Immunotherapy in Advanced Prostate Cancer: Current Knowledge and Future Directions
Abstract
1. Overview of Prostate Cancer Immunology
- Imbalance of cytotoxic cells and Tregs in favor of the latter. Prostate cancer has a low number of tumor-infiltrating lymphocytes, although with a predominance of CD4+ Tregs and M2 macrophages as opposed to CD8+ T lymphocytes and natural killer (NK) cells.
- Exhaustion of cytotoxic and antigen-presenting dendritic cells due to the overexpression of antigens that block the immune response, such as programmed-cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4). This is known as an exhausted phenotype. Despite this high expression of PD-1 and CTLA-4 (though not of programmed-cell death ligand 1 or PD-L1), the response to immune checkpoint inhibitors in these types of tumors is poor.
- Preponderance of suppressive cytokines released by CD4+ T lymphocytes and M2 macrophages. These cytokines, in addition to inhibiting the immune response, favor tumoral angiogenesis, metastasis and castration resistance. The most relevant suppressive cytokines present in prostate cancer are interleukin (IL) 10, IL-23, TGFß, and certain compounds such as chemokine (C-C motif) ligand (CCL) 2 and CCL22.
- Intratumoral molecules such as decoy receptor 3 (Dcr3), a soluble receptor and member of the superfamily of tumor necrosis factor receptor (TNFR) that favors tumor growth through TNFR inhibition.
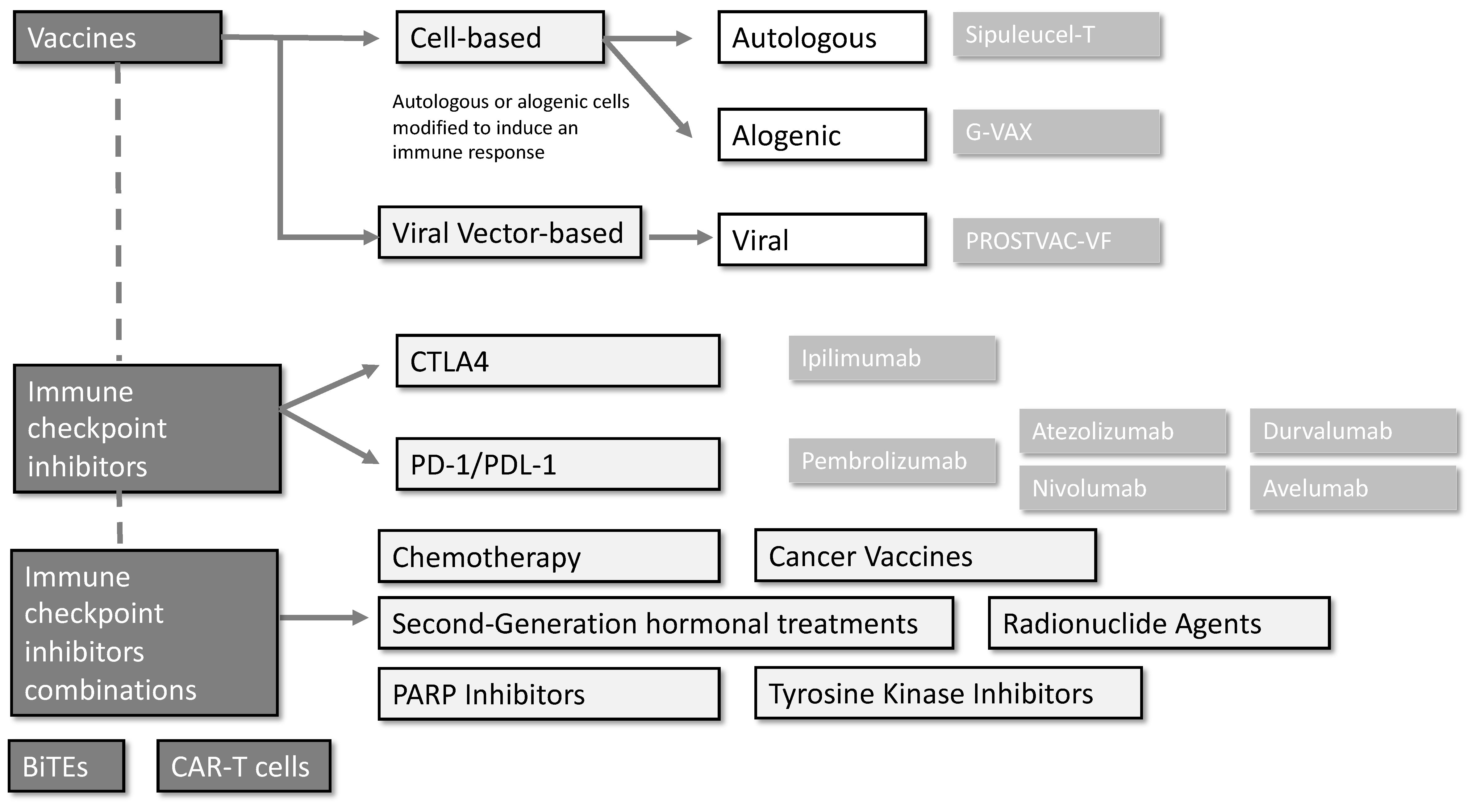
2. Vaccines
2.1. Cell-Based Vaccines
2.1.1. Sipuleucel-T (Provenge®)
2.1.2. G-VAX
2.2. Viral Vector-Based Vaccines
PROSTVAC-VF
3. Single-Agent Immune Checkpoint Inhibitors
3.1. CTLA-4 Inhibitors
3.2. PD-1 and PD-L1 Inhibitors
3.3. Biomarkers
4. Immune Checkpoint Inhibitor Combinations
4.1. Anti-CTLA-4 and anti-PD-1/PD-L1 Combinations
4.2. Immune Checkpoint Inhibitors and Chemotherapy
4.3. Immune Checkpoint Inhibitors and Second-Generation Hormonal Treatments
4.4. Immune Checkpoint Inhibitors and PARP Inhibitors
4.5. Immune Checkpoint Inhibitors and Cancer Vaccines
4.6. Immune Checkpoint Inhibitors with Tyrosine Kinase Inhibitors
4.7. Immune Checkpoint Inhibitors with Radionuclide Agents
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Rhea, L.P.; Mendez-Marti, S.; Kim, D.; Aragon-Ching, J.B. Role of immunotherapy in bladder cancer. Cancer Treat. Res. Commun. 2021, 26, 100296. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Bakouny, Z.; Hirsch, L.; Flippot, R.; Van Allen, E.M.; Wu, C.J.; Choueiri, T.K. Beyond conventional immune-checkpoint inhibition—Novel immunotherapies for renal cell carcinoma. Nat. Rev. Clin. Oncol. 2021, 18, 199–214. [Google Scholar] [CrossRef]
- Bansal, D.; Reimers, M.A.; Knoche, E.M.; Pachynski, R.K. Immunotherapy and immunotherapy combinations in metastatic castration-resistant prostate cancer. Cancers 2021, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Davar, D.; Lin, Y.; Kirkwood, J.M. Unfolding the mutational landscape of human melanoma. J. Investig. Dermatol. 2015, 135, 659–662. [Google Scholar] [CrossRef]
- Subudhi, S.K.; Vence, L.; Zhao, H.; Blando, J.; Yadav, S.S.; Xiong, Q.; Reuben, A.; Aparicio, A.; Corn, P.G.; Chapin, B.F.; et al. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci. Transl. Med. 2020, 12, eaaz3577. [Google Scholar] [CrossRef]
- Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Sun, B.L. Immunotherapy in treatment of metastatic prostate cancer: An approach to circumvent immunosuppressive tumor microenvironment. Prostate 2021, 81, 1125–1134. [Google Scholar] [CrossRef]
- Jiao, S.; Subudhi, S.K.; Aparicio, A.; Ge, Z.; Guan, B.; Miura, Y.; Sharma, P. Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell 2019, 179, 1177–1190.e13. [Google Scholar] [CrossRef]
- Prokhnevska, N.; Emerson, D.A.; Kissick, H.T.; Redmond, W.L. Immunological Complexity of the Prostate Cancer Microenvironment Influences the Response to Immunotherapy. Adv. Exp. Med. Biol. 2019, 1210, 121–147. [Google Scholar] [PubMed]
- Arlen, P.M.; Gulley, J.L.; Parker, C.; Skarupa, L.; Pazdur, M.; Panicali, D.; Beetham, P.; Tsang, K.Y.; Grosenbach, D.W.; Feldman, J.; et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Uhlman, M.A.; Bing, M.T.; Lubaroff, D.M. Prostate cancer vaccines in combination with additional treatment modalities. Immunol. Res. 2014, 59, 236–242. [Google Scholar] [CrossRef]
- Comber, J.D.; Philip, R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther. Adv. Vaccines 2014, 2, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Rahbar, M.R.; Dezfulian, M.H.; Jahangiri, A. In silico analyses of Wilms’ tumor protein to designing a novel multi-epitope DNA vaccine against cancer. J. Theor. Biol. 2015, 379, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Theoret, M.R.; Touloukian, C.E.; Surman, D.R.; Garman, S.C.; Feigenbaum, L.; Baxter, T.K.; Baker, B.M.; Restifo, N.P. Poor immunogenicity of a self/tumor antigen derives from peptide–MHC-I instability and is independent of tolerance. J. Clin. Investig. 2004, 114, 551–559. [Google Scholar] [CrossRef]
- Engels, B.; Engelhard, V.H.; Sidney, J.; Sette, A.; Binder, D.C.; Liu, R.B.; Kranz, D.M.; Meredith, S.C.; Rowley, D.A.; Schreiber, H. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell 2013, 23, 516–526. [Google Scholar] [CrossRef]
- Adamaki, M.; Zoumpourlis, V. Immunotherapy as a precision medicine tool for the treatment of prostate cancer. Cancers 2021, 13, 173. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Drake, C.G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 2010, 10, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M. Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006, 24, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009, 115, 3670–3679. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Bernstein, G.T.; Corman, J.M.; Glode, L.M.; Hall, S.J.; Poll, W.L.; Schellhammer, P.F.; Jones, L.A.; Xu, Y.; Kylstra, J.W.; et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin. Cancer Res. 2011, 17, 4558–4567. [Google Scholar] [CrossRef]
- Mark, D.; Samson, D.J.; Bonnell, C.J.; Ziegler, K.M.; Aronson, N. Outcomes of Sipuleucel-T Therapy; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2011. [Google Scholar]
- Higano, C.S.; Armstrong, A.J.; Sartor, A.O.; Vogelzang, N.J.; Kantoff, P.W.; McLeod, D.G.; Pieczonka, C.M.; Penson, D.F.; Shore, N.D.; Vacirca, J.; et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer 2019, 125, 4172–4180. [Google Scholar] [CrossRef]
- Rosser, C.J.; Hirasawa, Y.; Acoba, J.D.; Tamura, D.J.; Pal, S.K.; Huang, J.; Scholz, M.C.; Dorffet, T.B. Phase Ib study assessing different sequencing regimens of atezolizumab (anti-PD-L1) and sipuleucel-T (SipT)in patients who have asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer. J. Clin. Oncol. 2020, 38, e17564. [Google Scholar] [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Park, J.C.; DeWeese, T.L.; Tran, P.T.; Song, D.Y.; King, S.; Afful, M.; Hurrelbrink, J.; et al. Randomized phase II trial of sipuleucel-T with or without radium-223 in men with bone-metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2021, 27, 1623–1630. [Google Scholar] [CrossRef]
- Warren, T.L.; Weiner, G.J. Uses of granulocyte-macrophage colony-stimulating factor in vaccine development. Curr. Opin. Hematol. 2000, 7, 168–173. [Google Scholar] [CrossRef]
- Simons, J.W.; Carducci, M.A.; Mikhak, B.; Lim, M.; Biedrzycki, B.; Borellini, F.; Clift, S.M.; Hege, K.M.; Ando, D.G.; Piantadosi, S.; et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin. Cancer Res. 2006, 12, 3394–3401. [Google Scholar] [CrossRef]
- Simmons, A.D.; Li, B.; Gonzalez-Edick, M.; Lin, C.; Moskalenko, M.; Du, T.; Creson, J.; VanRoey, M.J.; Jooss, K. GM-CSF-secreting cancer immunotherapies: Preclinical analysis of the mechanism of action. Cancer Immunol. Immunother. 2007, 56, 1653–1665. [Google Scholar] [CrossRef]
- Handa, S.; Hans, B.; Goel, S.; Bashorun, H.O.; Dovey, Z.; Tewari, A. Immunotherapy in prostate cancer: Current state and future perspectives. Ther. Adv. Urol. 2020, 12, 1756287220951404. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Madan, R.A.; Tsang, K.Y.; Jochems, C.; Marté, J.L.; Farsaci, B.; Tucker, J.A.; Hodge, J.W.; Liewehr, D.J.; Steinberg, S.M.; et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol. Res. 2014, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Arlen, P.M.; Mohebtash, M.; Hodge, J.W.; Gulley, J.L. Prostvac-VF: A vector-based vaccine targeting PSA in prostate cancer. Expert Opin. Investig. Drugs 2009, 18, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Siobhan, N.; Agarwal, N.; Parker, C.C.; Pook, D.W.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III Trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Picardo, S.L.; Hansen, A.R. The PD-1/PD-L1 pathway in advanced prostate cancer—Have we milked this cow? Ann. Transl. Med. 2019, 7, 346. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marté, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFb, in advanced solid tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; De Guillebon, E.; Blanc, C.; Roussel, H.; Badoual, C.; Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017, 2, e000213. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The next immune-checkpoint inhibitors: Pd-1/pd-l1 blockade in melanoma. Clin. Ther. 2015, 37, 764–782. [Google Scholar] [CrossRef]
- Bylicki, O.; Barazzutti, H.; Paleiron, N.; Margery, J.; Assié, J.B.; Chouaïd, C. First-Line Treatment of Non-Small-Cell Lung Cancer (NSCLC) with Immune Checkpoint Inhibitors. BioDrugs 2019, 33, 159–171. [Google Scholar] [CrossRef]
- Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, S.E.; Salenius, S.; Mantz, C.A.; Shore, N.D.; Fernandez, E.B.; Shulman, J.; Myslicki, F.A.; Agassi, A.M.; Rotterman, Y.; DeVries, T.; et al. Combining immunotherapy and radiation for prostate cancer. Clin. Genitourin Cancer 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.F.; Higano, C.S.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.J.; Scher, H.I.; Chin, K.; Gagnier, P.; McHenry, M.B.; et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase i/ii study. Ann. Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Fakhrejahani, F.; Madan, R.A.; Dahut, W.L.; Karzai, F.; Cordes, L.M.; Schlom, J.; Liow, E.; Bennett, C.; Zheng, T.; Yu, J.; et al. Avelumab in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 159. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.-P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Graff, J.N.; Tagawa, S.T.; Hwang, C.; Kilari, D.; Ten Tije, A.J.; Omlin, A.U.; McDermott, R.S.; Vaishampayan, U.N.; Tony Elliott, T.; et al. KEYNOTE-199 cohorts (C) 4 and 5: Phase II study of pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 5543. [Google Scholar] [CrossRef]
- Tesi, R.J. MDSC; the Most Important Cell You Have Never Heard of. Trends Pharmacol. Sci. 2019, 40, 4–7. [Google Scholar]
- Zhang, S.; Ma, X.; Zhu, C.; Liu, L.; Wang, G.; Yuan, X. The role of myeloid-derived suppressor cells in patients with solid tumors: A meta-analysis. PLoS ONE 2016, 11, e0164514. [Google Scholar] [CrossRef] [PubMed]
- Calagua, C.; Russo, J.; Sun, Y.; Schaefer, R.; Lis, R.; Zhang, Z.; Mahoney, K.; Bubley, G.J.; Loda, M.; Taplin, M.E.; et al. Expression of PD-L1 in hormone-naïve and treated prostate cancer patients receiving neoadjuvant abiraterone acetate plus prednisone and leuprolide. Clin. Cancer Res. 2017, 23, 6812–6822. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Ciccarese, C.; Caliò, A.; Munari, E.; Cima, L.; Porcaro, A.B.; Novella, G.; Artibani, W.; Sava, T.; Eccher, A.; et al. Magnitude of PD-1, PD-L1 and T Lymphocyte Expression on Tissue from Castration-Resistant Prostate Adenocarcinoma: An Exploratory Analysis. Target. Oncol. 2016, 11, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Boudadi, K.; Suzman, D.L.; Anagnostou, V.; Fu, W.; Luber, B.; Wang, H.; Niknafs, N.; White, J.R.; Silberstein, J.L.; Sullivan, R.; et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 2018, 9, 28561–28571. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, L.; Cong, Z.; Amoozgar, Z.; Kiner, E.; Xing, D.; Orsulic, S.; Matulonis, U.; Goldberg, M.S. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1-/- murine model of ovarian cancer. Biochem. Biophys. Res. Commun. 2015, 463, 551–556. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef]
- Karzai, F.; Vanderweele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef]
- Wu, Y.M.; Cieślik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; Ning, Y.; Wang, L.; Kunju, L.P.; de Sarkar, N.; et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018, 173, 1770–1782.e14. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Isaacsson Velho, P.; Fu, W.; Wang, H.; Agarwal, N.; Santos, V.S.; Maughan, B.L.; Pili, R.; Adra, N.; Sternberg, C.N.; et al. CDK12 -Altered Prostate Cancer: Clinical Features and Therapeutic Outcomes to Standard Systemic Therapies, Poly (ADP-Ribose) Polymerase Inhibitors, and PD-1 Inhibitors. JCO Precis. Oncol. 2020, 4, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. Prostate cancer, version 1.2021: Featured updates to the nccn guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Joshi, H.; Pinski, J.K. Association of ARV7 expression with molecular and clinical characteristics in prostate cancer. J. Clin. Oncol. 2016, 34, 109. [Google Scholar] [CrossRef]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, K.S.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef]
- Hotte, S.J.; Winquist, E.; Chi, K.N.; Ellard, S.L.; Sridhar, S.; Emmenegger, U.; Jindal, S.; Tidwell, R.S.S.; Varma, A.; Logothetis, C.J.; et al. CCTG IND 232: A phase II study of durvalumab with or without tremelimumab in patients with metastatic castration resistant prostate cancer (mCRPC). Ann. Oncol. 2019, 30, v885. [Google Scholar] [CrossRef]
- Hodge, J.W.; Garnett, C.T.; Farsaci, B.; Palena, C.; Tsang, K.Y.; Ferrone, S.; Gameiro, S.R. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int. J. Cancer 2013, 133, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef]
- Chan, O.T.M.; Yang, L.X. The immunological effects of taxanes. Cancer Immunol. Immunother. 2000, 49, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Gonzalez Mella, P.; Castellano, D.; Minatta, J.N.; Rezazadeh Kalebasty, A.; Shaffer, D.; Vazquez-Limon, J.C.; Armstrong, A.J.; Sanchez-Lopez, H.M.; Sharkey, B.; et al. Efficacy and safety of nivolumab in combination with docetaxel in men with metastatic castration-resistant prostate cancer in CheckMate 9KD. Ann. Oncol. 2019, 30, v885–v886. Available online: https://www.sciencedirect.com/science/article/pii/S0923753419604059 (accessed on 29 January 2022). [CrossRef]
- Sridhar, S.S.; Kolinsky, M.P.; Gravis, G.; Mourey, L.; Piulats Rodriguez, J.M.M.; Romano, E.; Berry, W.R.; Gurney, H.; Retz, M.; Appleman, L.J.; et al. Pembrolizumab (pembro) plus docetaxel and prednisone in patients (pts) with abiraterone acetate (abi) or enzalutamide (enza)-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort B efficacy, safety and, biomarker results. J. Clin. Oncol. 2020, 38, 5550. [Google Scholar] [CrossRef]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar] [CrossRef]
- Ardiani, A.; Gameiro, S.R.; Kwilas, A.R.; Donahue, R.N.; Hodge, J.W. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget 2014, 5, 9335–9348. [Google Scholar] [CrossRef]
- Pal, S.K.; Moreira, D.; Won, H.; White, S.W.; Duttagupta, P.; Lucia, M.; Jones, J.; Hsu, J.; Kortylewski, M. Reduced T-cell numbers and elevated levels of immunomodulatory cytokines in metastatic prostate cancer patients de novo resistant to abiraterone and/or enzalutamide therapy. Int. J. Mol. Sci. 2019, 20, 1831. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Gillessen, S.; Rathkopf, D.; Matsubara, N.; Drake, C.; Fizazi, K.; Piulats, J.M.; Wysocki, P.J.; Buchschacher, G.L., Jr.; Doss, J.; et al. Abstract CT014: IMbassador250: A phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC). Cancer Res. 2020, 80, CT014. Available online: http://cancerres.aacrjournals.org/content/80/16_Supplement/CT014.abstract (accessed on 29 January 2022).
- Conter, H.J.; Shore, N.D.; Berry, W.R.; Fong, P.C.C.; Piulats Rodriguez, J.M.M.; Appleman, L.J.; Todenhöfer, T.; Gravis, G.; Laguerre, B.; Gurney, H.; et al. Pembrolizumab (pembro) plus enzalutamide (enza) in patients (pts) with abiraterone acetate (abi)-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort C efficacy, safety, and biomarker results. J. Clin. Oncol. 2020, 38, 5545. [Google Scholar] [CrossRef]
- Peyraud, F.; Italiano, A. Combined parp inhibition and immune checkpoint therapy in solid tumors. Cancers 2020, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Fenerty, K.E.; Padget, M.; Wolfson, B.; Gameiro, S.R.; Su, Z.; Lee, J.H.; Rabizadeh, S.; Soon-Shiong, P.; Hodge, J.W. Immunotherapy utilizing the combination of natural killer- and antibody dependent cellular cytotoxicity (ADCC)-mediating agents with poly (ADP-ribose) polymerase (PARP) inhibition 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis 11 Medical. J. Immunother. Cancer 2018, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Piulats, J.M.; Gravis, G.; Laguerre, B.; Arranz Arija, J.A.; Oudard, S.; Fong, P.C.C.; Kolinsky, M.P.; Augustin, M.; Feyerabend, S.; et al. KEYNOTE-365 cohort A updated results: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 100. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Perez-Gracia, J.L.; Lacombe, L.; Bastos, D.A.; Mahammedi, H.; Kwan, E.M.; Zschäbitz, S.; Armstrong, A.J.; Pachynski, R.K.; Goh, J.C.; et al. 579MO CheckMate 9KD cohort A2 final analysis: Nivolumab (NIVO) + rucaparib for chemotherapy (CT)-naïve metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 2021, 32, S629–S630. [Google Scholar] [CrossRef]
- Sinha, M.; Zhang, L.; Subudhi, S.; Chen, B.; Marquez, J.; Liu, E.V.; Allaire, K.; Cheung, A.; Ng, S.; Nguyen, C.; et al. Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J. Immunother. Cancer 2021, 9, e002254. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Nandana, S.; Billet, S.; Cavassani, K.A.; Mishra, R.; Chung, L.W.K.; Posadas, E.M.; Bhowmick, N.A. Modulation of cabozantinib efficacy by the prostate tumor microenvironment. Oncotarget 2017, 8, 87891–87902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, N.; McGregor, B.A.; Maughan, B.L.; Dorff, T.; Kelly, W.; Fang, B.; McKay, R.; Singh, P.; Pagliaro, L.; Dreicer, R.; et al. LBA24 Cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): Results of expanded cohort 6 of the COSMIC-021 study. Ann. Oncol. 2021, 32, S1299–S1300. [Google Scholar] [CrossRef]
- Morris, M.J.; Fong, L.; Petrylak, D.P.; Sartor, A.O.; Higano, C.S.; Pagliaro, L.C.; Alva, A.S.; Appleman, L.J.; Tan, W.; Vaishampayan, U.N.; et al. Safety and clinical activity of atezolizumab (atezo) + radium-223 dichloride (r-223) in 2L metastatic castration-resistant prostate cancer (mCRPC): Results from a phase Ib clinical trial. J. Clin. Oncol. 2020, 38, 5565. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Hadda, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Joshua, A.M.; Emmett, L.; Spain, L.; Horvath, L.G.; Crumbaker, M.; Anton, A.; Wallace, R.; Pasam, A.; Bressel, M.; et al. 577O PRINCE: Interim analysis of the phase Ib study of 177Lu-PSMA-617 in combination with pembrolizumab for metastatic castration resistant prostate cancer (mCRPC). Ann. Oncol. 2021, 32, S626–S627. [Google Scholar] [CrossRef]
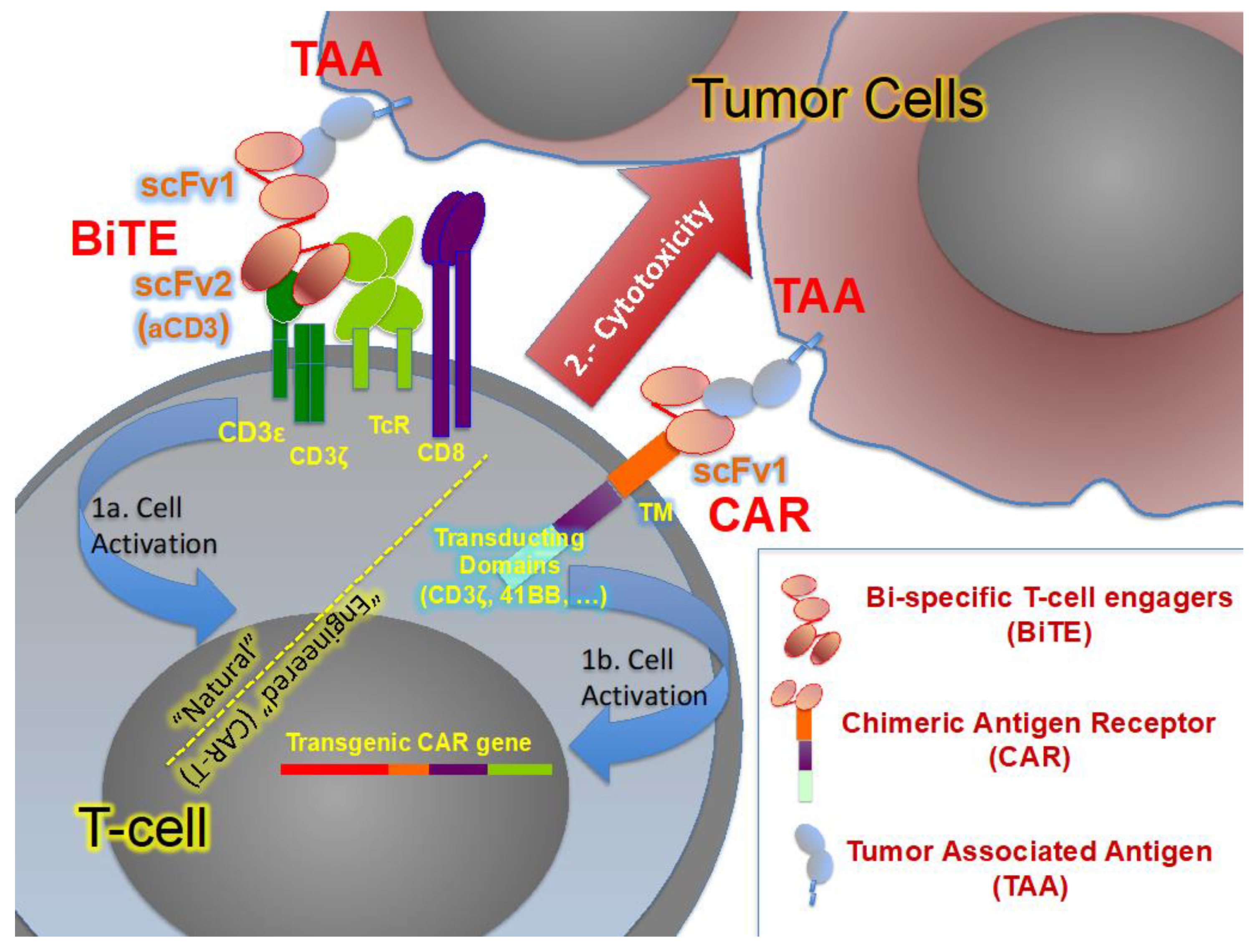
- Pantuck, M.; Palaskas, N.; Drakaki, A. Next generation T-cell therapy for genitourinary malignancies, part B: Overcoming obstacles and future strategies for success. Cancer Treat. Res. Commun. 2018, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Cursano, M.C.; Casadei, C.; Menna, C.; Altavilla, A.; Lolli, C.; Cerchione, C.; Paganelli, G.; Santini, D.; Tonini, G.; et al. CAR-T cell therapy: A potential new strategy against prostate cancer. J. Immunother. Cancer 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.P.; Solomon, D.; Rosenberg, S.A. Tumor-infiltrating lymphocytes from nonrenal urological malignancies. Cancer Immunol. Immunother. 1990, 30, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Yunger, S.; Bar El, A.; Zeltzer, L.; Fridman, E.; Raviv, G.; Laufer, M.; Schachter, J.; Markel, G.; Itzhaki, O.; Besser, M.J. Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy. Oncoimmunology 2019, 8, e1672494. [Google Scholar] [CrossRef]
- Yang, S.; Wei, W.; Zhao, Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int. J. Biol. Sci. 2020, 16, 1767–1773. [Google Scholar] [CrossRef]
- Yu, Y.D.; Kim, T.J. Chimeric antigen receptor-engineered T cell therapy for the management of patients with metastatic prostate cancer: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 640. [Google Scholar] [CrossRef]
| Treatment | Clinical Phase | Eligibility | Sample Size | Current Stage | Trial Identification |
|---|---|---|---|---|---|
| ICI combinations | |||||
| Nivolumab + Ipilimumab | II | mCRPC expressing AR-V7 | 15 | Active, not recruiting | NCT02601014 |
| Nivolumab + Ipilimumab | II | mCRPC Cohort 1 (pre-chemotherapy), cohort 2 (post-chemotherapy) | 90 | Active, recruiting | NCT02985957 |
| Durvalumab +/− Tremelimumab | II | mCRPC after prior NHT, and no more than one taxane | 52 | Active, not recruiting | NCT02788773 |
| ICI + chemotherapy | |||||
| Nivolumab + Docetaxel | II | Chemotherapy naïve mCRPC after prior NHT | 41 | Active, not recruiting | NCT03338790 |
| Nivolumab + Docetaxel | III | Chemotherapy naïve mCRPC after prior NHT | 984 | Active, recruiting | NCT04100018 |
| Pembrolizumab + Docetaxel | Ib/II | Chemotherapy naïve mCRPC after prior NHT | 104 | Active, recruiting | NCT02861573 |
| Pembrolizumab + Docetaxel | III | Chemotherapy naïve mCRPC after prior NHT | 1000 | Active, recruiting | NCT03834506 |
| ICI + Novel hormonal therapies | |||||
| Pembrolizumab + Enzalutamide | II | Chemotherapy naïve mCRPC after prior enzalutamide | 126 | Active, not recruiting | NCT02787005 |
| Pembrolizumab + Enzalutamide | Ib/II | Chemotherapy naïve mCRPC after prior abiraterone | 103 | Active, recruiting | NCT02861573 |
| Pembrolizumab + Enzalutamide | III | Chemotherapy naïve mCRPC | 1200 | Active, recruiting | NCT03834493 |
| Atezolizumab + Enzalutamide | III | mCRPC after prior abiraterone and docetaxel | 759 | Active, not recruiting | NCT03016312 |
| Nivolumab + Enzalutamida | II | mCRPC | 330 | Active, not recruiting | NCT03338790 |
| ICI + PARP inhibitors | |||||
| Pembrolizumab + Olaparib | Ib/II | mCRPC after prior docetaxel and ≤2 NHT | 84 | Active, recruiting | NCT02861573 |
| Pembrolizumab + Olaparib | III | mCRPC after prior docetaxel and 1 NHT | 780 | Active, not recruiting | NCT03834519 |
| Nivolumab + Rucaparib | II | mCRPC Cohort 1 (pre-chemotherapy), cohort 2 (post-chemotherapy) | 71 (Cohort 1) | Active, not recruiting | NCT03338790 |
| Durvalumab + Olaparib | II | mCRPC after prior NHT | 17 | Completed | NCT02484404 |
| ICI + vaccines | |||||
| Atezolizumab + Sipuleucel-T | Ib | Asymptomatic or minimally symptomatic progressive mCRPC | 37 | Completed | NCT03024216 |
| Ipilimumab + Sipuleucel-T | II | mCRPC | 50 | Completed | NCT01804465 |
| Nivolumab + PROSTVAC | I/II | mCRPC | 29 | Active, recruiting | NCT02933255 |
| Nivolumab + ChAdOx1-MVA 5T4 | II | mCRPC | 23 | Active, not recruiting | NCT03815942 |
| ICI + tyrosine kinase inhibitors | |||||
| Atezolizumab + Cabozantinib | Ib | mCRPC after 1 prior NHT | 132 | Active, recruiting | NCT03170960 |
| ICI + radionuclide | |||||
| Atezolizumab + Radium 223 | Ib | mCRPC | 44 | Completed | NCT02814669 |
| Pembrolizumab + Radium 223 | II | mCRPC | 45 | Active, not recruiting | NCT03093428 |
| Nivolumab + Radium 223 | II | mCRPC | 36 | Active, recruiting | NCT04109729 |
| Pembrolizumab + 177Lu-PSMA | Ib | mCRPC after prior abiraterone and docetaxel | 37 | Active, not recruiting | NCT03658447 |
| NCT Number | Title | CAR | Location | Sponsors |
|---|---|---|---|---|
| NCT04768608 | PD1 Integrated Anti-PSMA CART in Treating Patients with Castrate-Resist Prostate Cancer. | PD1 integrat -PSMA CART | Hangzhou, Zhejiang, China (1) | Zhejiang University |
| PSCA-CAR T Cells in Treating Patients With PSCA+ Metastatic Castration Resistant Prostate Cancer | PSCA CART | Duarte, CA, United States (1) | CHMC/NCI | |
| NCT04227275 | A Study of CART-PSMA-TGFβRDN in Patients With Metastatic Castration Resistant Prostate Cancer | PSMA-TGFβRdn CART | United States (9) | Tmunity Therapeutics |
| NCT04249947 | P-PSMA-101 CAR-T Cells in the Treatment of Subjects With Metastatic Castration-Resistant Prostate Cancer (mCRPC) | pPSMA CART | United States (7) | Poseida Therapeutics, Inc. |
| NCT05022849 | A Study of JNJ-75229414 for Metastatic Castration-resistant Prostate Cancer Participants | KLK2 CART | United States (7) | Janssen Research & Development |
| NCT02744287 | Safety and Activity Study of PSCA-Targeted CAR-T Cells (BPX-601) in Subjects With Selected Advanced Solid Tumors | PSCA CART | United States (7) | Bellicum Pharmaceuticals |
| NCT03013712 | Clinical Research of CAR T Cells Targeting EpCAM Positive Cancer (CARTEPC) | EpCAM CART | Chendu, China (1) | 1st Affiliated Hos Chengdu Med College |
| NCT04107142 | Haplo/Allogen NKG2DL-targeting Chimeric Antigen Receptor-grafted γδ T Cells for Relapsed or Refractory Solid Tumor | NKG2DL CART | Malaysia (1) | CytoMed Therapeutics Pte Ltd. |
| NCT04660929 | CAR-macrophages for the Treatment of HER2 Overexpressing Solid Tumors | HER2 CARm | United States (3) | Carisma Therap Inc |
| NCT04633148 | Dose-escalating trial with UniCAR02-T Cells and PSMA Target Module (TMpPSMA) in patients with Progressive Disease After Standard Systemic Therapy in Cancers with Positive PSMA Marker | UniCAR02-T pPSMA | Germany (4) | Cellex Patient Treatment/PHARMALOG |
| NCT04429451 | PSMA-specific CAR-T Cell Therapy | PSMA CART | Shenzhen, Guangdong, China (4) | Shenzhen Geno-Imm |
| NCT Number | Title | BiTE | Location (n. of Centers) | Sponsors |
|---|---|---|---|---|
| NCT04631601 | Safety and Efficacy of Therapy for Metastatic Castration-resistant Prostate Cancer (mCRPC) | Acapatamab (HLE anti-PSMA-CD3) | USA, Canada Europe, Australia (13) | Amgen |
| NCT03792841 | Safety, Tolerability, Pharmacokinetics, and Efficacy of Acapatamab in Subjects With mCRPC | Acapatamab (HLE anti-PSMA-CD3) | USA, Europe, Australia, Japan, Singapore (27) | Amgen |
| NCT01723475 | First-in-man Dose Escalation Study of BAY2010112 in … Prostate Cancer | MT110 (anti-PSMA-CD3) | Austria, Germany (5) | Bayer |
| NCT00635596 | Phase I Study of MT110 in Lung … Prostate and Ovarian Cancer (MT110-101) | MT110 (anti-PSMA-CD3) | Germany (4) | Amgen |
| NCT04221542 | Study of AMG 509 in Subjects With Metastatic Castration-Resistant Prostate Cancer | AMG 509 (anti-STEAP1-CD3) | USA, Canada, East Asia, Australia (17) | Amgen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Campos, F.; Gajate, P.; Romero-Laorden, N.; Zafra-Martín, J.; Juan, M.; Hernando Polo, S.; Conde Moreno, A.; Couñago, F. Immunotherapy in Advanced Prostate Cancer: Current Knowledge and Future Directions. Biomedicines 2022, 10, 537. https://doi.org/10.3390/biomedicines10030537
López-Campos F, Gajate P, Romero-Laorden N, Zafra-Martín J, Juan M, Hernando Polo S, Conde Moreno A, Couñago F. Immunotherapy in Advanced Prostate Cancer: Current Knowledge and Future Directions. Biomedicines. 2022; 10(3):537. https://doi.org/10.3390/biomedicines10030537
Chicago/Turabian StyleLópez-Campos, Fernando, Pablo Gajate, Nuria Romero-Laorden, Juan Zafra-Martín, Manel Juan, Susana Hernando Polo, Antonio Conde Moreno, and Felipe Couñago. 2022. "Immunotherapy in Advanced Prostate Cancer: Current Knowledge and Future Directions" Biomedicines 10, no. 3: 537. https://doi.org/10.3390/biomedicines10030537
APA StyleLópez-Campos, F., Gajate, P., Romero-Laorden, N., Zafra-Martín, J., Juan, M., Hernando Polo, S., Conde Moreno, A., & Couñago, F. (2022). Immunotherapy in Advanced Prostate Cancer: Current Knowledge and Future Directions. Biomedicines, 10(3), 537. https://doi.org/10.3390/biomedicines10030537







