossRef] [PubMed]
- Brönnimann, D.; Bouchet, A.; Schneider, C.; Potez, M.; Serduc, R.; Bräuer-Krisch, E.; Graber, W.; Von Gunten, S.; Laissue, J.A.; Djonov, V. Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo. Sci. Rep. 2016, 6, 33601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Holt, M.; Yin, H.; Li, G.; Hu, C.-J.; Ju, C. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem. Pharmacol. 2013, 86, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigmond, E.; Samia-Grinberg, S.; Pasmanik-Chor, M.; Brazowski, E.; Shibolet, O.; Halpern, Z.; Varol, C. Infiltrating Monocyte-Derived Macrophages and Resident Kupffer Cells Display Different Ontogeny and Functions in Acute Liver Injury. J. Immunol. 2014, 193, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Heymann, F.; Peusquens, J.; Ludwig-Portugall, I.; Kohlhepp, M.; Ergen, C.; Niemietz, P.; Martin, C.; van Rooijen, N.; Ochando, J.C.; Randolph, G.J.; et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015, 62, 279–291. [Google Scholar] [CrossRef]
- Tacke, F.; Zimmermann, H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Dal-Secco, D.; Wang, J.; Zeng, Z.; Kolaczkowska, E.; Wong, C.; Petri, B.; Ransohoff, R.M.; Charo, I.F.; Jenne, C.N.; Kubes, P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med. 2015, 212, 447–456. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Landsman, L.; Jung, S. Lung Macrophages Serve as Obligatory Intermediate between Blood Monocytes and Alveolar Macrophages. J. Immunol. 2007, 179, 3488–3494. [Google Scholar] [CrossRef]
- Abernathy, L.M.; Fountain, M.D.; Rothstein, S.E.; David, J.M.; Yunker, C.K.; Rakowski, J.; Lonardo, F.; Joiner, M.C.; Hillman, G.G. Soy Isoflavones Promote Radioprotection of Normal Lung Tissue by Inhibition of Radiation-Induced Activation of Macrophages and Neutrophils. J. Thorac. Oncol. 2015, 10, 1703–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.-I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Xie, J.; Zhao, L.; Fei, X.; Zhang, H.; Tan, Y.; Nie, X.; Zhou, L.; Liu, Z.; Ren, Y.; et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine 2020, 57, 102833. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.D.; Romanelli, P.; Bravin, A.; Le Duc, G.; Brauer-Krisch, E.; Requardt, H.; Bartzsch, S.; Hlushchuk, R.; Laissue, J.-A.; Djonov, V. Non-conventional Ultra-High Dose Rate (FLASH) Microbeam Radiotherapy Provides Superior Normal Tissue Sparing in Rat Lung Compared to Non-conventional Ultra-High Dose Rate (FLASH) Radiotherapy. Cureus 2021, 13, e19317. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef]
- Mehrabi, M.; Amini, F.; Mehrabi, S. Active Role of the Necrotic Zone in Desensitization of Hypoxic Macrophages and Regulation of CSC-Fate: A hypothesis. Front. Oncol. 2018, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Crosbie, J.; Anderson, R.; Rothkamm, K.; Restall, C.M.; Cann, L.; Ruwanpura, S.; Meachem, S.; Yagi, N.; Svalbe, I.; Lewis, R.A.; et al. Tumor Cell Response to Synchrotron Microbeam Radiation Therapy Differs Markedly from Cells in Normal Tissues. Int. J. Radiat. Oncol. 2010, 77, 886–894. [Google Scholar] [CrossRef]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front. Immunol. 2017, 8, 828. [Google Scholar] [CrossRef] [Green Version]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Leblond, M.M.; Pérès, E.A.; Helaine, C.; Gérault, A.N.; Moulin, D.; Anfray, C.; Divoux, D.; Petit, E.; Bernaudin, M.; Valable, M.B.S. M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma. Oncotarget 2017, 8, 72597–72612. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, S.J.; Ruckerl, D.; Cook, P.C.; Jones, L.H.; Finkelman, F.D.; van Rooijen, N.; MacDonald, A.S.; Allen, J.E. Local Macrophage Proliferation, Rather than Recruitment from the Blood, Is a Signature of T H 2 Inflammation. Science 2011, 332, 1284–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollenberg, A.; Oppel, T.; Schottdorf, E.-M.; Günther, S.; Moderer, M.; Mommaas, M. Expression and Function of the Mannose Receptor CD206 on Epidermal Dendritic Cells in Inflammatory Skin Diseases. J. Investig. Dermatol. 2002, 118, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahrendorf, M.; Swirski, F.K. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016, 119, 414–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanagavelu, S.; Gupta, S.; Wu, X.; Philip, S.; Wattenberg, M.; Hodge, J.W.; Couto, M.D.; Chung, K.D.; Ahmed, M.M. In VivoEffects of Lattice Radiation Therapy on Local and Distant Lung Cancer: Potential Role of Immunomodulation. Radiat. Res. 2014, 182, 149–162. [Google Scholar] [CrossRef]
- Lobachevsky, P.N.; Ventura, J.; Giannakandropoulou, L.; Forrester, H.; Palazzolo, J.S.; Haynes, N.M.; Stevenson, A.; Hall, C.J.; Mason, J.; Pollakis, G.; et al. A Functional Immune System Is Required for the Systemic Genotoxic Effects of Localized Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1184–1193. [Google Scholar] [CrossRef]

| Tissue Sample | MRT Parameters | Marker | Time Points |
|---|---|---|---|
| Liver | 50/400 µm 400 Gy x2′ cross-fired | γH2AX, F4/80, CD11b, GATA-6 | 12, 24 h, 48 h |
| 7 days | |||
| Lung | γH2AX, CD68 | 12, 24, 48 h | |
| 7 days | |||
| Ear | 50/200 µm | γH2AX, CD68, CD11b | 2, 7 days |
| 800 Gy | |||
| Lung Carcinoma | 50/400 µm | γH2AX, CD68, CD206, Dectin-1, Ly6C | 3, 7, 16 days |
| 400 Gy x2′ cross-fired | |||
| Melanoma | 50/200 µm | γH2AX, C68, CD206 | 2 h |
| 400 Gy | 2, 7 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trappetti, V.; Fazzari, J.; Fernandez-Palomo, C.; Smyth, L.; Potez, M.; Shintani, N.; de Breuyn Dietler, B.; Martin, O.A.; Djonov, V. Targeted Accumulation of Macrophages Induced by Microbeam Irradiation in a Tissue-Dependent Manner. Biomedicines 2022, 10, 735. https://doi.org/10.3390/biomedicines10040735
Trappetti V, Fazzari J, Fernandez-Palomo C, Smyth L, Potez M, Shintani N, de Breuyn Dietler B, Martin OA, Djonov V. Targeted Accumulation of Macrophages Induced by Microbeam Irradiation in a Tissue-Dependent Manner. Biomedicines. 2022; 10(4):735. https://doi.org/10.3390/biomedicines10040735
Chicago/Turabian StyleTrappetti, Verdiana, Jennifer Fazzari, Cristian Fernandez-Palomo, Lloyd Smyth, Marine Potez, Nahoko Shintani, Bettina de Breuyn Dietler, Olga A. Martin, and Valentin Djonov. 2022. "Targeted Accumulation of Macrophages Induced by Microbeam Irradiation in a Tissue-Dependent Manner" Biomedicines 10, no. 4: 735. https://doi.org/10.3390/biomedicines10040735
APA StyleTrappetti, V., Fazzari, J., Fernandez-Palomo, C., Smyth, L., Potez, M., Shintani, N., de Breuyn Dietler, B., Martin, O. A., & Djonov, V. (2022). Targeted Accumulation of Macrophages Induced by Microbeam Irradiation in a Tissue-Dependent Manner. Biomedicines, 10(4), 735. https://doi.org/10.3390/biomedicines10040735







