Increased Placental Anti-Oxidant Response in Asymptomatic and Symptomatic COVID-19 Third-Trimester Pregnancies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Tissue Collection
2.2. Transmission Electron Microscopy (TEM)
2.3. Lipid Peroxidation Measurement
2.4. RNA Isolation and Real-Time PCR
2.5. Assessment of SOD and CAT Enzymatic Activities
2.6. Statistical Analysis
3. Results
3.1. Clinical Features of Study Population
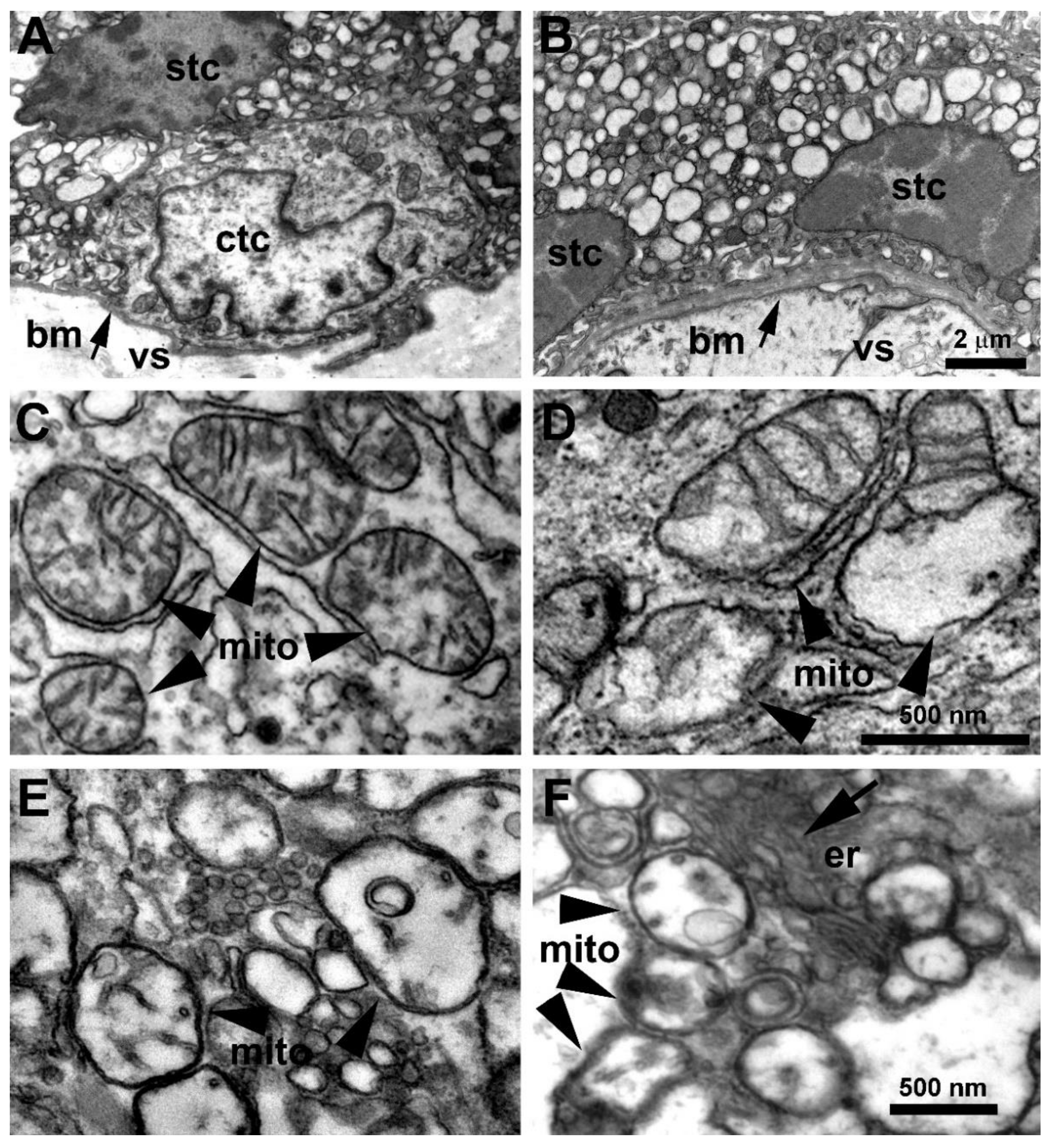
3.2. Placenta Ultrastructural Morphology in a-COVID-19, s-COVID-19, and CTRL Pregnant Women
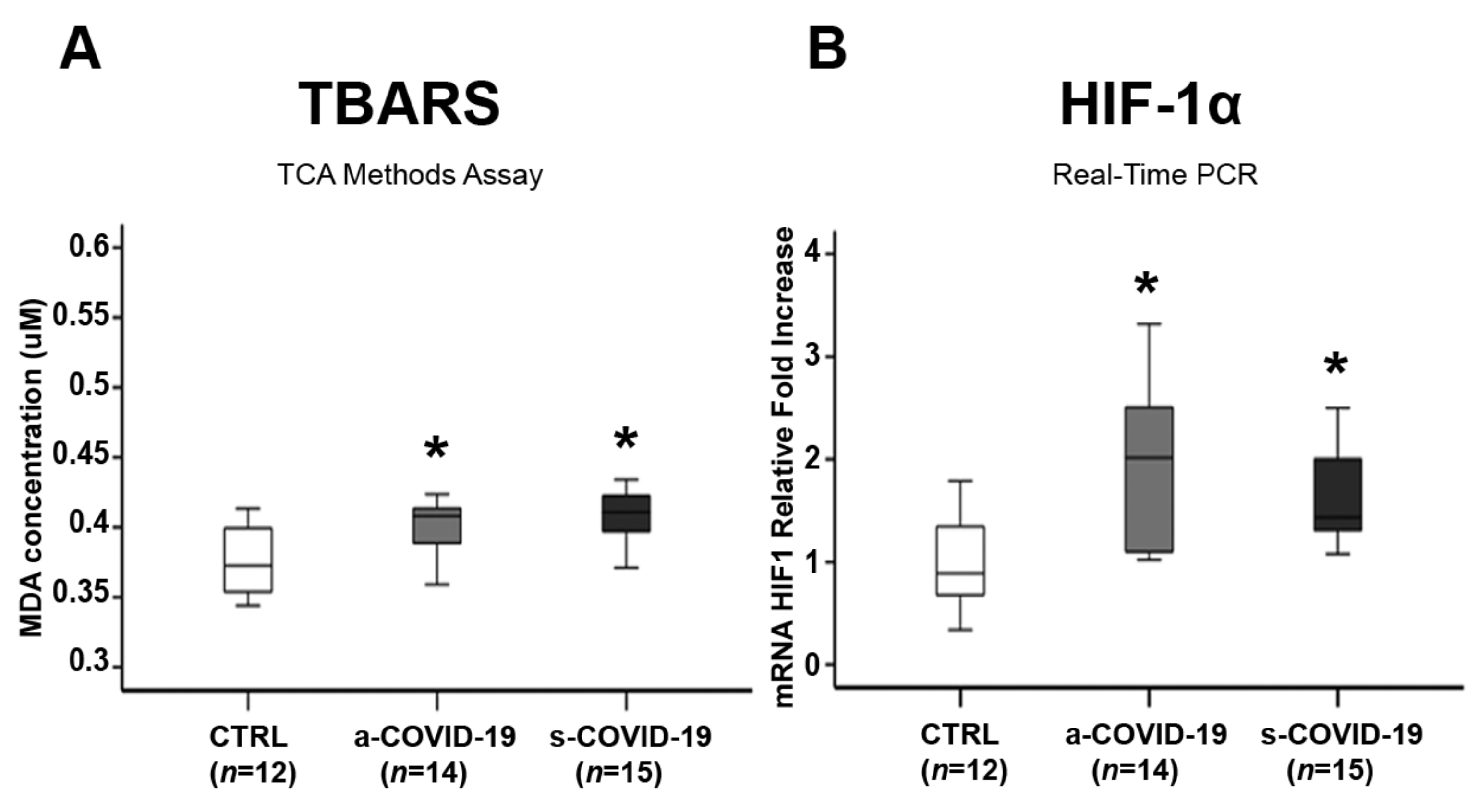
3.3. Assessment of Placental Oxidative Stress Markers
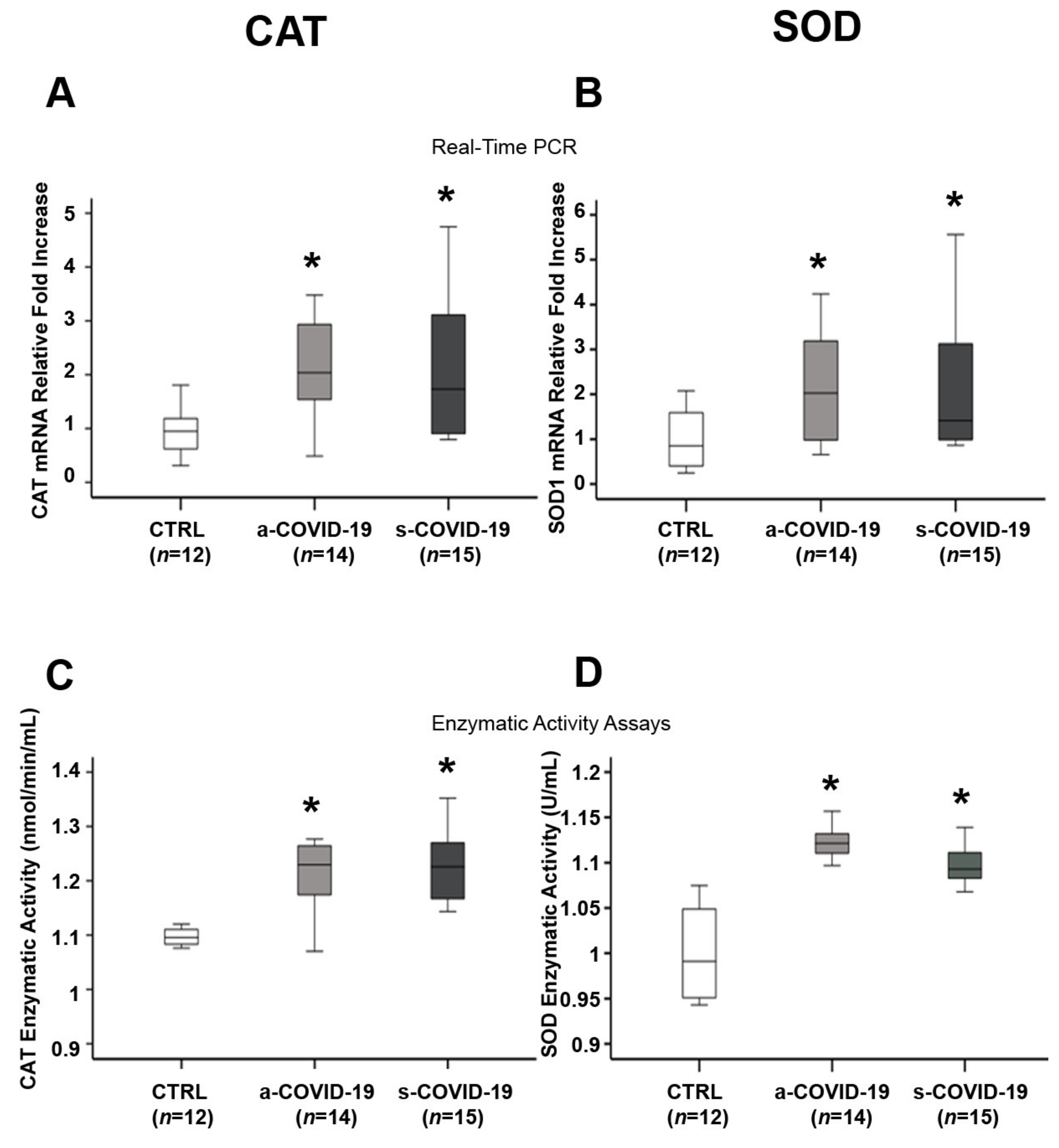
3.4. Assessment of Placental Antioxidant Defense Markers
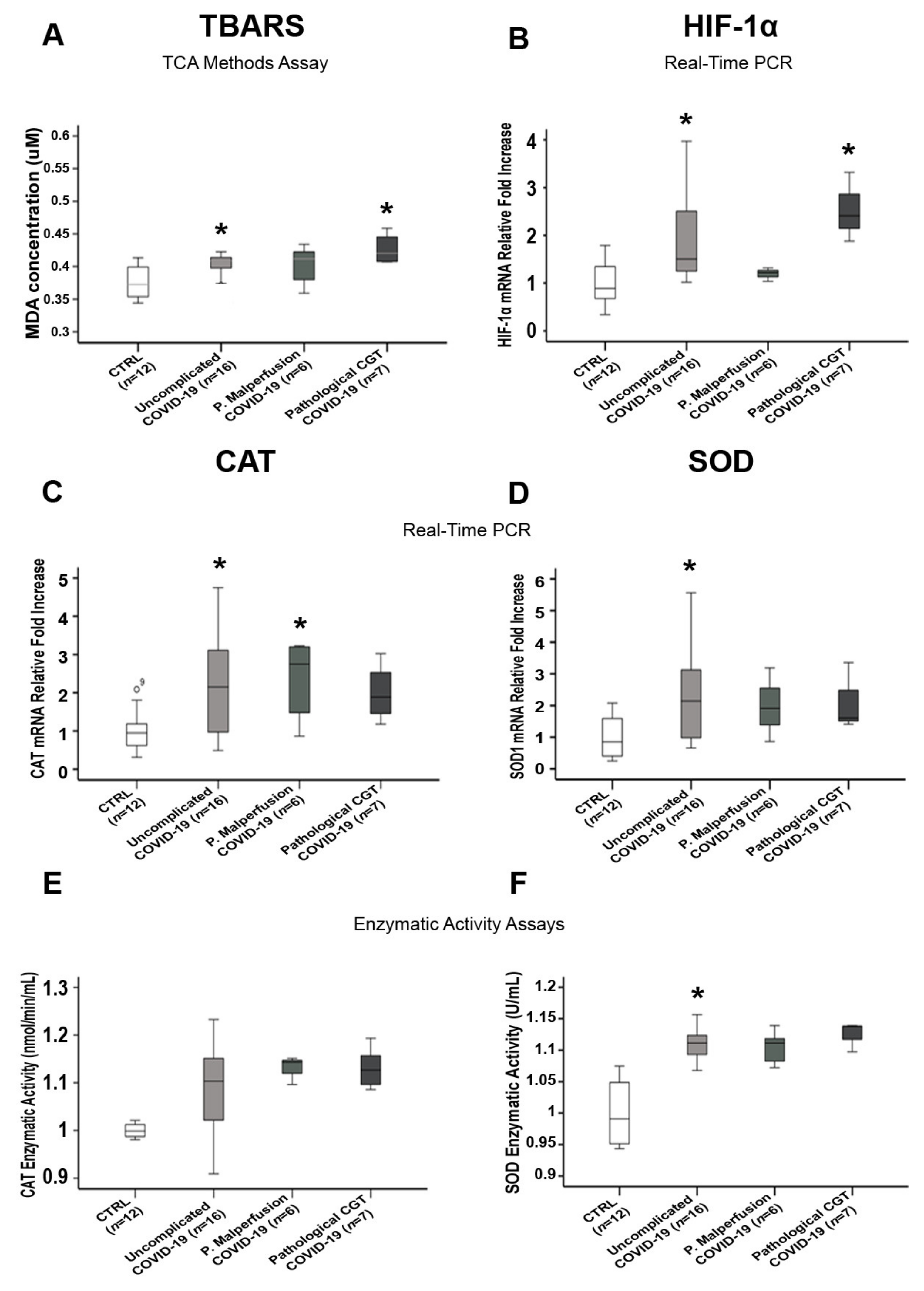
3.5. Comparisons of Placental Oxidative Stress and Antioxidant Defense Markers in SARS-CoV-2-Infected Women with and without Pregnancy-Related Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertero, L.; Borella, F.; Botta, G.; Carosso, A.; Cosma, S.; Bovetti, M.; Carosso, M.; Abbona, G.; Collemi, G.; Papotti, M.; et al. Placenta histopathology in SARS-CoV-2 infection: Analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021, 479, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Carosso, A.; Cosma, S.; Serafini, P.; Benedetto, C.; Mahmood, T. How to reduce the potential risk of vertical transmission of SARS-CoV-2 during vaginal delivery? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Li, W.; Zhou, Z.; Liu, S.; Rong, Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med. 2020, 14, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Mascio, D.; Buca, D.; Berghella, V.; Khalil, A.; Rizzo, G.; Odibo, A.; Saccone, G.; Galindo, A.; Liberati, M.; D’Antonio, F. Counseling in maternal–fetal medicine: SARS-CoV-2 infection in pregnancy. Ultrasound Obstet. Gynecol. 2021, 57, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, A.-E.; Skaltsounis, P.; Eleftheriades, M.; Antsaklis, P.; Charitou, A.; Anatolitou, F.; Moutafi, A.; Petropoulos, P.; Daskalakis, G. Pharyngeal sampling for PCR-testing in the investigation of SARS-COV-2 vertical transmission in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Solís-García, G.; Gutiérrez-Vélez, A.; Chamorro, I.P.; Zamora-Flores, E.; Vigil-Vázquez, S.; Rodríguez-Corrales, E.; Sánchez-Luna, M. Epidemiology, management and risk of SARS-CoV-2 transmission in a cohort of newborns born to mothers diagnosed with COVID-19 infection. Ann. Pediatr. (Engl. Ed.) 2021, 94, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Cosma, S.; Carosso, A.; Borella, F.; Cusato, J.; Bovetti, M.; Bevilacqua, F.; Carosso, M.; Gervasoni, F.; Sciarrone, A.; Marozio, L.; et al. Prenatal Biochemical and Ultrasound Markers in COVID-19 Pregnant Patients: A Prospective Case-Control Study. Diagnostics 2021, 11, 398. [Google Scholar] [CrossRef]
- Cosma, S.; Carosso, A.R.; Cusato, J.; Borella, F.; Carosso, M.; Bovetti, M.; Filippini, C.; D’Avolio, A.; Ghisetti, V.; Di Perri, G.; et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: A case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2021, 224, 391.e1–391.e7. [Google Scholar] [CrossRef]
- Yang, H.; Sun, G.; Tang, F.; Peng, M.; Gao, Y.; Peng, J.; Xie, H.; Zhao, Y.; Jin, Z. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J. Infect. 2020, 81, e40–e44. [Google Scholar] [CrossRef]
- Bian, J.; Li, Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm. Sin. B 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuffe, J.; Walton, S.; Steane, S.; Singh, R.; Simmons, D.; Moritz, K. The effects of gestational age and maternal hypoxia on the placental renin angiotensin system in the mouse. Placenta 2014, 35, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Lister, R.; Leitzke, A.; Goyal, D.; Gheorghe, C.; Longo, L. Antenatal maternal hypoxic stress: Adaptations of the placental renin-angiotensin system in the mouse. Placenta 2011, 32, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Yellon, S.; Longo, L.; Mata-Greenwood, E. Placental Gene Expression in a Rat ‘Model’ of Placental Insufficiency. Placenta 2010, 31, 568–575. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef] [Green Version]
- Gjyshi, O.; Bottero, V.; Veettil, M.V.; Dutta, S.; Singh, V.V.; Chikoti, L.; Chandran, B. Kaposi’s Sarcoma-Associated Herpesvirus Induces Nrf2 during De Novo Infection of Endothelial Cells to Create a Microenvironment Conducive to Infection. PLoS Pathog. 2014, 10, e1004460. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef]
- Garofalo, R.P.; Kolli, D.; Casola, A. Respiratory Syncytial Virus Infection: Mechanisms of Redox Control and Novel Therapeutic Opportunities. Antioxid. Redox Signal. 2013, 18, 186–217. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.G.; De Brito, C.A.; Dos Reis, V.M.S.; Sato, M.N.; Pereira, N.Z. SARS-CoV-2 and Other Respiratory Viruses: What Does Oxidative Stress Have to Do with It? Oxidative Med. Cell. Longev. 2020, 2020, 8844280. [Google Scholar] [CrossRef]
- Vassilaki, N.; Frakolaki, E. Virus–host interactions under hypoxia. Microbes Infect. 2017, 19, 193–203. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Chong, E.Y.; Serebrovska, T.V.; Tumanovska, L.V.; Xi, L. Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020, 41, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.V.D.; Haagmans, B.; van Riel, D.; Osterhaus, A.; Kuiken, T. The Pathology and Pathogenesis of Experimental Severe Acute Respiratory Syndrome and Influenza in Animal Models. J. Comp. Pathol. 2014, 151, 83–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, S.L.; De Lang, A.; Brand, J.M.A.V.D.; Leijten, L.M.; van Ijcken, W.; Eijkemans, M.J.C.; Van Amerongen, G.; Kuiken, T.; Andeweg, A.C.; Osterhaus, A.; et al. Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates. PLoS Pathog. 2010, 6, e1000756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heras, N.D.L.; Giménez, V.M.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants 2020, 9, 897. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of Oxidative Stress in Fetal Programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef] [PubMed]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxidative Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef]
- Santos-Rosendo, C.; Bugatto, F.; González-Domínguez, A.; Lechuga-Sancho, A.M.; Mateos, M.R.; Visiedo, F. Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity. Antioxidants 2020, 9, 794. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, B.; Perrelli, M.-G.; Aragno, M.; Mastrocola, R.; Corvetti, G.; Durazzo, M.; Poli, G.; Cutrín, J.C. Ischemic preconditioning attenuates the oxidant-dependent mechanisms of reperfusion cell damage and death in rat liver. Liver Transplant. 2002, 8, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Jendrach, M.; Mai, S.; Pohl, S.; Vöth, M.; Bereiter-Hahn, J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion 2008, 8, 293–304. [Google Scholar] [CrossRef]
- Jezek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Olowe, R.; Sandouka, S.; Saadi, A.; Shekh-Ahmad, T. Approaches for Reactive Oxygen Species and Oxidative Stress Quantification in Epilepsy. Antioxidants 2020, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Gao, L.; Ren, J.; Xu, L.; Ke, X.; Xiong, L.; Tian, X.; Fan, C.; Yan, H.; Yuan, J. Placental pathology of the third trimester pregnant women from COVID-19. Diagn. Pathol. 2021, 16, 8. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Qiancheng, X.; Jian, S.; Lingling, P.; Lei, H.; Xiaogan, J.; Weihua, L.; Gang, Y.; Shirong, L.; Zhen, W.; GuoPing, X.; et al. Coronavirus disease 2019 in pregnancy. Int. J. Infect. Dis. 2020, 95, 376–383. [Google Scholar] [CrossRef]
- Ellington, S.; Strid, P.; Tong, V.T.; Woodworth, K.; Galang, R.R.; Zambrano, L.D.; Nahabedian, J.; Anderson, K.; Gilboa, S.M. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–June 7, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 769–775. [Google Scholar] [CrossRef]
- Vaezi, M.; Mirghafourvand, M.; Hemmatzadeh, S. Characteristics, clinical and laboratory data and outcomes of pregnant women with confirmed SARS-CoV-2 infection admitted to Al-Zahra tertiary referral maternity center in Iran: A case series of 24 patients. BMC Pregnancy Childbirth 2021, 21, 378. [Google Scholar] [CrossRef]
- Yan, J.; Guo, J.; Fan, C.; Juan, J.; Yu, X.; Li, J.; Feng, L.; Li, C.; Chen, H.; Qiao, Y.; et al. Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am. J. Obstet. Gynecol. 2020, 223, 111.e1–111.e14. [Google Scholar] [CrossRef] [PubMed]
- Zaigham, M.; Andersson, O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020, 99, 823–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zhang, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship between the ABO Blood Group and the Coronavirus Disease 2019 (COVID-19) Susceptibility. Clin. Infect. Dis. 2021, 73, 328–331. [Google Scholar] [CrossRef]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020, 369, m2107. [Google Scholar] [CrossRef]
- Sahin, D.; Tanacan, A.; Erol, S.A.; Anuk, A.T.; Yetiskin, F.D.; Keskin, H.L.; Ozcan, N.; Ozgu-Erdinc, A.S.; Eyi, E.G.; Yucel, A.; et al. Updated experience of a tertiary pandemic center on 533 pregnant women with COVID-19 infection: A prospective cohort study from Turkey. Int. J. Gynecol. Obstet. 2021, 152, 328–334. [Google Scholar] [CrossRef]
- Cortese, M.; Lee, J.-Y.; Cerikan, B.; Neufeldt, C.J.; Oorschot, V.M.J.; Köhrer, S.; Hennies, J.; Schieber, N.L.; Ronchi, P.; Mizzon, G.; et al. Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies. Cell Host Microbe 2020, 28, 853–866.e5. [Google Scholar] [CrossRef]
- Yao, Y.; Lawrence, D.A. Susceptibility to COVID-19 in populations with health disparities: Posited involvement of mitochondrial disorder, socioeconomic stress, and pollutants. J. Biochem. Mol. Toxicol. 2021, 35, e22626. [Google Scholar] [CrossRef]
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Physiol. 2020, 319, C258–C267. [Google Scholar] [CrossRef]
- Burtscher, J.; Cappellano, G.; Omori, A.; Koshiba, T.; Millet, G.P. Mitochondria: In the Cross Fire of SARS-CoV-2 and Immunity. IScience 2020, 23, 101631. [Google Scholar] [CrossRef]
- Sims, A.C.; Burkett, S.E.; Yount, B.; Pickles, R.J. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res. 2008, 133, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Caldas, L.A.; Freitas, T.R.P.; Azevedo, R.C.; De Souza, W. Prostaglandin A1 inhibits the replication of bovine viral diarrhea virus. Braz. J. Microbiol. 2018, 49, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Caldas, L.A.; Carneiro, F.A.; Higa, L.M.; Monteiro, F.L.; da Silva, G.P.; da Costa, L.J.; Durigon, E.L.; Tanuri, A.; de Souza, W. Ultrastructural analysis of SARS-CoV-2 interactions with the host cell via high resolution scanning electron microscopy. Sci. Rep. 2020, 10, 16099. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, Y.; Juttukonda, L.; Sabharwal, V.; Boateng, J.; Khan, A.R.; Yarrington, C.; Wachman, E.M.; Taglauer, E. Differential Expression of Rab5 and Rab7 Small GTPase Proteins in Placental Tissues From Pregnancies Affected by Maternal Coronavirus Disease 2019. Clin. Ther. 2021, 43, 308–318. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Cekerevac, I.; Turnic, T.N.; Draginic, N.; Andjic, M.; Zivkovic, V.; Simovic, S.; Susa, R.; Novkovic, L.; Mijailovic, Z.; Andjelkovic, M.; et al. Predicting Severity and Intrahospital Mortality in COVID-19: The Place and Role of Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 6615787. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Ntyonga-Pono, M.-P. COVID-19 infection and oxidative stress: An under-explored approach for prevention and treatment? Pan Afr. Med. J. 2020, 35, 12. [Google Scholar] [CrossRef]
- Loffredo, L.; Violi, F. COVID-19 and cardiovascular injury: A role for oxidative stress and antioxidant treatment? Int. J. Cardiol. 2020, 312, 136. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; de Brito Magalhães, C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2017, 162, 907–917. [Google Scholar] [CrossRef]
- Reshi, L.; Su, Y.-C.; Hong, J.-R. RNA Viruses: ROS-Mediated Cell Death. Int. J. Cell Biol. 2014, 2014, 467452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrobot, A.M.; Szaflarska-Szczepanik, A.; Drewa, G. Antioxidant defense in children with chronic viral hepatitis B and C. Med. Sci. Monit. 2000, 6, 713–718. [Google Scholar] [PubMed]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; Ahmad, A.E.-F.; Kabir, M.B.; Bindawa, K.U.; Ahmed, A. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021, 9, 2050312121991246. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.; Ashmore, T.J.; Yung, H.-W.; Cindrova-Davies, T.; Spiroski, A.-M.; Sutherland, M.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef] [Green Version]
- Holland, O.J.; Cuffe, J.S.M.; Nitert, M.D.; Callaway, L.; Cheung, K.A.K.; Radenkovic, F.; Perkins, A.V. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018, 9, 1150. [Google Scholar] [CrossRef] [Green Version]
| CTRL (n = 12) | a-COVID-19 (n = 14) | s-COVID-19 (n = 15) | p Value | |
|---|---|---|---|---|
| Nulliparae (%) | 14.3 (n = 2) | 35.7 (n = 5) | 53.3 (n = 8) | p > 0.05 |
| Maternal age at delivery (years) | 35.1 ± 0.8 | 32 ± 1 | 33.1 ± 0.7 | p > 0.05 |
| Gestational age at delivery (weeks) | 39.8 ± 0.2 | 38.8 ± 0.8 | 37.8 ± 0.5 | p > 0.05 |
| Pre-Pregnancy comorbidity: | ||||
| Asthma (%) Diabetes (%) Tobacco (%) Chronic Hypertension (%) Gestational Overweight (%) Hematological Disease (%) | 0 0 0 0 0 0 | 0 21.4 (n = 3) 0 0 35.7 ^ (n = 5) 14.3 (n = 2) | 0 6.7 (n = 1) 6.7 (n = 1) 0 13.3 (n = 2) 6.7 (n = 1) | p > 0.05 p > 0.05 p > 0.05 p > 0.05 ^ p = 0.021 p > 0.05 |
| Pregnancy complications: | ||||
| Gestational Hypertension (%) IUGR (%) HELLP (%) PE (%) | 0 0 0 0 | 21.4 (n = 3) 7.1 (n = 1) 0 0 | 6.7 (n = 1) 0 0 6.7 (n = 1) | p > 0.05 p > 0.05 p > 0.05 p > 0.05 |
| SARS-CoV-2 symptoms: | ||||
| Dyspnea (%) Fever (%) Anosmia/Ageusia/Asthenia (%) Cough (%) Rhinitis (%) | 0 0 0 0 0 | 0 0 0 0 0 | 40 (n = 6) 86.7 ^,* (n = 13) 66.7 ^,* (n = 10) 66.7 ^,* (n = 10) 13.3 (n = 2) | p > 0.05 ^,* p = 0.001 ^,* p = 0.001 ^,* p = 0.001 p > 0.05 |
| Obstetrics & Neonatal outcomes | ||||
| Pathological Doppler (%) Pathological CTG (%) Cesarean section (%) Birth weight (g) Placental weight (g) Positive neonatal swab (%) APGAR < 7 at 5 min (%) Female fetus (%) Male fetus (%) | 0 0 41.7 (n = 5) 3358 ± 121.2 571 ± 22.2 0 0 41.7 (n = 5) 58.3 (n = 7) | 0 21.4 (n = 3) 35.7 (n = 5) 3306.4 ± 191.7 604.1 ± 46.6 0 7.1 (n = 1) 71.4 (n = 10) 28.6 (n = 4) | 6.7 (n = 1) 26.7 (n = 4) 60 (n = 9) 2993 ± 171.7 554.3 ± 40.3 0 6.7 (n = 1) 60 (n = 9) 40 (n = 6) | p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolfo, A.; Cosma, S.; Nuzzo, A.M.; Salio, C.; Moretti, L.; Sassoè-Pognetto, M.; Carosso, A.R.; Borella, F.; Cutrin, J.C.; Benedetto, C. Increased Placental Anti-Oxidant Response in Asymptomatic and Symptomatic COVID-19 Third-Trimester Pregnancies. Biomedicines 2022, 10, 634. https://doi.org/10.3390/biomedicines10030634
Rolfo A, Cosma S, Nuzzo AM, Salio C, Moretti L, Sassoè-Pognetto M, Carosso AR, Borella F, Cutrin JC, Benedetto C. Increased Placental Anti-Oxidant Response in Asymptomatic and Symptomatic COVID-19 Third-Trimester Pregnancies. Biomedicines. 2022; 10(3):634. https://doi.org/10.3390/biomedicines10030634
Chicago/Turabian StyleRolfo, Alessandro, Stefano Cosma, Anna Maria Nuzzo, Chiara Salio, Laura Moretti, Marco Sassoè-Pognetto, Andrea Roberto Carosso, Fulvio Borella, Juan Carlos Cutrin, and Chiara Benedetto. 2022. "Increased Placental Anti-Oxidant Response in Asymptomatic and Symptomatic COVID-19 Third-Trimester Pregnancies" Biomedicines 10, no. 3: 634. https://doi.org/10.3390/biomedicines10030634
APA StyleRolfo, A., Cosma, S., Nuzzo, A. M., Salio, C., Moretti, L., Sassoè-Pognetto, M., Carosso, A. R., Borella, F., Cutrin, J. C., & Benedetto, C. (2022). Increased Placental Anti-Oxidant Response in Asymptomatic and Symptomatic COVID-19 Third-Trimester Pregnancies. Biomedicines, 10(3), 634. https://doi.org/10.3390/biomedicines10030634











