New Frontiers of Extracorporeal Shock Wave Medicine in Urology from Bench to Clinical Studies
Abstract
1. Introduction
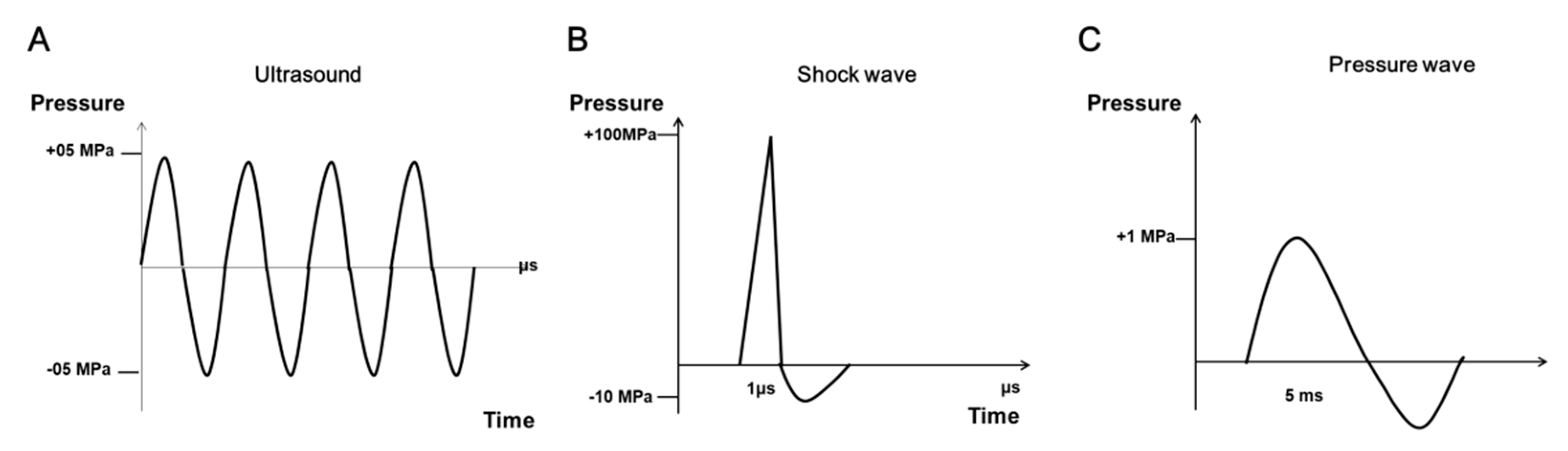
2. Characteristics of SWs
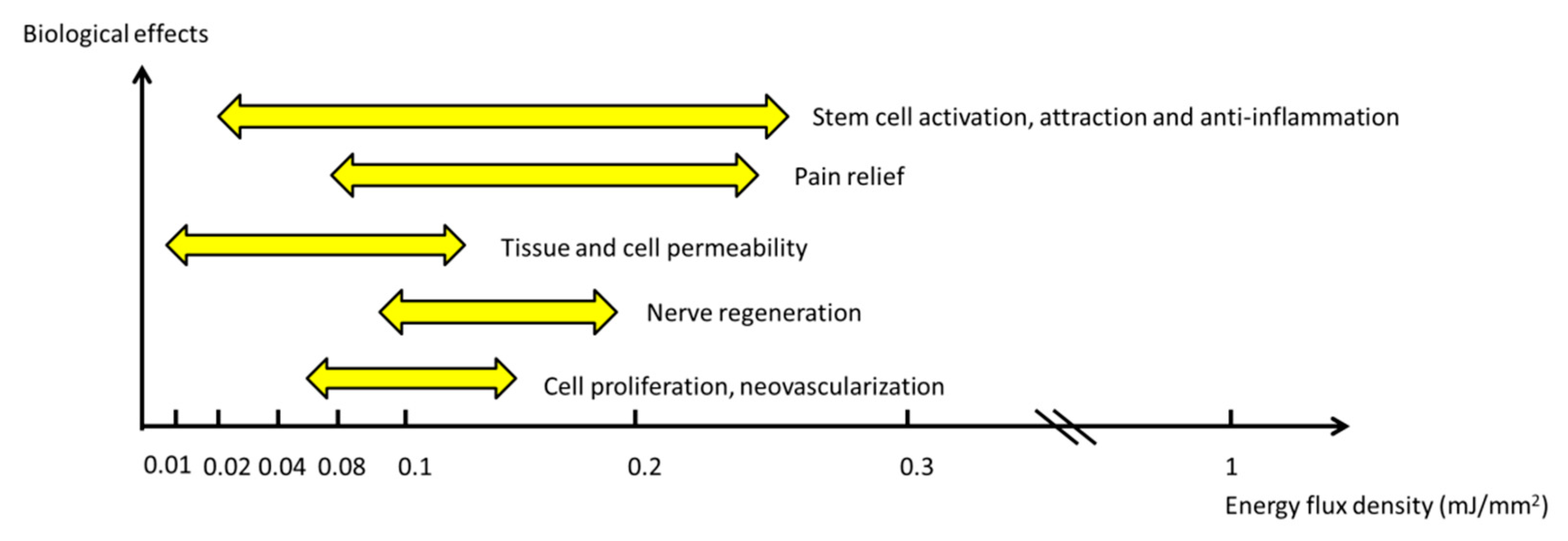
3. Dosage of ESWT
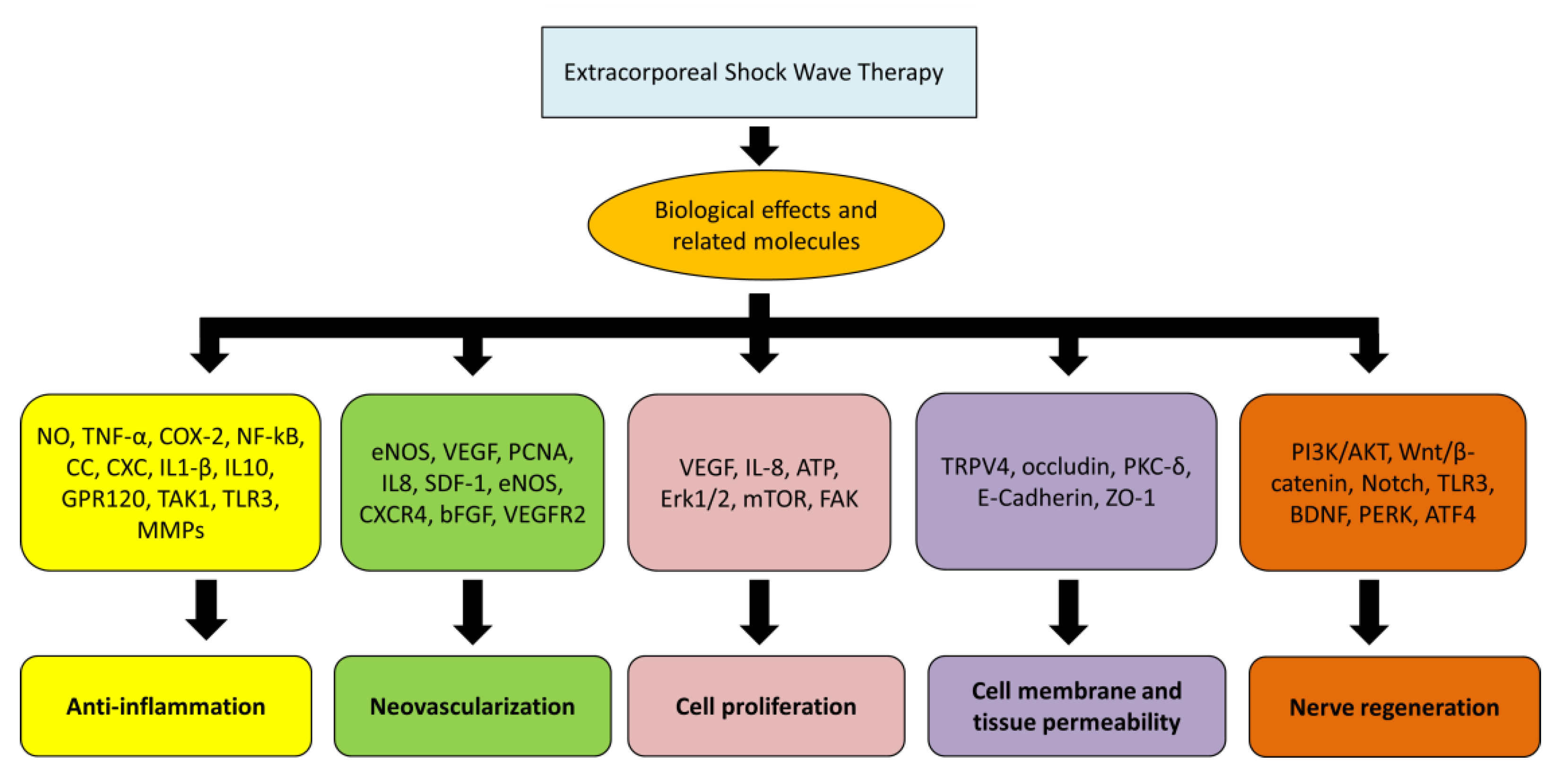
4. Biological Effects of ESWT
5. Anti-Inflammation
6. Angiogenesis
7. Cell Proliferation
8. Cell Membrane Permeability
9. Nerve Regeneration
10. Application of Low Energy Shock Wave (LESW) in Urology
10.1. Chronic Prostatitis/Chronic Pelvic Pain Syndrome
10.2. Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS), Ketamine Cystitis, and Radiation Cystitis
10.3. Overactive Bladder (OAB)
10.4. Erectile Dysfunction (ED)
10.5. Stress Urinary Incontinence
10.6. Detrusor Underactivity/Underactive Bladder
10.7. Drug Delivery
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaussy, C.H.; Brendel, W.; Schmiedt, E. Extracorporeally Induced Destruction of Kidney Stones by Shock Waves. Lancet 1980, 316, 1265–1268. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Oster, D.M.; Park, J.B.; Park, S.H.; Loening, S. The effect of the extracorporeal shock wave lithotriptor on the bone-cement interface in dogs. Clin. Orthop. Relat. Res. 1988, 235, 261–267. [Google Scholar] [CrossRef]
- Wang, C.J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Moya, D.; Ramon, S.; Schaden, W.; Wang, C.J.; Guiloff, L.; Cheng, J.H. The Role of Extracorporeal Shockwave Treatment in Musculoskeletal Disorders. J. Bone Jt. Surg. Am. 2018, 100, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Burneikaite, G.; Shkolnik, E.; Celutkiene, J.; Zuoziene, G.; Butkuviene, I.; Petrauskiene, B.; Serpytis, P.; Laucevicius, A.; Lerman, A. Cardiac shock-wave therapy in the treatment of coronary artery disease: Systematic review and meta-analysis. Cardiovasc. Ultrasound 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Meirer, R.; Kamelger, F.S.; Huemer, G.M.; Wanner, S.; Piza-Katzer, H. Extracorporal shock wave may enhance skin flap survival in an animal model. Br. J. Plast. Surg. 2005, 58, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, X.-B.; Chen, S.; Zhao, Z.-B.; Schmitz, C.; Weng, C.-S. Efficacy and safety of extracorporeal shock wave therapy for acute and chronic soft tissue wounds: A systematic review and meta-analysis. Int. Wound J. 2018, 15, 590–599. [Google Scholar] [CrossRef]
- Hitchman, L.H.; Totty, J.P.; Raza, A.; Cai, P.; Smith, G.E.; Carradice, D.; Wallace, T.; Harwood, A.E.; Chetter, I.C. Extracorporeal Shockwave Therapy for Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2019, 56, 330–339. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Permits Marketing of Device to Treat Diabetic Foot Ulcers [Press Release]; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Wang, C.-J.K.; Schaden, W.V.; Ko, J.-Y.K. Shockwave Medicine. In Translational Research in Biomedicine; Karger: Basel, Switzerland, 2018; Volume 6. [Google Scholar] [CrossRef]
- Beisteiner, R.; Matt, E.; Fan, C.; Baldysiak, H.; Schönfeld, M.; Philippi Novak, T.; Amini, A.; Aslan, T.; Reinecke, R.; Lehrner, J.; et al. Transcranial Pulse Stimulation with Ultrasound in Alzheimer’s Disease—A New Navigated Focal Brain Therapy. Adv. Sci. 2019, 7, 1902583–1902593. [Google Scholar] [CrossRef]
- Porst, H. Review of the Current Status of Low Intensity Extracorporeal Shockwave Therapy (Li-ESWT) in Erectile Dysfunction (ED), Peyronie’s Disease (PD), and Sexual Rehabilitation After Radical Prostatectomy With Special Focus on Technical Aspects of the Different Marketed ESWT Devices Including Personal Experiences in 350 Patients. Sex. Med. Rev. 2021, 9, 93–122. [Google Scholar] [CrossRef]
- Fojecki, G.L.; Tiessen, S.; Osther, P.J.S. Extracorporeal shock wave therapy (ESWT) in urology: A systematic review of outcome in Peyronie’s disease, erectile dysfunction and chronic pelvic pain. World J. Urol. 2016, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Cheng, J.-H.; Chuang, Y.-C. Potential applications of low-energy shock waves in functional urology. Int. J. Urol. 2017, 24, 573–581. [Google Scholar] [CrossRef]
- Ogden, J.A.; Tóth-Kischkat, A.; Schultheiss, R. Principles of Shock Wave Therapy. Clin. Orthop. Relat. Res. 2001, 387, 8–17. [Google Scholar] [CrossRef]
- D’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Rompe, J.D.; Kirkpatrick, C.J.; Küllmer, K.; Schwitalle, M.; Krischek, O. Dose-related effects of shock waves on rabbit tendo Achillis: A sonographic and histological study. J. Bone Jt. Surg. 1998, 80, 546–552. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Flavin, N.E.; Vaysbrot, E.; Harvey, W.; McAlindon, T. High-Energy Extracorporeal Shock-Wave Therapy for Treating Chronic Calcific Tendinitis of the Shoulder. Ann. Intern. Med. 2014, 160, 542. [Google Scholar] [CrossRef]
- Wang, B.; Reed-Maldonado, A.B.; Ly, K.; Lin, G.; Lue, T.F. Potential Applications of Low-Intensity Extracorporeal Shock Wave Therapy in Urological Diseases via Activation of Tissue Resident Stem Cells. Urol. Sci. 2022, 33, 3–8. [Google Scholar] [CrossRef]
- Haupt, G. Use of Extracorporeal Shock Waves in the Treatment of Pseudarthrosis, Tendinopathy and Other Orthopedic Diseases. J. Urol. 1997, 158, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Junger, W.G.; Yuan, C.; Jin, A.; Zhao, Y.; Zheng, X.; Zeng, Y.; Liu, J. Shockwaves increase T-cell proliferation and IL-2 expression through ATP release, P2X7 receptors, and FAK activation. Am. J. Physiol.-Cell Physiol. 2010, 298, C457–C464. [Google Scholar] [CrossRef]
- Weihs, A.M.; Fuchs, C.; Teuschl, A.H.; Hartinger, J.; Slezak, P.; Mittermayr, R.; Redl, H.; Junger, W.G.; Sitte, H.H.; Rünzler, D. Shock Wave Treatment Enhances Cell Proliferation and Improves Wound Healing by ATP Release-coupled Extracellular Signal-regulated Kinase (ERK) Activation. J. Biol. Chem. 2014, 289, 27090–27104. [Google Scholar] [CrossRef]
- Jan, C.-R.; Huang, J.-K.; Tseng, C.-J. High-Energy Shock Waves Alter Cytosolic Calcium Mobilization in Single MDCK Cells. Nephron 1998, 78, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Wang, F.-S.; Yang, K.D.; Weng, L.-H.; Hsu, C.-C.; Huang, C.-S.; Yang, L.-C. Shock wave therapy induces neovascularization at the tendon–bone junction. A study in rabbits. J. Orthop. Res. 2003, 21, 984–989. [Google Scholar] [CrossRef]
- Pölzl, L.; Nägele, F.; Hirsch, J.; Graber, M.; Grimm, M.; Gollmann-Tepeköylü, C.; Holfeld, J. Exosome Isolation after in vitro Shock Wave Therapy. J. Vis. Exp. 2020, 163, e61508. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Wang, C.-J.; Su, S.-H.; Huang, C.-Y.; Hsu, S.-L. Next-generation sequencing identifies articular cartilage and subchondral bone miRNAs after ESWT on early osteoarthritis knee. Oncotarget 2016, 7, 84398–84407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hausner, T.; Pajer, K.; Halat, G.; Hopf, R.; Schmidhammer, R.; Redl, H.; Nógrádi, A. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp. Neurol. 2012, 236, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Doukas, A.G.; Hamblin, M.R. Shock wave-mediated molecular delivery into cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2002, 1542, 186–194. [Google Scholar] [CrossRef]
- Alshihri, A.; Niu, W.; Kämmerer, P.W.; Al-Askar, M.; Yamashita, A.; Kurisawa, M.; Spector, M. The effects of shock wave stimulation of mesenchymal stem cells on proliferation, migration, and differentiation in an injectable gelatin matrix for osteogenic regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Veysset, D.; Kooi, S.E.; Martynowych, D.; Nakagawa, K.; Nelson, K.A. Interferometric and fluorescence analysis of shock wave effects on cell membrane. Commun. Phys. 2020, 3, 124–130. [Google Scholar] [CrossRef]
- Leu, S.; Huang, T.-H.; Chen, Y.-L.; Yip, H.-K. Effect of Extracorporeal Shockwave on Angiogenesis and Anti-Inflammation: Molecular-Cellular Signaling Pathways. Shock. Med. 2018, 6, 109–116. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lin, Y.-P.; Sun, C.-K.; Huang, T.-H.; Yip, H.-K.; Chen, Y.-T. Extracorporeal shockwave against inflammation mediated by GPR120 receptor in cyclophosphamide-induced rat cystitis model. Mol. Med. 2018, 24, 60–73. [Google Scholar] [CrossRef]
- Sukubo, N.G.; Tibalt, E.; Respizzi, S.; Locati, M.; d’Agostino, M.C. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int. J. Surg. 2015, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Holfeld, J.; Tepeköylü, C.; Kozaryn, R.; Urbschat, A.; Zacharowski, K.; Grimm, M.; Paulus, P. Shockwave Therapy Differentially Stimulates Endothelial Cells: Implications on the Control of Inflammation via Toll-Like Receptor 3. Inflammation 2013, 37, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, A.; Elster, E.A.; Anam, K.; Tadaki, D.; Amare, M.; Zins, S.; Davis, T.A. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis 2008, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Yang, C.C.; Sun, C.K.; Chiang, H.J.; Chen, Y.L.; Sung, P.H.; Zhen, Y.Y.; Huang, T.H.; Chang, C.L.; Chen, H.H.; et al. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am. J. Transl. Res. 2014, 6, 631–648. [Google Scholar] [PubMed]
- Davis, T.A.; Stojadinovic, A.; Anam, K.; Amare, M.; Naik, S.; Peoples, G.E.; Tadaki, D.; Elster, E.A. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int. Wound J. 2009, 6, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-R.; Wang, C.-T.; Wang, F.-S.; Yang, K.D.; Chiang, Y.-C.; Wang, C.-J. Extracorporeal shock wave treatment modulates skin fibroblast recruitment and leukocyte infiltration for enhancing extended skin-flap survival. Wound Repair Regen. 2009, 17, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F.; Chang, C.H.; Wang, C.T.; Yang, M.Y.; Wang, C.J.; Kuo, Y.R. Modulation of vascular endothelial growth factor and mitogen-activated protein kinase-related pathway involved in extracorporeal shockwave therapy accelerate diabetic wound healing. Wound Repair Regen. 2018, 27, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Tao, G.; Yun-zhu, P.; Yu, W.; Hong-yan, C. GW24-e0232 Effects of cardiac shock wave therapy on the angiogenesis related cytokine of CAD patients. Heart 2013, 99, A155–A156. [Google Scholar] [CrossRef]
- Biondi-Zoccai, G.; Fu, M.; Sun, C.-K.; Lin, Y.-C.; Wang, C.-J.; Wu, C.-J.; Ko, S.-F.; Chua, S.; Sheu, J.-J.; Chiang, C.-H.; et al. Extracorporeal Shock Wave Therapy Reverses Ischemia-Related Left Ventricular Dysfunction and Remodeling: Molecular-Cellular and Functional Assessment. PLoS ONE 2011, 6, e24342. [Google Scholar] [CrossRef]
- Cai, H.-Y.; Li, L.I.N.; Guo, T.A.O.; Wang, Y.U.; Ma, T.-K.; Xiao, J.-M.; Zhao, L.; Fang, Y.I.N.; Yang, P.; Zhao, H.U. Cardiac shockwave therapy improves myocardial function in patients with refractory coronary artery disease by promoting VEGF and IL-8 secretion to mediate the proliferation of endothelial progenitor cells. Exp. Ther. Med. 2015, 10, 2410–2416. [Google Scholar] [CrossRef][Green Version]
- Huang, T.-H.; Sun, C.-K.; Chen, Y.-L.; Wang, C.-J.; Yin, T.-C.; Lee, M.S.; Yip, H.-K. Shock Wave Therapy Enhances Angiogenesis through VEGFR2 Activation and Recycling. Mol. Med. 2016, 22, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Zhen, Y.Y.; Yuen, C.M.; Fan, R.; Chen, Y.T.; Sheu, J.J.; Chen, Y.L.; Wang, C.J.; Sun, C.K.; Yip, H.K. The mTOR-FAK mechanotransduction signaling axis for focal adhesion maturation and cell proliferation. Am. J. Transl. Res. 2017, 9, 1603–1617. [Google Scholar] [PubMed]
- Luh, J.-J.; Huang, W.-T.; Lin, K.-H.; Huang, Y.-Y.; Kuo, P.-L.; Chen, W.-S. Effects of Extracorporeal Shock Wave-Mediated Transdermal Local Anesthetic Drug Delivery on Rat Caudal Nerves. Ultrasound Med. Biol. 2018, 44, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Hoon Ha, C.; Cheol Lee, S.; Kim, S.; Chung, J.; Bae, H.; Kwon, K. Novel mechanism of gene transfection by low-energy shock wave. Sci. Rep. 2015, 5, 12843–12856. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.; Lan, C.; Hsiao, M.-Y.; Sun, M.-K.; Hsu, Y.-H.; Huang, A.P.H.; Liao, W.-H.; Liu, H.-L.; Inserra, C.; Chen, W.-S. Focused shockwave induced blood-brain barrier opening and transfection. Sci. Rep. 2018, 8, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Huang, T.-L.; Tyagi, P.; Huang, C.-C. Urodynamic and Immunohistochemical Evaluation of Intravesical Botulinum Toxin A Delivery Using Low Energy Shock Waves. J. Urol. 2016, 196, 599–608. [Google Scholar] [CrossRef]
- Luo, H.-L.; Liu, H.-Y.; Chang, Y.-L.; Su, Y.-L.; Huang, C.-C.; Lin, X.-J.; Chuang, Y.-C. Extracorporeal Shock Wave Enhances the Cisplatin Efficacy by Improving Tissue Infiltration and Cellular Uptake in an Upper Urinary Tract Cancer Animal and Human-Derived Organoid Model. Cancers 2021, 13, 4558. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, S.-H. Effect of Extracorporeal Shock Wave Therapy on Denervation Atrophy and Function Caused by Sciatic Nerve Injury. J. Phys. Ther. Sci. 2013, 25, 1067–1069. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Kang, N.; Yu, X.; Ma, Y.; Pang, X. Radial Extracorporeal Shock Wave Therapy Enhances the Proliferation and Differentiation of Neural Stem Cells by Notch, PI3K/AKT, and Wnt/β-catenin Signaling. Sci. Rep. 2017, 7, 15321–15331. [Google Scholar] [CrossRef]
- Gollmann-Tepeköylü, C.; Nägele, F.; Graber, M.; Pölzl, L.; Lobenwein, D.; Hirsch, J.; An, A.; Irschick, R.; Röhrs, B.; Kremser, C.; et al. Shock waves promote spinal cord repair via TLR3. JCI Insight 2020, 5, e134552. [Google Scholar] [CrossRef]
- Wang, B.; Ning, H.; Reed-Maldonado, A.; Zhou, J.; Ruan, Y.; Zhou, T.; Wang, H.; Oh, B.; Banie, L.; Lin, G.; et al. Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 433. [Google Scholar] [CrossRef] [PubMed]
- Habermacher, G.M.; Chason, J.T.; Schaeffer, A.J. Prostatitis/Chronic Pelvic Pain Syndrome. Annu. Rev. Med. 2006, 57, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Cai, Z.; Li, N.; Li, H. The Lifetime Risk and Prognosis of Chronic Prostatitis/Chronic Pelvic Pain Syndrome in the Middle-Aged Chinese Males. Am. J. Men’s Health 2019, 13, 155798831986538. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Fischer, K.A.; Goralnick, S.J.; Litt, M.; Burleson, J.A.; Albertsen, P.; Kreutzer, D.L. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology 2002, 59, 603–608. [Google Scholar] [CrossRef]
- Miller, L.J.; Fischer, K.A.; Goralnick, S.J.; Litt, M.; Burleson, J.A.; Albertsen, P.; Kreutzer, D.L. Interleukin-10 levels in seminal plasma: Implications for chronic prostatitis-chronic pelvic pain syndrome. J. Urol. 2002, 167, 753–756. [Google Scholar] [CrossRef]
- Zimmermann, R.; Cumpanas, A.; Hoeltl, L.; Janetschek, G.; Stenzl, A.; Miclea, F. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: A feasibility study and the first clinical results. BJU Int. 2008, 102, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Cumpanas, A.; Miclea, F.; Janetschek, G. Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome in Males: A Randomised, Double-Blind, Placebo-Controlled Study. Eur. Urol. 2009, 56, 418–424. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Liang, C.; Ye, Z.Q. Extracorporeal shock wave treatment for non-inflammatory chronic pelvic pain syndrome: A prospective, randomized and sham-controlled study. Chin. Med. J. 2012, 125, 114–118. [Google Scholar] [CrossRef]
- Vahdatpour, B.; Alizadeh, F.; Moayednia, A.; Emadi, M.; Khorami, M.H.; Haghdani, S. Efficacy of Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome: A Randomized, Controlled Trial. ISRN Urol. 2013, 2013, 972601. [Google Scholar] [CrossRef]
- Moayednia, A.; Haghdani, S.; Khosrawi, S.; Yousefi, E.; Vahdatpour, B. Long-term effect of extracorporeal shock wave therapy on the treatment of chronic pelvic pain syndrome due to non bacterial prostatitis. J. Res. Med. Sci. 2014, 19, 293–296. [Google Scholar]
- Pajovic, B.; Radojevic, N.; Dimitrovski, A.; Vukovic, M. Comparison of the efficiency of combined extracorporeal shock-wave therapy and triple therapy versus triple therapy itself in Category III B chronic pelvic pain syndrome (CPPS). Aging Male 2016, 19, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Al Edwan, G.M.; Muheilan, M.M.; Atta, O.N.M. Long term efficacy of extracorporeal shock wave therapy [ESWT] for treatment of refractory chronic abacterial prostatitis. Ann. Med. Surg. 2017, 14, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Guu, S.-J.; Geng, J.-H.; Chao, I.T.; Lin, H.-T.; Lee, Y.-C.; Juan, Y.-S.; Liu, C.-C.; Wang, C.-J.; Tsai, C.-C. Efficacy of Low-Intensity Extracorporeal Shock Wave Therapy on Men With Chronic Pelvic Pain Syndrome Refractory to 3-As Therapy. Am. J. Men’s Health 2017, 12, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.B.; Abouelnaga, W.A. Effect of radial shock wave on chronic pelvic pain syndrome/chronic prostatitis. J. Phys. Ther. Sci. 2018, 30, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-X.; Zhang, D.; Yu, X.-T.; Ma, Y.-W. Efficacy of Radial Extracorporeal Shock Wave Therapy for Chronic Pelvic Pain Syndrome: A Nonrandomized Controlled Trial. Am. J. Men’s Health 2018, 13, 155798831881466. [Google Scholar] [CrossRef] [PubMed]
- Skaudickas, D.; Telksnys, T.; Veikutis, V.; Aniulis, P.; Jievaltas, M. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome. Open Med. 2020, 15, 580–585. [Google Scholar] [CrossRef]
- Li, G.; Man, L. Low-intensity extracorporeal shock wave therapy for III B chronic pelvic pain syndrome. Transl. Androl. Urol. 2020, 9, 1323–1328. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, Y.S.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.-H.; Lee, J.Y.; Ahn, S.T.; Moon, D.G.; Kim, S.W. Efficacy of Low-Intensity Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome IIIb: A Prospective-Randomized, Double-Blind, Placebo-Controlled Study. World J. Men’s Health 2021, 39, e40. [Google Scholar] [CrossRef]
- Mykoniatis, I.; Kalyvianakis, D.; Zilotis, F.; Kapoteli, P.; Fournaraki, A.; Poulios, E.; Hatzichristou, D. Evaluation of a low-intensity shockwave therapy for chronic prostatitis type IIIb/chronic pelvic pain syndrome: A double-blind randomized sham-controlled clinical trial. Prostate Cancer Prostatic Dis. 2021, 24, 370–379. [Google Scholar] [CrossRef]
- Sakr, A.M.; Fawzi, A.M.; Kamel, M.; Ali, M.M. Outcomes and clinical predictors of extracorporeal shock wave therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: A prospective randomized double-blind placebo-controlled clinical trial. Prostate Cancer Prostatic Dis. 2021. [Google Scholar] [CrossRef]
- Wu, W.-L.; Bamodu, O.A.; Wang, Y.-H.; Hu, S.-W.; Tzou, K.-Y.; Yeh, C.-T.; Wu, C.-C. Extracorporeal Shockwave Therapy (ESWT) Alleviates Pain, Enhances Erectile Function and Improves Quality of Life in Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J. Clin. Med. 2021, 10, 3602. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Zhu, G.Q.; Kwon, E.B.; Lee, K.W.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; Bae, W.J.; Kim, S.W. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFκB pathway in a prostatitis rat model. Prostate 2019, 79, 1498–1504. [Google Scholar] [CrossRef]
- Wang, H.-J.; Tyagi, P.; Chen, Y.-M.; Chancellor, M.B.; Chuang, Y.-C. Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats. Int. J. Mol. Sci. 2019, 20, 4777. [Google Scholar] [CrossRef]
- Song, Z.; Jin, C.; Bian, Z.; Liang, C. Extracorporeal shock wave therapy decreases the number of total and degranulated mast cells and alleviates pelvic pain in a rat model of prostatitis. Mol. Cell. Biochem. 2021, 476, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Hanno, P.; Dmochowski, R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol. Urodyn. 2009, 28, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Lee, W.-C.; Tyagi, P.; Huang, C.-C.; Chuang, Y.-C. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol. Urodyn. 2017, 36, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Meng, E.; Chancellor, M.; Kuo, H.C. Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome—A prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol. Urodyn. 2020, 39, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Tyagi, P.; Lee, W.-C.; Chancellor, M.; Chuang, Y.-C. Improves symptoms and urinary biomarkers in refractory interstitial cystitis/bladder pain syndrome patients randomized to extracorporeal shock wave therapy versus placebo. Sci. Rep. 2021, 11, 7558–7567. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, K.H.; Sung, P.H.; Yang, C.C.; Cheng, B.C.; Chen, C.H.; Chang, C.L.; Sheu, J.J.; Lee, F.Y.; Shao, P.L.; et al. Extracorporeal shock wave markedly alleviates radiation-induced chronic cystitis in rat. Am. J. Transl. Res. 2018, 10, 1036–1052. [Google Scholar] [PubMed]
- Chen, Y.T.; Yang, C.C.; Sung, P.H.; Lin, K.C.; Chiang, J.Y.; Huang, C.R.; Huang, K.H.; Chuang, F.C.; Chu, Y.C.; Huang, E.Y.; et al. Long-term effect of extracorporeal shock wave therapy on attenuating radiation-induced chronic cystitis in rat. Am. J. Transl. Res. 2020, 12, 999–1015. [Google Scholar] [PubMed]
- Chen, Y.-T.; Huang, K.-H.; Chiang, J.Y.; Sung, P.-H.; Huang, C.-R.; Chu, Y.-C.; Chuang, F.-C.; Yip, H.-K. Extracorporeal Shock Wave Therapy Protected the Functional and Architectural Integrity of Rodent Urinary Bladder against Ketamine-Induced Damage. Biomedicines 2021, 9, 1391. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.J.; Gomelsky, A.; Souter, L.; Vasavada, S.P. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 2019, 202, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chuang, S.-M.; Lin, K.-L.; Chen, W.-C.; Lu, J.-H.; Chueh, K.-S.; Shen, M.-C.; Liu, L.-W.; Long, C.-Y.; Juan, Y.-S. Low-Intensity Extracorporeal Shock Wave Therapy Ameliorates the Overactive Bladder: A Prospective Pilot Study. BioMed Res. Int. 2020, 2020, 9175676. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-H.; Chueh, K.-S.; Chuang, S.-M.; Wu, Y.-H.; Lin, K.-L.; Long, C.-Y.; Lee, Y.-C.; Shen, M.-C.; Sun, T.-W.; Juan, Y.-S. Low Intensity Extracorporeal Shock Wave Therapy as a Potential Treatment for Overactive Bladder Syndrome. Biology 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Angulo, J.; Chitaley, K.; Dai, Y.-T.; Kim, N.N.; Paick, J.-S.; Simonsen, U.; Ückert, S.; Wespes, E.; Andersson, K.E.; et al. Anatomy, Physiology, and Pathophysiology of Erectile Dysfunction. J. Sex. Med. 2010, 7, 445–475. [Google Scholar] [CrossRef] [PubMed]
- Mykoniatis, I.; Pyrgidis, N.; Sokolakis, I.; Ouranidis, A.; Sountoulides, P.; Haidich, A.-B.; van Renterghem, K.; Hatzichristodoulou, G.; Hatzichristou, D. Assessment of Combination Therapies vs Monotherapy for Erectile Dysfunction. JAMA Netw. Open 2021, 4, e2036337. [Google Scholar] [CrossRef]
- McMahon, C.N.; Smith, C.J.; Shabsigh, R. Treating erectile dysfunction when PDE5 inhibitors fail. bmj 2006, 332, 589–592. [Google Scholar] [CrossRef]
- Skolarikos, A.; Alargof, E.; Rigas, A.; Deliveliotis, C.; Konstantinidis, E. Shockwave Therapy as First-Line Treatment for Peyronie’s Disease: A Prospective Study. J. Endourol. 2005, 19, 11–14. [Google Scholar] [CrossRef]
- Poulakis, V.; Skriapas, K.; Vries, R.; Dillenburg, W.; Ferakis, N.; Witzsch, U.; Melekos, M.; Becht, E. Extracorporeal shockwave therapy for Peyronie’s disease: An alternative treatment? Asian J. Androl. 2006, 8, 361–366. [Google Scholar] [CrossRef]
- Chitale, S.; Morsey, M.; Swift, L.; Sethia, K. Limited shock wave therapy vs sham treatment in men with Peyronie’s disease: Results of a prospective randomized controlled double-blind trial. BJU Int. 2010, 106, 1352–1356. [Google Scholar] [CrossRef]
- Gruenwald, I.; Appel, B.; Vardi, Y. Low-Intensity Extracorporeal Shock Wave Therapy—A Novel Effective Treatment for Erectile Dysfunction in Severe ED Patients Who Respond Poorly to PDE5 Inhibitor Therapy. J. Sex. Med. 2012, 9, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Vardi, Y.; Appel, B.; Kilchevsky, A.; Gruenwald, I. Does Low Intensity Extracorporeal Shock Wave Therapy Have a Physiological Effect on Erectile Function? Short-Term Results of a Randomized, Double-Blind, Sham Controlled Study. J. Urol. 2012, 187, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, A.; Imbimbo, C.; Creta, M.; Verze, P.; Fusco, F.; Mirone, V. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie’s disease and erectile dysfunction: Results from a prospective randomized trial. Int. J. Androl. 2012, 35, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.-H.; Chan, E.S.Y.; Hou, S.S.-M.; Ng, C.-F. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: A prospective, randomized, double-blinded, placebo controlled study. Int. J. Urol. 2014, 21, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Srini, V.S.; Reddy, R.K.; Shultz, T.; Denes, B. Low intensity extracorporeal shockwave therapy for erectile dysfunction: A study in an Indian population. Can. J. Urol. 2015, 22, 7614–7622. [Google Scholar]
- Pelayo-Nieto, M.; Linden-Castro, E.; Alias-Melgar, A.; Espinosa-Pérez Grovas, D.; Carreño-de la Rosa, F.; Bertrand-Noriega, F.; Cortez-Betancourt, R. Terapia de ondas de choque lineales en el tratamiento de la disfunción eréctil. Actas Urológicas Españolas 2015, 39, 456–459. [Google Scholar] [CrossRef]
- Chung, E.; Cartmill, R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: An Australian first open-label single-arm prospective clinical trial. BJU Int. 2015, 115, 46–49. [Google Scholar] [CrossRef]
- Bechara, A.; Casabe, A.; De Bonis, W.; Nazar, J. Effectiveness of low-intensity extracorporeal shock wave therapy on patients with Erectile Dysfunction (ED) who have failed to respond to PDE5i therapy. A pilot study. Arch. Esp. Urol. 2015, 68, 152–160. [Google Scholar]
- Frey, A.; Sønksen, J.; Fode, M. Low-intensity extracorporeal shockwave therapy in the treatment of postprostatectomy erectile dysfunction: A pilot study. Scand. J. Urol. 2015, 50, 123–127. [Google Scholar] [CrossRef]
- Olsen, A.B.; Persiani, M.; Boie, S.; Hanna, M.; Lund, L. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand. J. Urol. 2014, 49, 329–333. [Google Scholar] [CrossRef]
- Hisasue, S.-I.; China, T.; Horiuchi, A.; Kimura, M.; Saito, K.; Isotani, S.; Ide, H.; Muto, S.; Yamaguchi, R.; Horie, S. Impact of aging and comorbidity on the efficacy of low-intensity shock wave therapy for erectile dysfunction. Int. J. Urol. 2016, 23, 80–84. [Google Scholar] [CrossRef]
- Kalyvianakis, D.; Memmos, E.; Mykoniatis, I.; Kapoteli, P.; Memmos, D.; Hatzichristou, D. Low-Intensity Shockwave Therapy for Erectile Dysfunction: A Randomized Clinical Trial Comparing 2 Treatment Protocols and the Impact of Repeating Treatment. J. Sex. Med. 2018, 15, 334–345. [Google Scholar] [CrossRef]
- Vinay, J.; Moreno, D.; Rajmil, O.; Ruiz-Castañe, E.; Sanchez-Curbelo, J. Penile low intensity shock wave treatment for PDE5I refractory erectile dysfunction: A randomized double-blind sham-controlled clinical trial. World J. Urol. 2020, 39, 2217–2222. [Google Scholar] [CrossRef]
- De Oliveira, P.S.; De Oliveira, T.R.; Nunes, Á.; Martins, F.; Lopes, T. Low-intensity shock wave therapy for erectile dysfunction and the influence of disease duration. Arch. Ital. Urol. Androl. 2019, 90, 276–282. [Google Scholar] [CrossRef]
- Huang, Y.P.; Liu, W.; Liu, Y.D.; Zhang, M.; Xu, S.R.; Lu, M.J. Effect of low-intensity extracorporeal shockwave therapy on nocturnal penile tumescence and rigidity and penile haemodynamics. Andrologia 2020, 52, e13745. [Google Scholar] [CrossRef]
- Sramkova, T.; Motil, I.; Jarkovsky, J.; Sramkova, K. Erectile Dysfunction Treatment Using Focused Linear Low-Intensity Extracorporeal Shockwaves: Single-Blind, Sham-Controlled, Randomized Clinical Trial. Urol. Int. 2020, 104, 417–424. [Google Scholar] [CrossRef]
- Palmieri, A.; Arcaniolo, D.; Palumbo, F.; Verze, P.; Liguori, G.; Mondaini, N.; Falcone, M.; Scroppo, F.I.; Salonia, A.; Cai, T.; et al. Low intensity shockwave therapy in combination with phosphodiesterase-5 inhibitors is an effective and safe treatment option in patients with vasculogenic ED who are PDE5i non-responders: A multicenter single-arm clinical trial. Int. J. Impot. Res. 2021, 33, 634–640. [Google Scholar] [CrossRef]
- Shendy, W.S.; Elsoghier, O.M.; El Semary, M.M.; Ahmed, A.A.; Ali, A.F.; Saber-Khalaf, M. Effect of low-intensity extracorporeal shock wave therapy on diabetic erectile dysfunction: Randomised control trial. Andrologia 2021, 53, e13997. [Google Scholar] [CrossRef]
- Müller, A.; Akin-Olugbade, Y.; Deveci, S.; Donohue, J.F.; Tal, R.; Kobylarz, K.A.; Palese, M.; Mulhall, J.P. The Impact of Shock Wave Therapy at Varied Energy and Dose Levels on Functional and Structural Changes in Erectile Tissue. Eur. Urol. 2008, 53, 635–643. [Google Scholar] [CrossRef]
- Behr-Roussel, D.; Giuliano, F. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. Transl. Androl. Urol. 2016, 5, 977–979. [Google Scholar] [CrossRef]
- Qiu, X.; Lin, G.; Xin, Z.; Ferretti, L.; Zhang, H.; Lue, T.F.; Lin, C.S. Effects of Low-Energy Shockwave Therapy on the Erectile Function and Tissue of a Diabetic Rat Model. J. Sex. Med. 2013, 10, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, F.C.C.; Bosch, J.L.H.R.; Cruz, F.; Lemack, G.E.; Nambiar, A.K.N.; Thiruchelvam, N.; Tubaro, A. Guidelines Associates. In Urinary Incontinence in Adults; Ambühl, D., Bedretdinova, D., Farag, F., Lombardo, R., Schneider, M.P., Eds.; European Association of Urology: Arnhem, The Netherlands, 2020. [Google Scholar]
- Wu, A.K.; Zhang, X.; Wang, J.; Ning, H.; Zaid, U.; Villalta, J.D.; Wang, G.; Banie, L.; Lin, G.; Lue, T.F. Treatment of stress urinary incontinence with low-intensity extracorporeal shock wave therapy in a vaginal balloon dilation induced rat model. Transl. Androl. Urol. 2018, 7, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ruan, Y.; Wu, A.K.; Zaid, U.; Villalta, J.D.; Wang, G.; Banie, L.; Reed-Maldonado, A.B.; Lin, G.; Lue, T.F. Delayed Treatment With Low-intensity Extracorporeal Shock Wave Therapy in an Irreversible Rat Model of Stress Urinary Incontinence. Urology 2020, 141, 187.e181–187.e187. [Google Scholar] [CrossRef] [PubMed]
- Long, C.-Y.; Lin, K.-L.; Lee, Y.-C.; Chuang, S.-M.; Lu, J.-H.; Wu, B.-N.; Chueh, K.-S.; Ker, C.-R.; Shen, M.-C.; Juan, Y.-S. Therapeutic effects of Low intensity extracorporeal low energy shock wave therapy (LiESWT) on stress urinary incontinence. Sci. Rep. 2020, 10, 5818–5828. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Gammie, A.; Kaper, M.; Dorrepaal, C.; Kos, T.; Abrams, P. Signs and Symptoms of Detrusor Underactivity: An Analysis of Clinical Presentation and Urodynamic Tests From a Large Group of Patients Undergoing Pressure Flow Studies. Eur. Urol. 2016, 69, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Chapple, C.R.; Abrams, P.; Dmochowski, R.; Haab, F.; Nitti, V.; Koelbl, H.; van Kerrebroeck, P.; Wein, A.J. Detrusor Underactivity and the Underactive Bladder: A New Clinical Entity? A Review of Current Terminology, Definitions, Epidemiology, Aetiology, and Diagnosis. Eur. Urol. 2014, 65, 389–398. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Nomiya, M.; Andersson, K.-E. Functional consequences of chronic bladder ischemia. Neurourol. Urodyn. 2014, 33, 54–58. [Google Scholar] [CrossRef] [PubMed]
- van Koeveringe, G.A.; Rademakers, K.L.J.; Birder, L.A.; Korstanje, C.; Daneshgari, F.; Ruggieri, M.R.; Igawa, Y.; Fry, C.; Wagg, A. Detrusor underactivity: Pathophysiological considerations, models and proposals for future research. ICI-RS 2013. Neurourol. Urodyn. 2014, 33, 591–596. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Tyagi, P.; Wang, H.-J.; Huang, C.-C.; Lin, C.-C.; Chancellor, M.B. Urodynamic and molecular characteristics of detrusor underactivity in a rat cryoinjury model and effects of low energy shock wave therapy. Neurourol. Urodyn. 2018, 37, 708–715. [Google Scholar] [CrossRef]
- Wang, H.S.; Oh, B.S.; Wang, B.; Ruan, Y.; Zhou, J.; Banie, L.; Lee, Y.C.; Tamaddon, A.; Zhou, T.; Wang, G.; et al. Low-intensity extracorporeal shockwave therapy ameliorates diabetic underactive bladder in streptozotocin-induced diabetic rats. BJU Int. 2018, 122, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Cruz, F.; Cruz, C.D.; Avelino, A. Spread of OnabotulinumtoxinA After Bladder Injection. Experimental Study Using the Distribution of Cleaved SNAP-25 as the Marker of the Toxin Action. Eur. Urol. 2012, 61, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Nageib, M.; El-Hefnawy, A.S.; Zahran, M.H.; El-Tabey, N.A.; Sheir, K.Z.; Shokeir, A.A. Delivery of intravesical botulinum toxin A using low-energy shockwaves in the treatment of overactive bladder: A preliminary clinical study. Arab J. Urol. 2019, 17, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Elkashef, A.; Barakat, N.; Khater, S.M.; Awadalla, A.; Belal, F.; El-Assmy, A.M.; Sheir, K.Z.; Shokeir, A.A. Effect of low-energy shock wave therapy on intravesical epirubicin delivery in a rat model of bladder cancer. BJU Int. 2020, 127, 80–89. [Google Scholar] [CrossRef] [PubMed]
| Study Design | N | Treatment Setting Treatment Course (mJouls/mm2) | Following Duration (Weeks) | Treatment Effect | |
|---|---|---|---|---|---|
| Zimmermann et al., 2008 [58] | Cohort | 34 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 1, 4, 12 | Improvements in pain and QoL. |
| Zimmermann et al., 2009 [59] | Double-blind. RCT | 60 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 1,4,12 | Improved QoL, IIEF, CPSI, VAS, and IPSS at 1, 4, and 12 weeks. |
| Zeng et al., 2012 [60] | RCT | 80 | 3000 impulses, 5 times a week, 2 weeks. 0.06-maximum tolerated dose. | 4, 12 | Decreased NIH-CPSI score, improved QOL, 71.1% vs. 28.9% at week 2. |
| Vahdatpour et al., 2013 [61] | RCT | 40 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz, increased to 0.3, 0.35, 0.4. | 1,2,3, 12 | Pain scores decreases at 2, 3, and 12 weeks. Urinary scores improved in weeks 3 and 12. |
| Moayednia et al., 2014 [62] | RCT | 19 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 16, 20, 24 | NIH-CPSI, VAS, IPSS decreased. Not statistically different at week 24. |
| Pajovic et al., 2016 [63] | RCT | 30 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 12, 24, 36 | Improved NIH-CPSI. No change for PVR or QMAX. |
| Al Edwan et al., 2017 [64] | Cohort | 41 | 2500 impulses per week, 4 weeks. 0.25, 3 Hz. | 2, 24, 48 | Improved NIH-CPSI, IIEF, IPSS. |
| Guu et al., 2018 [65] | Cohort | 33 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 1, 4, 12 | Improved NIH-CPSI, IIEF, IPSS. |
| Salama et al., 2018 [66] | RCT | 40 | 3000 pulses, 12 Hz at 3 to 5 bar, twice a week for 4 weeks. | 1, 4, 8 | Four domains of the NIH-CPSI decreased at weeks 1, 4, and 8. |
| Zhang et al., 2019 [67] | nRCT | 40 | 3000 pulses per week, 8 weeks. 1.8–2.0 bar; 10 Hz. | 4, 8, 12 | Both groups improved NIH-CPSI, QoL, VAS, IPSS, and IIEF-5. Only 8 weeks CPSI. IIEF statistically significant. |
| Skaudickas et al., 2020 [68] | Cohort | 40 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 0, 4, 12 | NIH-CPSI, IPSS, VAS, and IIEF-5 showed greatest improvement at week 4; VAS and IPSS, improvement at week 12. |
| Li et al., 2020 [69] | Cohort | 32 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 1, 2, 4, 12 | VAS and the NIH-CPSI showed substantial improvement at week 4 and 12. |
| Kim et al., 2021 [70] | Double-blind RCT | 34 | 3000 impulses per week, 8 weeks. 0.25, 3 Hz. | 0, 4 | NIH-CPSI, QoL, IIEF, and VAS decrease at Week 0 and 4. |
| Mykoniatis et al., 2021 [71] | Double-blind RCT | 45 | 5000 shockwaves per week, 6 weeks, 0.1. | 4, 12, 24 | NIH-CPSI, pain, and QoL improved, persisted at 24 weeks. No improvement of NIH-CPSI urinary subdomain and IPSS. |
| Sakr et al., 2021 [72] | RCT | 155 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 4, 12, 24, 48 | NIH-CPSI, IPSS, VAS, and IIEF-5. |
| Wu et al., 2021 [73] | Cohort | 215 | 3000 impulses per week, 6 weeks. 0.25, 4 Hz. | 4, 12, 24, 48 | Improved NIH-CPSI, IIEF, IPSS, and AUA QoL_US at 4, 12, 24, and 48 weeks |
| Study Design | N | Treatment Setting Treatment Course (mJouls/mm2) | Following Duration (Months) | Treatment Effect | |
|---|---|---|---|---|---|
| Skolarikos et al., 2005 [90] | Cohort | 40 | 3000 impulses, 6 weeks. | 3, 12 | 64.2% improve IIEF Improvement in penile angulation, with a mean reduction of 35 degrees No significant change in plaque size |
| Poulakis et al., 2006 [91] | RCT | 68 | 2000 impulses per week, 5 weeks, 0.25. | 1, 3, 6 | Improvement in pain, IIEF-5 score and plaque size, but no difference compared to another group. |
| Zimmermann et al., 2009 [59] | RCT | 60 | 3000 impulses per week, 4 weeks, 0.25, 3 Hz. | 1, 3 | Improvement of pain, QoL, and voiding conditions IIEF-5. |
| Chitale et al., 2010 [92] | RCT | 36 | 3000 impulses per week, 6 weeks. | 3, 6 | Improved IIEF-5 and VAS score. No significant change in Peyronie’s disease. |
| Gruenwald et al., 2012 [93] | Cohort | 29 | 1500 impulses twice per week, 3 weeks, 0.09. | 1, 2 | DE-5i poor responders. Improved EHS and IIEF-ED. |
| Vardi et al., 2012 [94] | RCT | 67 | 1500 impulses twice per week, 9 weeks, 0.09. | 1 | Improved IIEF-5, EHS, and penile blood flow. |
| Palmieri et al., 2012 [95] | Cohort | 50 | 2000 impulses per week, 4 weeks, 0.25. | 3, 6 | Improved IIEF-5 and quality of life. |
| Yee et al., 2014 [96] | RCT | 70 | 1500 impulses twice per week, 9 weeks, 0.09. | 1 | Clinical improvement in IIEF-ED and EHS, but no significant difference between two groups. |
| Srini et al., 2015 [97] | RCT | 135 | NA | 1, 3, 6, 9, 12 | Improvement in IIEF-EF, EHS, and CGIC. |
| Pelayo-Nieto et al., 2015 [98] | Cohort | 15 | 5000 impulses per week, 4 weeks, 0.09. | 1, 6 | Improvement in IIEF, SEP, and GAQ. |
| Chung and Cartmill 2015 [99] | Cohort | 30 | 3000 impulses twice per week, 6 weeks, 0.25. | 1, 4 | PDE5i non-responders; 18 (60%) patients ≥ 5 points improvement in IIEF-5 score; 21 (70%) patients >50% in EDITS index score, last 4-months. |
| Bechara et al., 2015 [100] | Cohort | 25 | 5000 impulses once per week, 4 weeks, 0.09. | 3 | PDE-5i non-responders. Improved IIEF-6, SEP2, SEP3, and GAQ. Restoring PDE5i response in >50% of patients. |
| Frey et al., 2015 [101] | Cohort | 18 | 3000 impulses twice per week, 6 weeks. 20, 15, and 12. | 1, 12 | post-prostatectomy erectile dysfunction. Improved IIEF-5 scores at 1 and 12 months. |
| Olsen et al., 2015 [102] | RCT | 112 | 3000 impulses per week, 5 weeks, 0.15. | 1, 3, 6 | Improved IIEF-5 and EHS. |
| Hisasue 2016 [103] | Cohort | 57 | 1500 impulses twice per week, 9 weeks, 0.09. | 1, 3, 6 | Improvement in IIEF, EHS, and MPCC. |
| Kalyvianakis et al., 2018 [104] | RCT | 44 | 5000 impulses once/twice per week, 6 weeks, 2 phase treatment, 0.05. | 1,3, 6 | Vasculogenic ED, PDE5 responders. Improvement in IIEF-EF score, MCID, and SEP3 score. 12 sessions twice per week were better than 6 sessions once a week. |
| Vinay et al., 2021 [105] | RCT | 76 | 5000 impulses once per week, 4 weeks, 0.09. | 1, 3, 6 | *PDE5I refractory patients. Improved IIEF-EF in 3 and 6 months. |
| Oliveira et al., 2019 [106] | Cohort | 25 | 2000 impulses on perineum + 2000 on dorsum penis once per week, 6 weeks, 0.16. | 1.5, 3 | Improved PSV and EDV. Disease duration dose not negative impact on treatment outcomes. |
| Huang et al., 2020 [107] | Cohort | 35 | NA | 1, | Improved IIEF-5, EHS, erectile rigidity, and nocturnal erection frequency. |
| Sramkova et al., 2020 [108] | RCT | 60 | 6000 impulses twice per week, 2 weeks, 0.160. | 1, 3 | Improve IIEF-5, EHS, GAQ, SEP 2, and SEP 3. |
| Palmieri et al., 2021 [109] | RCT | 106 | 3000 impulses twice per week, 3 weeks, 0.25. | 1 | vasculogenic ED PDE5i non-responders. Improved IIEF-EF, PSV, and EDV. |
| Shendy et al., 2021 [110] | RCT | 42 | 3 | Improved IIEF-5 and PSV. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Cheng, J.-H.; Wu, Z.-S.; Chuang, Y.-C. New Frontiers of Extracorporeal Shock Wave Medicine in Urology from Bench to Clinical Studies. Biomedicines 2022, 10, 675. https://doi.org/10.3390/biomedicines10030675
Chen P-Y, Cheng J-H, Wu Z-S, Chuang Y-C. New Frontiers of Extracorporeal Shock Wave Medicine in Urology from Bench to Clinical Studies. Biomedicines. 2022; 10(3):675. https://doi.org/10.3390/biomedicines10030675
Chicago/Turabian StyleChen, Po-Yen, Jai-Hong Cheng, Zong-Sheng Wu, and Yao-Chi Chuang. 2022. "New Frontiers of Extracorporeal Shock Wave Medicine in Urology from Bench to Clinical Studies" Biomedicines 10, no. 3: 675. https://doi.org/10.3390/biomedicines10030675
APA StyleChen, P.-Y., Cheng, J.-H., Wu, Z.-S., & Chuang, Y.-C. (2022). New Frontiers of Extracorporeal Shock Wave Medicine in Urology from Bench to Clinical Studies. Biomedicines, 10(3), 675. https://doi.org/10.3390/biomedicines10030675







