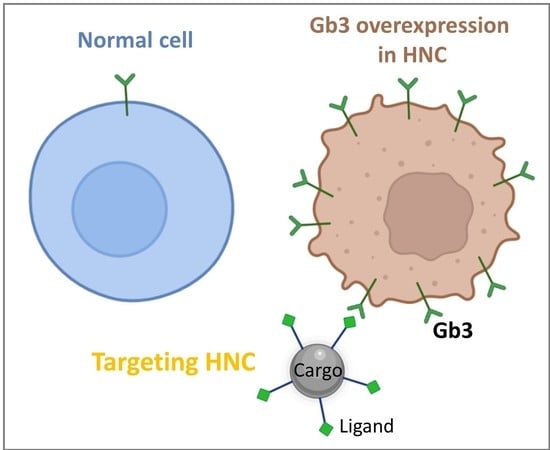
Gb3/cd77 Is a Predictive Marker and Promising Therapeutic Target for Head and Neck Cancer
Abstract
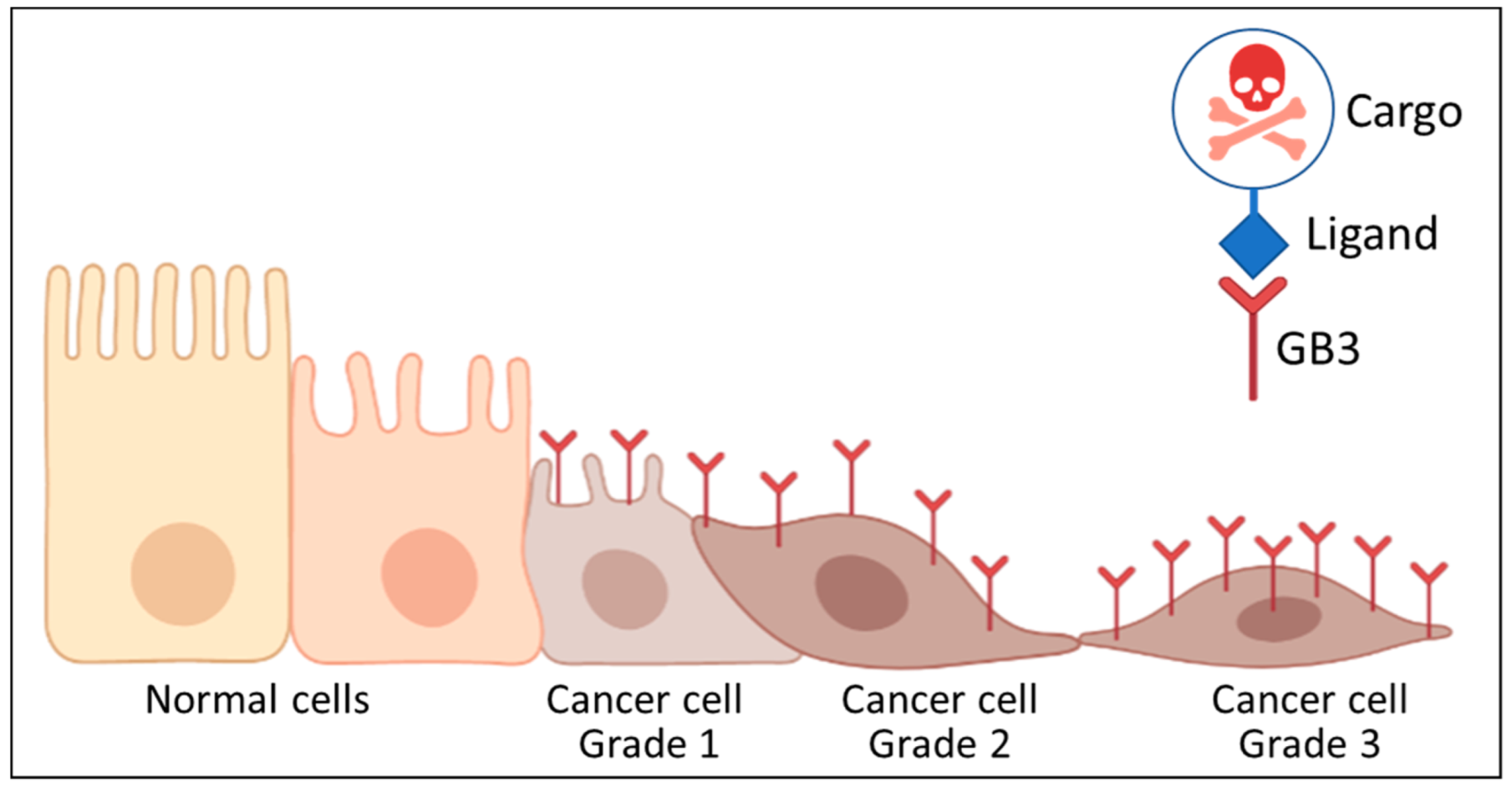
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunofluorescence
2.3. Flow Cytometry
2.4. Preclinical Murine Models
2.5. Frozen Animal Tissues
2.6. Human Biopsy Samples
2.7. Quantitative Examination of GB3 Expression
2.8. Statistical Analysis
3. Results
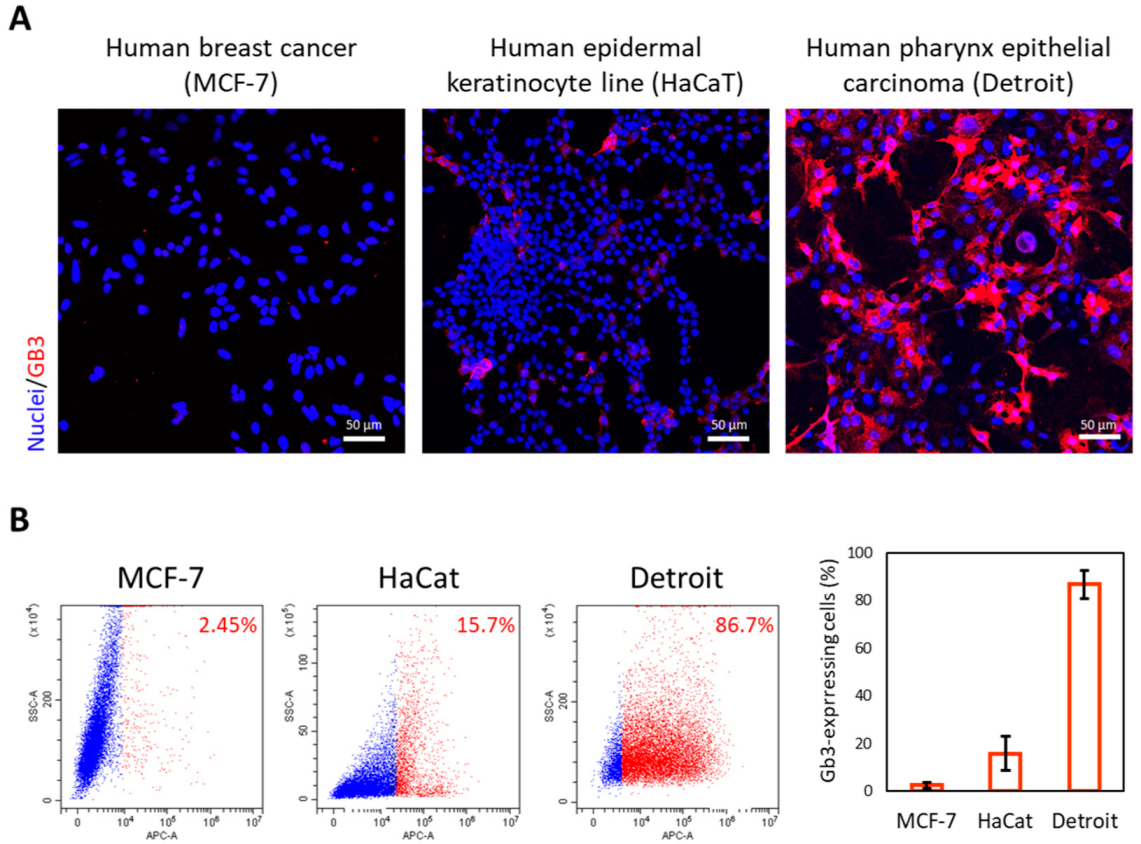
3.1. Detection of GB3 in Preneoplastic and Malignant Head and Neck Cells
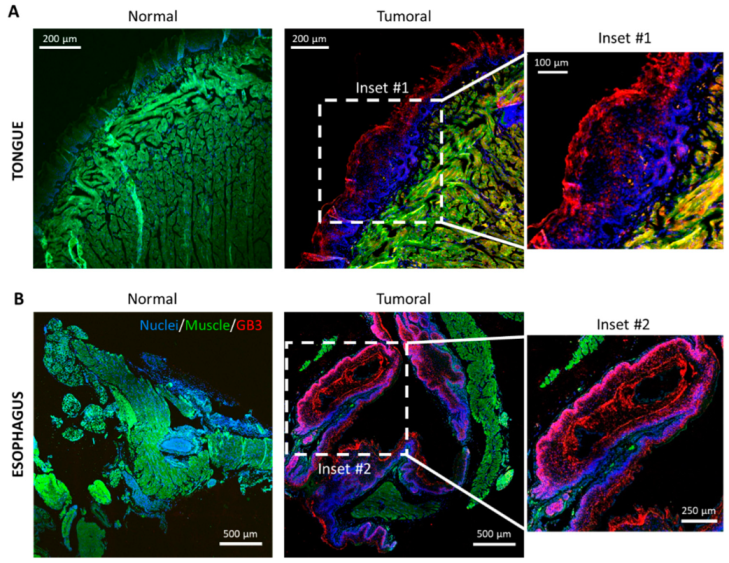
3.2. Gb3 Expression in an Hnc Mouse Model
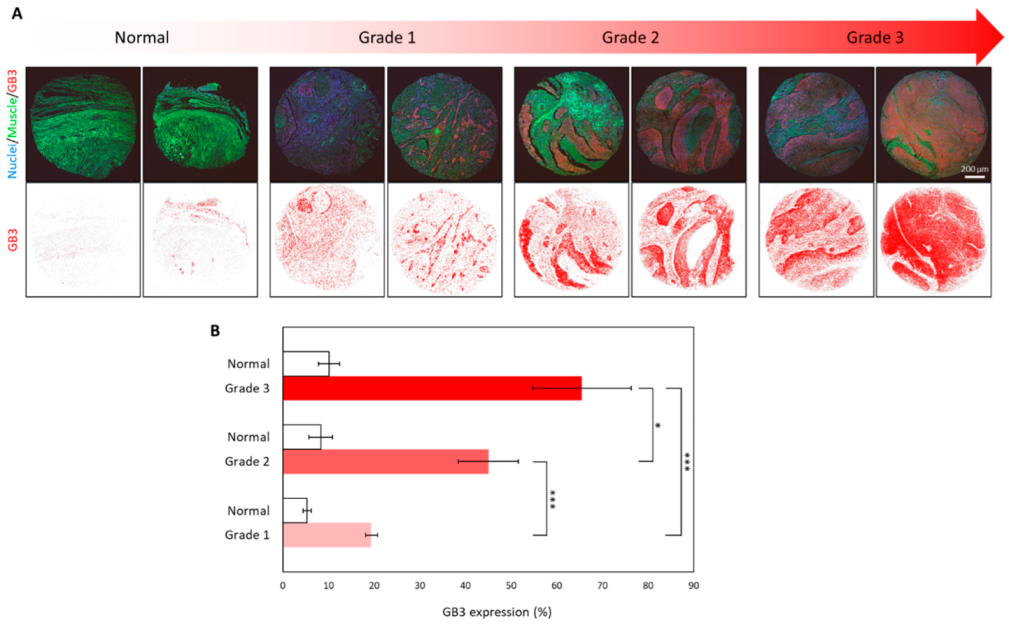
3.3. Overexpression of GB3 in Biopsy Samples of Human HNC Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Duprez, F.; Berwouts, D.; De Neve, W.; Bonte, K.; Boterberg, T.; Deron, P.; Huvenne, W.; Rottey, S.; Mareel, M. Distant metastases in head and neck cancer. Head Neck 2017, 39, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.A.; Dietz, A.; Gewelke, U.; Heller, W.; Weidauer, H. Tobacco and alcohol and the risk of head and neck cancer. Clin. Investig. 1992, 70, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Boffetta, P.; Zaridze, D.; Shangina, O.; Szeszenia-Dabrowska, N.; Mates, D.; Janout, V.; Fabiánová, E.; Bencko, V.; Moullan, N.; et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol. Prev. Biomark. 2006, 15, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef]
- Niya, M.H.K.; Tameshkel, F.S.; Panahi, M.; Salim, F.B.; Monavari, S.H.R.; Keyvani, H. Human Papillomavirus Investigation in Head and Neck Squamous Cell Carcinoma: Initial Report from the Low Risk HPV Types Associations. Asian Pac. J. Cancer Prev. 2017, 18, 2573. [Google Scholar]
- Udager, A.; McHugh, J. Human Papillomavirus-Associated Neoplasms of the Head and Neck. Surg. Pathol. Clin. 2017, 10, 35–55. [Google Scholar] [CrossRef]
- Mohebbi, E.; Hadji, M.; Rashidian, H.; Rezaianzadeh, A.; Marzban, M.; Haghdoost, A.A.; Ms, A.N.T.; Moradi, A.; Gholipour, M.; Najafi, F.; et al. Opium use and the risk of head and neck squamous cell carcinoma. Int. J. Cancer 2021, 148, 1066–1076. [Google Scholar] [CrossRef]
- Rabinovics, N.; Mizrachi, A.; Hadar, T.; Ad-El, D.; Feinmesser, R.; Guttman, D.; Shpitzer, T.; Bachar, G. Cancer of the head and neck region in solid organ transplant recipients. Head Neck 2014, 36, 181–186. [Google Scholar] [CrossRef]
- Maier, H.; Tisch, M. Occupation and Head and Neck Cancer. Oncol. Res. Treat. 2000, 23, 34–40. [Google Scholar] [CrossRef]
- Boeing, H.; Dietrich, T.; Hoffmann, K.; Pischon, T.; Ferrari, P.; Lahmann, P.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Allen, N.; Key, T.; et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: The prospective EPIC-study. Cancer Causes Control 2006, 17, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, H.; Sato, T.; Yoshino, K. Radiation-induced cancers of the head and neck region. Acta Otolaryngol. Suppl. 1998, 533, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, V.; Stojanovic, J.; Vecchioni, A.; Pastorino, R.; Boccia, S. Systematic Review and Meta-analysis of SNPs from Genome-Wide Association Studies of Head and Neck Cancer. Otolaryngol. Head Neck Surg. 2018, 159, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Lacko, M.; Braakhuis, B.J.; Sturgis, E.M.; Boedeker, C.C.; Suárez, C.; Rinaldo, A.; Ferlito, A.; Takes, R.P. Genetic susceptibility to head and neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Adkins, D.; Ley, J.; Neupane, P.; Worden, F.; Sacco, A.G.; Palka, K.; Grilley-Olson, J.E.; Maggiore, R.; Salama, N.; Trinkaus, K.; et al. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: A multicentre, multigroup, phase 2 trial. Lancet Oncol. 2019, 20, 1295–1305. [Google Scholar] [CrossRef]
- Leblanc, O.; Vacher, S.; Lecerf, C.; Jeannot, E.; Klijanienko, J.; Berger, F.; Hoffmann, C.; Calugaru, V.; Badois, N.; Chilles, A.; et al. Biomarkers of cetuximab resistance in patients with head and neck squamous cell carcinoma. Cancer Biol. Med. 2020, 17, 208–217. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Gougis, P.; Moreau-Bachelard, C.; Kamal, M.; Gan, H.K.; Borcoman, E.; Torossian, N.; Bièche, I.; Le Tourneau, C. Clinical Development of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. JNCI Cancer Spectr. 2019, 3, pkz055. [Google Scholar] [CrossRef]
- Cohen, R.B. Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC). Cancer Treat Rev. 2014, 40, 567–577. [Google Scholar] [CrossRef]
- Siu, L.L.; Soulieres, D.; Chen, E.X.; Pond, G.R.; Chin, S.F.; Francis, P.; Harvey, L.; Klein, M.; Zhang, W.; Dancey, J.; et al. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: A Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group Study. J. Clin. Oncol. 2007, 25, 2178–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenni, D.; Karpova, M.; Mühleisen, B.; Mangana, J.; Dreier, J.; Hafner, J.; Dummer, R. A prospective clinical trial to assess lapatinib effects on cutaneous squamous cell carcinoma and actinic keratosis. ESMO Open 2016, 1, e000003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, K.A.; Lee, H.-Y.; Kim, E.S. Targeted therapies in squamous cell carcinoma of the head and neck. Cancer 2009, 115, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.K.; Schuler, P.J. Antigen-specific immunotherapy in head and neck cancer. Adv. Cell. Mol. Otolaryngol. 2013, 1, 21758. [Google Scholar] [CrossRef]
- Picon, H.; Guddati, A.K. Mechanisms of resistance in head and neck cancer. Am. J. Cancer Res. 2020, 10, 2742. [Google Scholar]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef] [Green Version]
- Behnam-Motlagh, P.; Tyler, A.; Grankvist, K.; Johansson, A. Verotoxin-1 Treatment or Manipulation of its Receptor Globotriaosylceramide (Gb3) for Reversal of Multidrug Resistance to Cancer Chemotherapy. Toxins 2010, 2, 2467–2477. [Google Scholar] [CrossRef] [Green Version]
- Arab, S.; Russel, E.; Chapman, W.; Rosen, B.; Lingwood, C. Expression of the verotoxin receptor glycolipid, globotriaosylceramide, in ovarian hyperplasias. Oncol. Res. Treat. 1997, 9, 553–563. [Google Scholar]
- Bathi, R.J.; Nandimath, K.; Kannan, N.; Shetty, P. Evaluation of glycoproteins as prognosticators in head and neck malignancy. Indian J. Dent. Res. 2001, 12, 93–99. [Google Scholar]
- Gariépy, J. The use of Shiga-like toxin 1 in cancer therapy. Crit. Rev. Oncol. Hematol. 2001, 39, 99–106. [Google Scholar] [CrossRef]
- Geyer, P.E.; Maak, M.; Nitsche, U.; Perl, M.; Novotny, A.; Slotta-Huspenina, J.; Dransart, E.; Holtorf, A.; Johannes, L.; Janssen, K.-P. Gastric Adenocarcinomas Express the Glycosphingolipid Gb3/CD77: Targeting of Gastric Cancer Cells with Shiga Toxin B-Subunit. Mol. Cancer Ther. 2016, 15, 1008–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannes, L.; Römer, W. Shiga toxins from cell biology to biomedical applications. Nat. Rev. Microbiol. 2010, 8, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.W.; Moore, R.; De Breucker, S.; Lincicome, L.; Jacewicz, M.; Skutelsky, E.; Keusch, G.T. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect. Immun. 1996, 64, 3294–3300. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Bergan, J.; Dyve, A.-B.; Skotland, T.; Torgersen, M.L. Endocytosis and retrograde transport of Shiga toxin. Toxicon 2010, 56, 1181–1185. [Google Scholar] [CrossRef]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.E.; Fraisier, V.; Florent, J.-C.; Perrais, D.; et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Nichols, C.D.; Casanova, J.E. Salmonella-directed recruitment of new membrane to invasion foci via the host exocyst complex. Curr. Biol. 2010, 20, 1316–1320. [Google Scholar] [CrossRef] [Green Version]
- Pezeshkian, W.; Hansen, A.G.; Johannes, L.; Khandelia, H.; Shillcock, J.C.; Kumar, P.B.S.; Ipsen, J.H. Membrane invagination induced by Shiga toxin B-subunit: From molecular structure to tube formation. Soft Matter 2016, 12, 5164–5171. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Palomares, E.; García-Hevia, L.; Padín-González, E.; Bañobre-López, M.; Villegas, J.C.; Valiente, R.; Fanarraga, M.L. Targeting Nanomaterials to Head and Neck Cancer Cells Using a Fragment of the Shiga Toxin as a Potent Natural Ligand. Cancers 2021, 13, 4920. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, L.; Valiente, R.; Gonzalez, J.; Villegas, J.C.; Fanarraga, M.L. Multiwalled carbon nanotubes display microtubule biomimetic properties in vivo, enhancing microtubule assembly and stabilization. ACS Nano 2012, 6, 6614–6625. [Google Scholar] [CrossRef]
- Garcia-Hevia, L.; Valiente, R.; Gonzalez, J.A.; Fernandez-Luna, J.; Villegas, J.C.; Fanarraga, M. Anti-cancer cytotoxic effects of multiwalled carbon nanotubes. Curr. Pharm. Des. 2015, 21, 1920–1929. [Google Scholar] [CrossRef]
- Garcia-Hevia, L.; Valiente, R.; Luna, J.L.F.; Flahaut, E.; Rodríguez-Fernández, L.; Villegas, J.C.; Gonzalez, J.A.; Fanarraga, M.L. Inhibition of Cancer Cell Migration by Multiwalled Carbon Nanotubes. Adv. Healthc. Mater. 2015, 4, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- García-Hevia, L.; Villegas, J.C.; Fernández, F.; Casafont, Íñigo; González, J.; Valiente, R.; Fanarraga, M.L. Multiwalled Carbon Nanotubes Inhibit Tumor Progression in a Mouse Model. Adv. Healthc. Mater. 2016, 5, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, D.; Vaidya, M.M. 4-Nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006, 42, 655–667. [Google Scholar] [CrossRef]
- Schoop, R.A.L.; Noteborn, M.H.M.; de Jong, R.J.B. A mouse model for oral squamous cell carcinoma. J. Mol. Histol. 2009, 40, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.-H.; Knudsen, B.; Bemis, D.; Tickoo, S.; Gudas, L.J. Oral Cavity and Esophageal Carcinogenesis Modeled in Carcinogen-Treated Mice. Clin. Cancer Res. 2004, 10, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Kibble, E.A.; Sarkar-Tyson, M.; Coombs, G.W.; Kahler, C.M. The Detroit 562 Pharyngeal Immortalized Cell Line Model for the Assessment of Infectivity of Pathogenic Neisseria sp. Methods Mol. Biol. 2019, 1969, 123–133. [Google Scholar] [PubMed]
- List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools (accessed on 17 January 2022).
- Economopoulou, P.; De Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic tumor markers in head and neck squamous cell carcinoma (HNSCC) in the clinical setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef]
- Lingwood, C.A.; Rhine, A.A.; Arab, S. Globotriaosyl ceramide (Gb3) expression in human tumour cells: Intracellular trafficking defines a new retrograde transport pathway from the cell surface to the nucleus, which correlates with sensitivity to verotoxin. Acta Biochim. Pol. 1998, 45, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Lacasse, E.C.; Bray, M.; Patterson, B.; Lim, W.M.; Perampalam, S.; Radvanyi, L.G.; Keating, A.; Stewart, A.K.; Buckstein, R.; Sandhu, J.S.; et al. Shiga-Like Toxin-1 Receptor on Human Breast Cancer, Lymphoma, and Myeloma and Absence From CD34+ Hematopoietic Stem Cells: Implications for Ex Vivo Tumor Purging and Autologous Stem Cell Transplantation. Blood 1999, 94, 2901–2910. [Google Scholar]
- Falguières, T.; Maak, M.; von Weyhern, C.; Sarr, M.; Sastre, X.; Poupon, M.-F.; Robine, S.; Johannes, L.; Janssen, K.-P. Human colorectal tumors and metastases express Gb3 and can be targeted by an intestinal pathogen-based delivery tool. Mol. Cancer Ther. 2008, 7, 2498–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distler, U.; Souady, J.; Hülsewig, M.; Drmić-Hofman, I.; Haier, J.; Friedrich, A.W.; Karch, H.; Senninger, N.; Dreisewerd, K.; Berkenkamp, S.; et al. Shiga Toxin Receptor Gb3Cer/CD77: Tumor-Association and Promising Therapeutic Target in Pancreas and Colon Cancer. Häcker G, editor. PLoS ONE 2009, 4, e6813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, D.; Kosovac, E.; Moharer, J.; Ljuslinder, I.; Brännström, T.; Johansson, A.; Behnam-Motlagh, P. Expression of verotoxin-1 receptor Gb3 in breast cancer tissue and verotoxin-1 signal transduction to apoptosis. BMC Cancer 2009, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingwood, C.A. Verotoxin/globotriaosyl ceramide recognition: Angiopathy, angiogenesis and antineoplasia. Biosci. Rep. 1999, 19, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Hevia, L.; Muñoz-Guerra, D.; Casafont, Í.; Morales-Angulo, C.; Ovejero, V.J.; Lobo, D.; Fanarraga, M.L. Gb3/cd77 Is a Predictive Marker and Promising Therapeutic Target for Head and Neck Cancer. Biomedicines 2022, 10, 732. https://doi.org/10.3390/biomedicines10040732
García-Hevia L, Muñoz-Guerra D, Casafont Í, Morales-Angulo C, Ovejero VJ, Lobo D, Fanarraga ML. Gb3/cd77 Is a Predictive Marker and Promising Therapeutic Target for Head and Neck Cancer. Biomedicines. 2022; 10(4):732. https://doi.org/10.3390/biomedicines10040732
Chicago/Turabian StyleGarcía-Hevia, Lorena, Débora Muñoz-Guerra, Íñigo Casafont, Carmelo Morales-Angulo, Victor J. Ovejero, David Lobo, and Mónica L. Fanarraga. 2022. "Gb3/cd77 Is a Predictive Marker and Promising Therapeutic Target for Head and Neck Cancer" Biomedicines 10, no. 4: 732. https://doi.org/10.3390/biomedicines10040732
APA StyleGarcía-Hevia, L., Muñoz-Guerra, D., Casafont, Í., Morales-Angulo, C., Ovejero, V. J., Lobo, D., & Fanarraga, M. L. (2022). Gb3/cd77 Is a Predictive Marker and Promising Therapeutic Target for Head and Neck Cancer. Biomedicines, 10(4), 732. https://doi.org/10.3390/biomedicines10040732