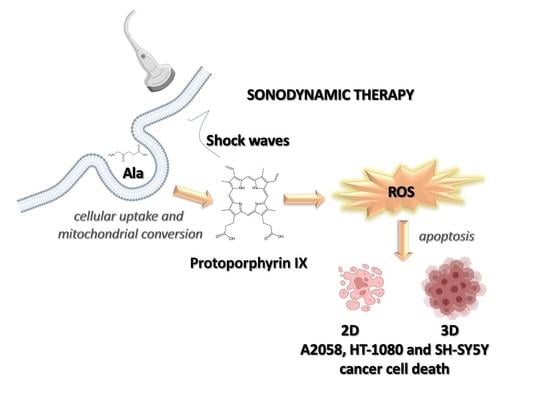
Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models
Abstract
1. Introduction
2. Materials and Methods
2.1. 2D and 3D Culture of Cell Lines
2.2. Glutathione Intracellular Concentration
2.3. Evaluation of Intracellular Ala-Derived PPIX
2.4. In Vitro Sonodynamic and Photodynamic Treatment
2.5. Cell Proliferation Assay
2.6. Flow Cytometric Analyses
2.7. Cytochrome c Analysis
2.8. Evaluation of DNA Damage
2.9. Real-Time RT-PCR
3. Results
3.1. Determination of Glutathione Intracellular Levels in A2058, HT-1080 and SH-SY5 Y Cells
3.2. Evaluation of Ala-Derived PPIX Intracellular Accumulation in A2058, HT-1080 and SH-SY5 Y Cells
3.3. Effect of SW-Mediated SDT and PDT on Cell Proliferation
3.4. Evaluation of Cell Death after SW-Mediated SDT and PDT
3.5. ROS Production after SW-Mediated SDT and PDT
3.6. Evaluation of Gene Expression and Cytochrome c Production after SW-Mediated SDT and PDT
3.7. Evaluation of SW-Mediated SDT Effect on DNA Damage
3.8. SW-Mediated SDT Effect on Spheroid Growth
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, V.; Rajora, M.A.; Zheng, G. Activating Drugs with Sound: Mechanisms Behind Sonodynamic Therapy and the Role of Nanomedicine. Bioconjug. Chem. 2020, 31, 967–989. [Google Scholar] [CrossRef] [PubMed]
- Lafond, M.; Yoshizawa, S.; Umemura, S.-I. Sonodynamic Therapy: Advances and Challenges in Clinical Translation. J. Ultrasound Med. 2018, 38, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Shibaguchi, H.; Tsuru, H.; Kuroki, M.; Kuroki, M. Sonodynamic cancer therapy: A non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011, 31, 2425–2429. [Google Scholar] [PubMed]
- Villeneuve, L.; Alberti, L.; Steghens, J.-P.; Lancelin, J.-M.; Mestas, J.-L. Assay of hydroxyl radicals generated by focused ultrasound. Ultrason. Sonochem. 2009, 16, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gielen, B.; Jordens, J.; Janssen, J.; Pfeiffer, H.; Wevers, M.; Thomassen, L.; Braeken, L.; Van Gerven, T. Characterization of stable and transient cavitation bubbles in a milliflow reactor using a multibubble sonoluminescence quenching technique. Ultrason. Sonochem. 2014, 25, 31–39. [Google Scholar] [CrossRef]
- Serpe, L.; Foglietta, F.; Canaparo, R. Nanosonotechnology: The next challenge in cancer sonodynamic therapy. Nanotechnol. Rev. 2012, 1, 173–182. [Google Scholar] [CrossRef]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef]
- Sun, X.; Xu, H.; Shen, J.; Guo, S.; Shi, S.; Dan, J.; Tian, F.; Tian, Y.; Tian, Y. Real-time detection of intracellular reactive oxygen species and mitochondrial membrane potential in THP-1 macrophages during ultrasonic irradiation for optimal sonodynamic therapy. Ultrason. Sonochem. 2015, 22, 7–14. [Google Scholar] [CrossRef]
- Giuntini, F.; Foglietta, F.; Marucco, A.M.; Troia, A.; Dezhkunov, N.V.; Pozzoli, A.; Durando, G.; Fenoglio, I.; Serpe, L.; Canaparo, R. Insight into ultrasound-mediated reactive oxygen species generation by various metal-porphyrin complexes. Free Radic. Biol. Med. 2018, 121, 190–201. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Francovich, A.; Arena, F.; Civera, S.; Cravotto, G.; Frairia, R.; Serpe, L. Sonodynamic treatment as an innovative bimodal anticancer approach: Shock wave-mediated tumor growth inhibition in a syngeneic breast cancer model. Discov. Med. 2015, 20, 197–205. [Google Scholar]
- Maruyama, M.; Asano, T.; Uematsu, T.; Nakagohri, T.; Hasegawa, M.; Miyauchi, H.; Iwashita, C.; Isono, K. Enhancement of the Antitumor Effect by Combined Use of High-energy Shock Waves and ATX-70. Jpn. J. Cancer Res. 1995, 86, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Wess, O.J. Physics and Technique of Shock Wave Lithotripsy (SWL). In Urolithiasis; Talati, J., Tiselius, H.G., Albala, D., Ye, Z., Eds.; Springer: London, UK, 2012. [Google Scholar]
- Serpe, L.; Canaparo, R.; Varchi, G.; Ballestri, M.; Foglietta, F.F.; Sotgiu, G.; Guerrini, A.; Francovich, A.; Civera, P.; Frairia, R. Polymeric nanoparticles enhance the sonodynamic activity of meso-tetrakis (4-sulfonatophenyl) porphyrin in an in vitro neuroblastoma model. Int. J. Nanomed. 2013, 8, 4247–4263. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Canaparo, R.; Berta, L.; Bargoni, A.; Zara, G.P.; Frairia, R. High Energy Shock Waves and 5-Aminolevulinic for Sonodynamic Therapy: Effects in a Syngeneic Model of Colon Cancer. Technol. Cancer Res. Treat. 2011, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Costantino, L.; Fortunati, N.; Bosco, O.; Pugliese, M.; Boccuzzi, G.; Berta, L.; Frairia, R. High Energy Shock Waves Activate 5′-Aminolevulinic Acid and Increase Permeability to Paclitaxel: Antitumor Effects of a New Combined Treatment on Anaplastic Thyroid Cancer Cells. Thyroid 2007, 17, 91–99. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Pape, J.; Emberton, M.; Cheema, U. 3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness. Front. Bioeng. Biotechnol. 2021, 9, 660502. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Muccioli, G.; Terreno, E.; Serpe, L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020, 254, 117784. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Wang, X.; Fang, H.; Huang, Z.; Shang, W.; Hou, T.; Cheng, A.; Cheng, H. Imaging ROS signaling in cells and animals. Klin. Wochenschr. 2013, 91, 917–927. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy––a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, T.; Zhu, Y.; Hao, Y. Ultrasound-Activated Oxygen and ROS Generation Nanosystem Systematically Modulates Tumor Microenvironment and Sensitizes Sonodynamic Therapy for Hypoxic Solid Tumors. Adv. Funct. Mater. 2019, 29, 1906195. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, Q.; Geng, J.; Meng, Q.; Wei, Z.; Ding, M.; Zhou, J.; Zeng, Y.; Cao, W.; Liu, F.; et al. Sonodynamic Effects of a Novel Ether-Group Modified Porphyrin Derivative Combined with Pulsed Low-Intensity Ultrasound on PC-9 Cells. Front. Pharmacol. 2021, 12, 792360. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.G.; Díaz-Quintana, A.; Pérez-Mejías, G.; Elena-Real, C.A.; González-Arzola, K.; García-Mauriño, S.M.; De la Rosa, M.A.; Díaz-Moreno, I. Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria. Proc. Natl. Acad. Sci. USA 2018, 115, 7955–7960. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome C-Mediated Apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Zhang, K.; Liu, J.; Wang, P.; Wang, X.; Liu, Q. Correction: Sonodynamic therapy induces oxidative stress, DNA damage and apoptosis in glioma cells. RSC Adv. 2021, 11, 19569. [Google Scholar] [CrossRef]
- Noodt, B.B.; Berg, K.S.; Stokke, T.; Peng, Q.; Nesland, J.M. Apoptosis and necrosis induced with light and 5-aminolaevulinic acid-derived protoporphyrin IX. Br. J. Cancer 1996, 74, 22–29. [Google Scholar] [CrossRef]
- Cheing, G.L.Y.; Chang, H. Extracorporeal Shock Wave Therapy. J. Orthop. Sports Phys. Ther. 2003, 33, 337–343. [Google Scholar] [CrossRef]
- Kato, K.; Fujimura, M.; Nakagawa, A.; Saito, A.; Ohki, T.; Takayama, K.; Tominaga, T. Pressure-dependent effect of shock waves on rat brain: Induction of neuronal apoptosis mediated by a caspase-dependent pathway. J. Neurosurg. 2007, 106, 667–676. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Messmann, H.; Rauch, J.; Seeger, S.; Knuechel, R. Metabolic characterization of tumor cell-specific proto-porphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem. Photobiol. 2002, 76, 518–525. [Google Scholar] [CrossRef]
- Gibson, S.L.; Nguyen, M.L.; Havens, J.J.; Barbarin, A.; Hilf, R. Relationship of δ-Aminolevulinic Acid-Induced Protoporphyrin IX Levels to Mitochondrial Content in Neoplastic Cells in Vitro. Biochem. Biophys. Res. Commun. 1999, 265, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Burn, J.L.; Reed, M.; Brown, N. Factors affecting aminolaevulinic acid-induced generation of protoporphyrin IX. Br. J. Cancer 1997, 76, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.L.; Chen, B.; O’Hara, J.A.; Hoopes, P.J.; Hasan, T.; Pogue, B.W. Protoporphyrin IX Level Correlates with Number of Mitochondria, But Increase in Production Correlates with Tumor Cell Size. Photochem. Photobiol. 2006, 82, 1334–1341. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Moor, A.C. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B Biol. 2000, 57, 1–13. [Google Scholar] [CrossRef]
- Beguin, E.; Shrivastava, S.; Dezhkunov, N.V.; McHale, A.P.; Callan, J.F.; Stride, E. Direct Evidence of Multibubble Sonoluminescence Using Therapeutic Ultrasound and Microbubbles. ACS Appl. Mater. Interfaces 2019, 11, 19913–19919. [Google Scholar] [CrossRef]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef]
- Shen, Y.; Ou, J.; Chen, X.; Zeng, X.; Huang, L.; Pi, Z.; Hu, Y.; Chen, S.; Chen, T. An in vitro study on sonodynamic treatment of human colon cancer cells using sinoporphyrin sodium as sonosensitizer. Biomed. Eng. Online 2020, 19, 1–15. [Google Scholar] [CrossRef]
- Zong, W.-X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Fettweis, G.; Rubio, N.; Agostinis, P.; Piette, J. 5-ALA-PDT induces RIP3-dependent necrosis in glioblastoma. Photochem. Photobiol. Sci. 2011, 10, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Rengeng, L.; Qianyu, Z.; Yuehong, L.; Zhongzhong, P.; Libo, L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.; da Silva, L.C.G. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Gao, L.; Zheng, L.; Kou, J.; Zhu, X.; Jiang, Y.; Zhong, Z.; Dan, J.; Xu, H.; et al. The efficacy and mechanism of apoptosis induction by hypericin-mediated sonodynamic therapy in THP-1 macrophages. Int. J. Nanomed. 2015, 10, 821–838. [Google Scholar] [CrossRef][Green Version]
- Rimann, M.; Graf-Hausner, U. Synthetic 3D multicellular systems for drug development. Curr. Opin. Biotechnol. 2012, 23, 803–809. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Logan, K.; Foglietta, F.; Nesbitt, H.; Sheng, Y.; McKaig, T.; Kamila, S.; Gao, J.; Nomikou, N.; Callan, B.; McHale, A.P.; et al. Targeted chemo-sonodynamic therapy treatment of breast tumours using ultrasound responsive microbubbles loaded with paclitaxel, doxorubicin and Rose Bengal. Eur. J. Pharm. Biopharm. 2019, 139, 224–231. [Google Scholar] [CrossRef]








| A2058–Ala [mM] | PPIX at 12 h | |
| Cell Population (%) | iMFI Ratio | |
| 0.45 | 90.89 ± 0.16 | 57.71 ± 0.77 |
| 1.5 | 90.73 ± 1.03 | 57.39 ± 1.96 |
| 3.0 | 89.55 ± 0.64 | 45.92 ± 1.29 |
| HT-1080–Ala [mM] | PPIX at 4 h | |
| Cell Population (%) | iMFI ratio | |
| 0.45 | 85.43 ± 0.80 | 37.20 ± 1.13 |
| 1.5 | 84.37 ± 0.90 | 35.99 ± 0.72 |
| 3.0 | 83.54 ± 0.76 | 37.02 ± 1.44 |
| SH-SY5 Y–Ala [mM] | PPIX at 24 h | |
| Cell Population (%) | iMFI ratio | |
| 0.45 | 54.36 ± 0.19 | 8.28 ± 1.29 |
| 0.9 | 69.73 ± 0.99 | 38.77 ± 1.78 |
| 1.8 | 76.23 ± 0.92 | 53.89 ± 1.30 |
| 3.6 | 49.38 ± 0.16 | 10.90 ± 0.83 |
| A2058 Cells | |||
| Cell Treatment 48 h | Viable Cells (%) | Apoptotic Cells (%) | Necrotic Cells (%) |
| Untreated cells | 81.62 ± 0.54 | 8.66 ± 0.48 | 9.94 ± 0.09 |
| SW 1 | 81.73 ± 0.38 | 9.58 ± 0.60 | 8.55 ± 0.64 |
| LB | 80.26 ± 1.05 | 6.80 ± 1.00 | 14.59 ± 0.60 |
| Ala | 72.27 ± 0.38 | 10.91 ± 0.13 | 16.02 ± 0.02 |
| Ala + SW 1 | 74.58 ± 0.81 | 17.51 ± 1.21 * | 8.60 ± 0.57 |
| Ala + LB | 26.58 ± 0.59 *** | 7.56 ± 0.62 | 65.88 ± 0.18 *** |
| HT-1080 cells | |||
| Cell treatment 24 h | Viable cells (%) | Apoptotic cells (%) | Necrotic cells (%) |
| Untreated cells | 79.18 ± 1.17 | 6.76 ± 0.35 | 15.19 ± 1.15 |
| SW 2 | 74.44 ± 2.03 | 10.93 ± 0.11 | 13.13 ± 1.24 |
| LB | 77.22 ± 1.11 | 15.03 ± 0.67 * | 9.11 ± 0.55 |
| Ala | 81.89 ± 1.25 | 7.11 ± 0.16 | 8.79 ± 0.41 * |
| Ala + SW 2 | 65.90 ± 1.27 * | 21.46 ± 0.76 *** | 11.19 ± 0.26 |
| Ala + LB | 60.04 ± 1.36 * | 10.18 ± 1.17 | 27.94 ± 0.73 ** |
| SH-SY5 Y cells | |||
| Cell treatment 24 h | Viable cells (%) | Apoptotic cells (%) | Necrotic cells (%) |
| Untreated cells | 87.14 ± 1.22 | 7.89 ± 0.16 | 5.89 ± 0.16 |
| SW 2 | 85.31 ± 0.98 | 9.63 ± 0. 53 | 6.25 ± 0.36 |
| LB | 84.84 ± 1.18 | 7.12 ± 0.16 | 6.95 ± 0.08 |
| Ala | 89.22 ± 1.10 | 6.42 ± 0.59 | 5.04 ± 0.66 |
| Ala + SW 2 | 72.99 ± 1.40 | 15.97 ± 1.36 ** | 8.96 ± 0.06 |
| Ala + LB | 53.84 ± 0.23 *** | 10.69 ± 0.98 | 34.31 ± 0.43 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglietta, F.; Panzanelli, P.; Serpe, L.; Canaparo, R. Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models. Biomedicines 2022, 10, 615. https://doi.org/10.3390/biomedicines10030615
Foglietta F, Panzanelli P, Serpe L, Canaparo R. Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models. Biomedicines. 2022; 10(3):615. https://doi.org/10.3390/biomedicines10030615
Chicago/Turabian StyleFoglietta, Federica, Patrizia Panzanelli, Loredana Serpe, and Roberto Canaparo. 2022. "Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models" Biomedicines 10, no. 3: 615. https://doi.org/10.3390/biomedicines10030615
APA StyleFoglietta, F., Panzanelli, P., Serpe, L., & Canaparo, R. (2022). Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models. Biomedicines, 10(3), 615. https://doi.org/10.3390/biomedicines10030615