In Vivo Metabolic Analysis of the Anticancer Effects of Plasma-Activated Saline in Three Tumor Animal Models
Abstract
:1. Introduction
2. Materials and Methods
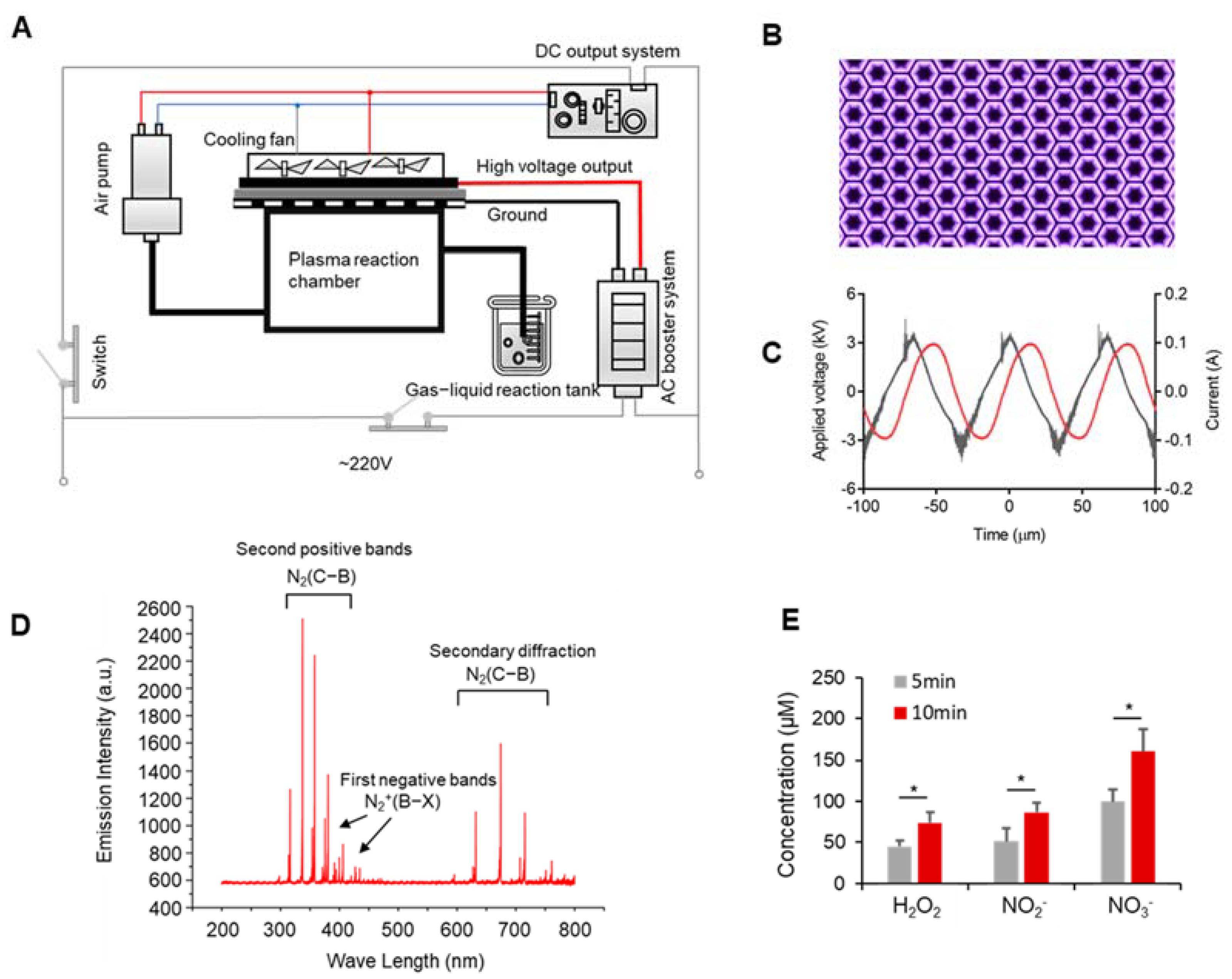
2.1. Experimental Device
2.2. Cell Culture
2.3. Cell Viability Assay
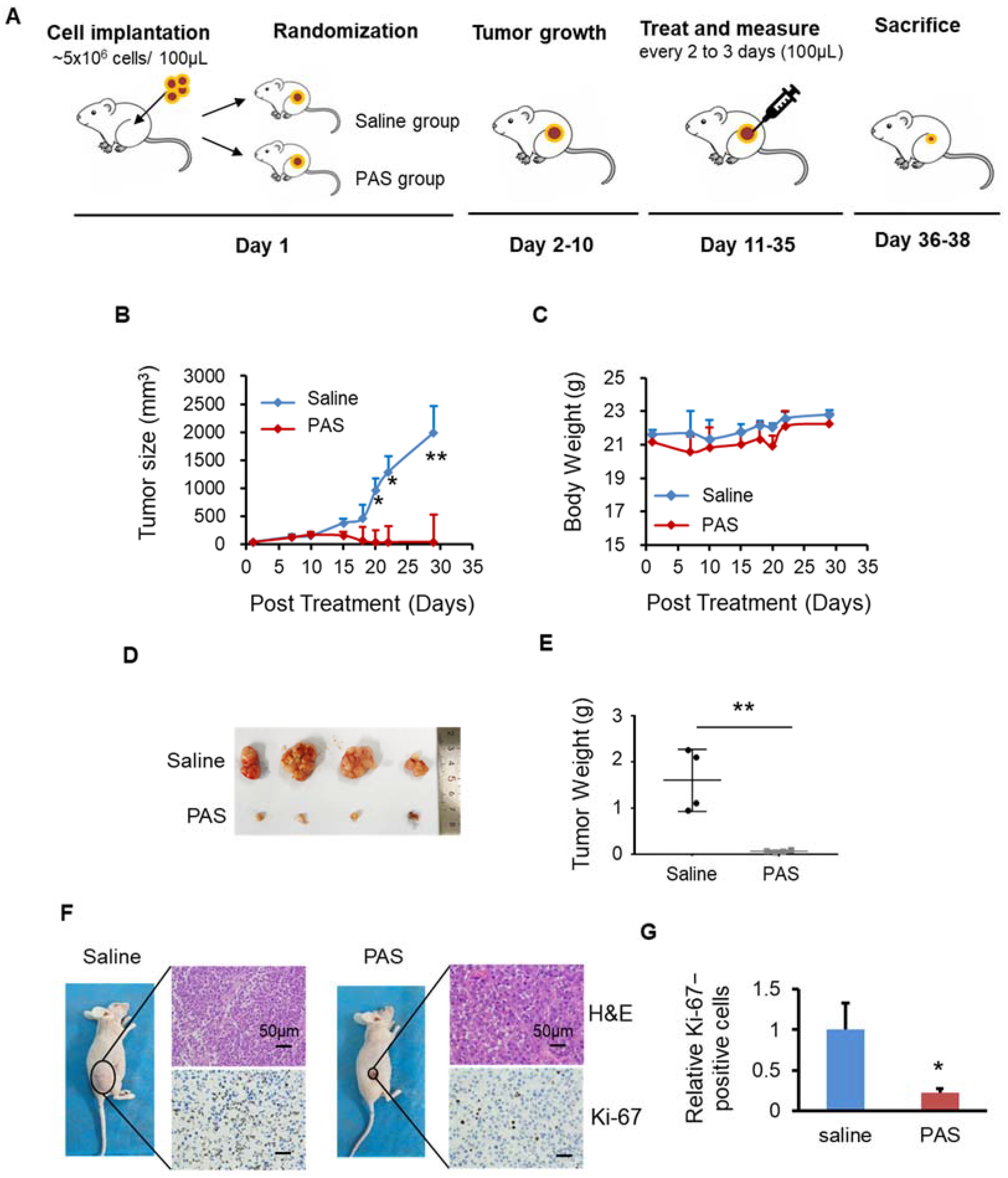
2.4. Animal Study
2.5. Immunohistochemical Staining
2.6. UHPLC-QTOF-MS Analysis
2.7. Emission Spectrum Detection
2.8. Statistical Analysis
3. Results
3.1. Discharge Plasma and Aqueous Reactive Species Generation
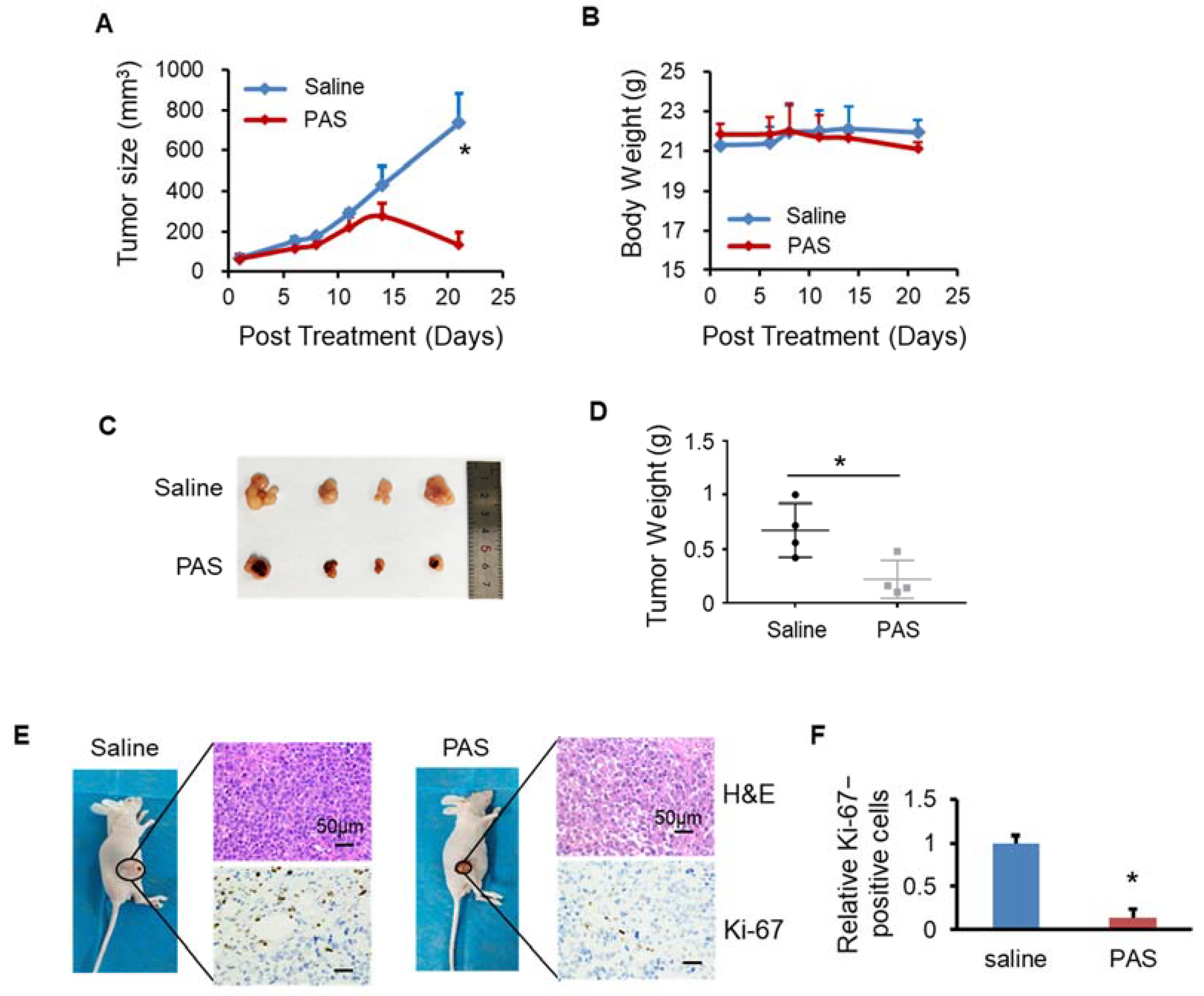
3.2. PAS Inhibited Melanoma Cell Growth In Vivo
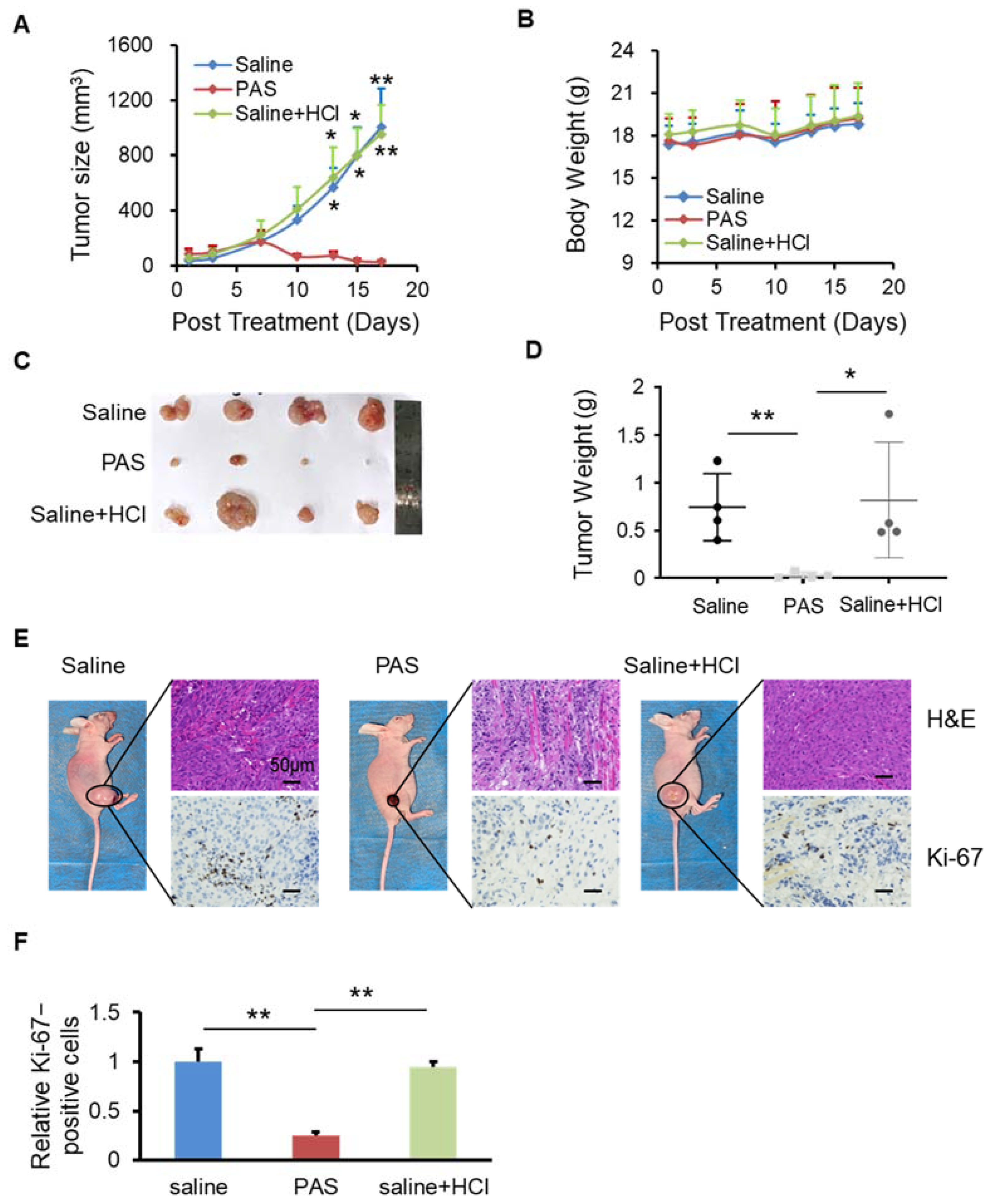
3.3. PAS Inhibited Cancer Cell Growth In Vivo in a pH-Independent Manner
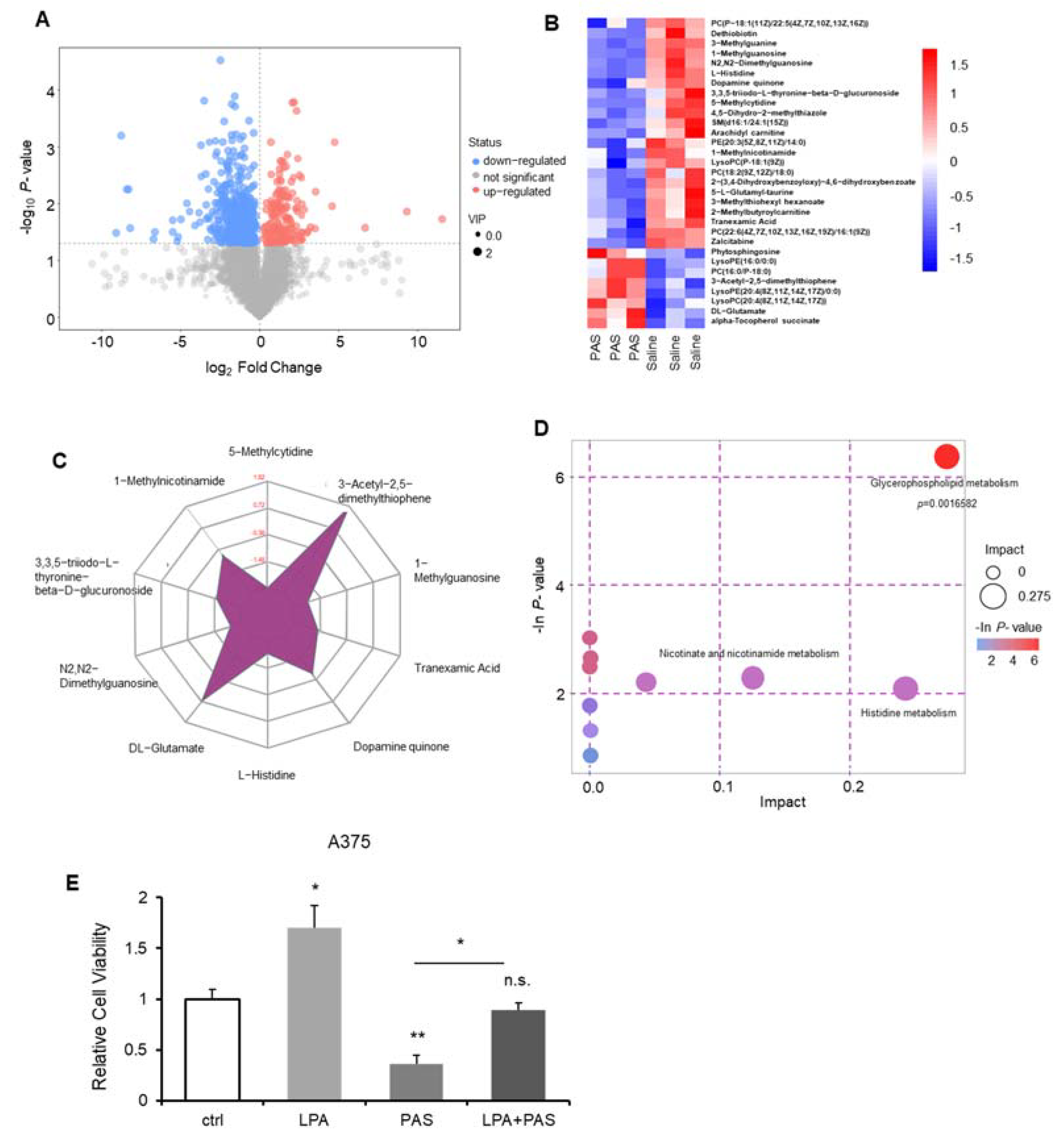
3.4. The Glycerophospholipid Metabolic Pathway Was the Most Susceptible Metabolic Pathway of PAS Therapy In Vivo
3.5. Biological Safety of PAS Injection
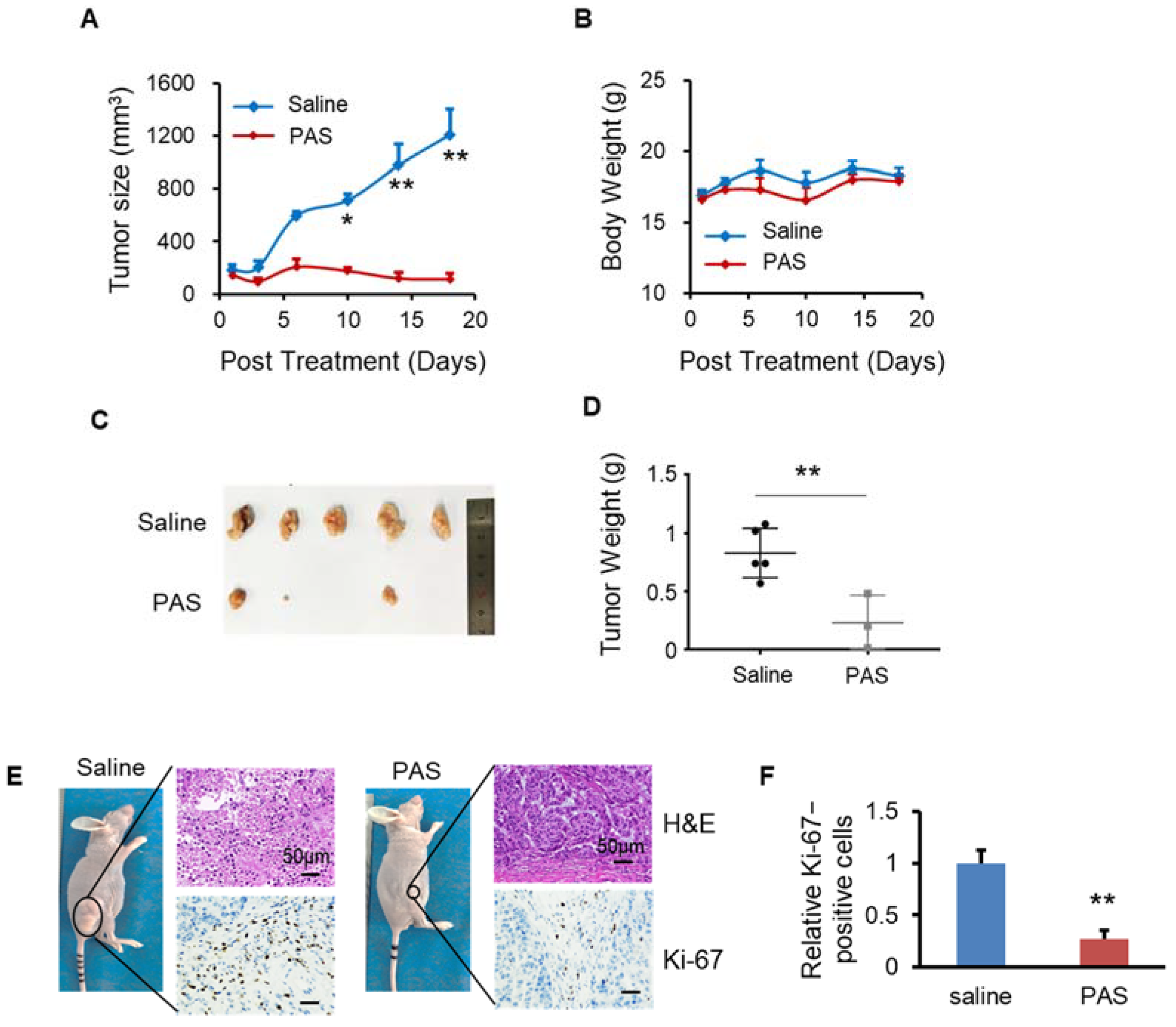
3.6. PAS Suppressed Oral Tongue Squamous-Cell Carcinoma (OTSCC) Cell Growth In Vivo
3.7. PAS Inhibited Non-Small-Cell Lung Cancer (NSCLC) Cell Growth In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.M.; Frame, F.M.; Arya, M.; Maitland, N.J.; O’Connell, D. Low temperature plasmas as emerging cancer therapeutics: The state of play and thoughts for the future. Tumor Biol. 2016, 37, 7021–7031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baránková, H.; Bardos, L. Cold Atmospheric Plasma. Plasma Processes Polym. 2008, 5, 299. [Google Scholar] [CrossRef]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.W.; Ostrikov, K. The Emerging Role of Gas Plasma in Oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.D.; Von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Cotrol. Fusion 2016, 59, 014031. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of plasma treatment on pancreatic cancer cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Peng, Y.; Utsumi, F.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, K.; Kajiyama, H. Novel Intraperitoneal Treatment With Non-Thermal Plasma-Activated Medium Inhibits Metastatic Potential of Ovarian Cancer Cells. Sci. Rep. 2017, 7, 6085. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yamada, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Fujii, T.; et al. Intraperitoneal Administration of Plasma-Activated Medium: Proposal of a Novel Treatment Option for Peritoneal Metastasis From Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yamada, S.; Takeda, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Kodera, Y. Effect of Plasma-Activated Lactated Ringer’s Solution on Pancreatic Cancer Cells In Vitro and In Vivo. Ann. Surg. Oncol. 2017, 25, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.-P.; Heidecke, C.-D.; Von Bernstorff, W.; Von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; Van Der Linde, J.; Metelmann, H.-R.; Heidecke, C.-D.; Partecke, L.-I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Liu, J.R.; Wu, Y.M.; Xu, G.M.; Gao, L.G.; Ma, Y.; Shi, X.M.; Zhang, G.J. Low-temperature plasma induced melanoma apoptosis by triggering a p53/PIGs/caspase-dependent pathway in vivo and in vitro. J. Phys. D: Appl. Phys. 2019, 52, 315204. [Google Scholar] [CrossRef]
- Lee, C.B.; Seo, I.H.; Chae, M.-W.; Park, J.W.; Choi, E.H.; Uhm, H.S.; Baik, K.Y. Anticancer Activity of Liquid Treated with Microwave Plasma-Generated Gas through Macrophage Activation. Oxidative Med. Cell. Longev. 2020, 2020, 2946820. [Google Scholar] [CrossRef]
- Maheshwari, K.; Turan, A.; Makarova, N.; Ma, C.; Esa, W.A.S.; Ruetzler, K.; Barsoum, S.; Kuhel, A.G.; Ritchey, M.R.; Higuera-Rueda, C.; et al. Saline versus Lactated Ringer’s Solution: The Saline or Lactated Ringer’s (SOLAR) Trial. Anesthesiology 2020, 132, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Clemen, R.; Nießner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical Gas Plasma Jet Technology Targets Murine Melanoma in an Immunogenic Fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Granja, S.; Tavares-Valente, D.; Queiros, O.; Baltazar, F. Value of pH regulators in the diagnosis, prognosis and treatment of cancer. Semin. Cancer Biol. 2017, 43, 17–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Wang, S.; Li, B.; Qi, M.; Feng, R.; Li, Q.; Zhang, H.; Chen, H.; Kong, M.G. Effects of Plasma-Activated Water on Skin Wound Healing in Mice. Microorganisms 2020, 8, 1091. [Google Scholar] [CrossRef]
- Dolce, V.; Rita Cappello, A.; Lappano, R.; Maggiolini, M. Glycerophospholipid Synthesis as a Novel Drug Target Against Cancer. Curr. Mol. Pharmacol. 2011, 4, 167–175. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Suh, M.J.; Lee, H.Y.; Lee, H.J.; Choi, E.H.; Moon, I.S.; Song, K. Anti-tumor effects of cold atmospheric pressure plasma on vestibular schwannoma demonstrate its feasibility as an intra-operative adjuvant treatment. Free. Radic. Biol. Med. 2018, 115, 43–56. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Brullé, L.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; et al. Plasma Medicine: Potential of cold atmospheric plasmas in cancer treatment. Bull. Group Int. Rec. Sci. Stomatol. Odontol. 2011, 61–69. [Google Scholar]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward understanding the selective anticancer ca-pacity of cold atmospheric plasma—A model based on aquaporins. Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef]
- Babington, P.; Rajjoub, K.; Canady, J.; Siu, A.; Keidar, M.; Sherman, J.H. Use of cold atmospheric plasma in the treatment of cancer. Biointerphases 2015, 10, 029403. [Google Scholar] [CrossRef] [Green Version]
- Pavri, S.N.; Clune, J.; Ariyan, S.; Narayan, D. Malignant Melanoma: Beyond the Basics. Plast. Reconstr. Surg. 2016, 138, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Mirili, C.; Yilmaz, A.; Demirkan, S.; Bilici, M.; Tekin, S.B. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int. J. Clin. Oncol. 2019, 24, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Chen, Z.; Wen, D.; Wang, Z.; Li, H.; Zeng, Y.; Dotti, G.; Wirz, R.E.; Gu, Z. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3687–3692. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.-K. Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Toyokuni, S.; Kajiyama, H.; Kikkawa, F.; Hori, M. Molecular mechanisms of non-thermal plasma-induced effects in cancer cells. Biol. Chem. 2018, 400, 87–91. [Google Scholar] [CrossRef]
- Yusupov, M.; Razzokov, J.; Cordeiro, R.M.; Bogaerts, A. Transport of Reactive Oxygen and Nitrogen Species Across Aquaporin: A Molecular Level Picture. Oxid. Med. Cell. Longev. 2019, 17, 2930504. [Google Scholar] [CrossRef] [Green Version]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [Green Version]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Tan, M.; Cai, Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015, 356, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Pyun, W.Y.; Park, H.H. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Metabolic Regulation of Apoptosis in Cancer. Int. Rev. Cell Mol. Biol. 2016, 327, 43–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eibl, H. Synthesis of glycerophospholipids. Chem. Phys. Lipids 1980, 26, 405–429. [Google Scholar] [CrossRef]
- Henderson, F.; Johnston, H.R.; Badrock, A.P.; Jones, E.A.; Forster, D.; Nagaraju, R.T.; Hurlstone, A. Enhanced Fatty Acid Scavenging and Glycerophospholipid Metabolism Ac-company Melanocyte Neoplasia Progression in Zebrafish. Cancer Res. 2019, 79, 2136–2151. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.L.; Zheng, J.Y.; Cheng, X.Q.; Xin, G.Z.; Wang, S.L.; Xie, T. Chemical Markers’ Knockout Coupled With UHPLC-HRMS-based Metabolomics Reveals Anti-Cancer Integration Effects of the Curcuminoids of Turmeric (Curcuma Longa L.) on Lung Cancer Cell Line. J. Pharm. Biomed. Anal. 2019, 175, 112738. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Y.; Ning, N.; Cui, Q.; Liu, Z.; Wang, X.; Liu, D.; Chen, H.; Kong, M.G. Alteration of metabolite profiling by cold atmospheric plasma treatment in human myeloma cells. Cancer Cell Int. 2018, 18, 42. [Google Scholar] [CrossRef]
- Xu, D.; Ning, N.; Xu, Y.; Xia, W.; Liu, D.; Chen, H.; Kong, M.G. Effect of He Plasma Jet Versus Surface Plasma on the Metabolites of Acute Myeloid Leukemia Cells. Front. Oncol. 2021, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Lefaki, M.; Papaevgeniou, N.; Chondrogianni, N. Redox regulation of proteasome function. Redox Biol. 2017, 13, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Shadyro, O.; Lisovskaya, A. ROS-induced lipid transformations without oxygen participation. Chem. Phys. Lipids 2019, 221, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Shadyro, O.; Samovich, S.; Edimecheva, I. Free-radical and biochemical reactions involving polar part of glycerophospholipids. Free Radic. Biol. Med. 2019, 144, 6–15. [Google Scholar] [CrossRef]
- Kulis-Horn, R.K.; Persicke, M.; Kalinowski, J. Histidine biosynthesis, its regulation and biotechnological application in Coryne-bacterium glutamicum. Microb. Biotechnol. 2014, 7, 5–25. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef] [Green Version]
- Frezza, C. Histidine metabolism boosts cancer therapy. Nature 2018, 559, 484–485. [Google Scholar] [CrossRef]
- Dickinson, A.; Saraswat, M.; Joenväärä, S.; Agarwal, R.; Jyllikoski, D.; Wilkman, T.; Mäkitie, A.; Silén, S. Mass spectrometry-based lipidomics of oral squamous cell carcinoma tissue reveals aberrant cholesterol and glycerophospholipid metabolism-A Pilot study. Transl. Oncol. 2020, 13, 100807. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Li, Y.; Hou, Y.; Sun, F.; Zhou, S.; Li, C.; Zhang, B. Plasma lipid profiling and diagnostic biomarkers for oral squamous cell carcinoma. Oncotarget 2017, 8, 92324–92332. [Google Scholar] [CrossRef] [Green Version]
- Hoang, C.D.; Wu, M.; Xu, Y.; Merrit, R.; Whyte, R.; Shrager, J.; Peltz, G. Abstract 5574: Altered glycerophospholipid metabolism in non-small cell lung carcinoma. Cancer Chem. 2010, 70, 5574. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Zhan, X.; Wang, B.; Wu, J.; Zhou, A. A UPLC-Q-TOF/MS-based plasma metabolomics approach reveals the mechanism of Compound Kushen Injection-based intervention against non-small cell lung cancer in Lewis tumor-bearing mice. Phytomedi-Cine 2020, 76, 153259. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Flight, R.M.; Moseley, H.N. Untargeted Lipidomics of Non-Small Cell Lung Carcinoma Demonstrates Differen-tially Abundant Lipid Classes in Cancer vs. Non-Cancer Tissue. Metabolites 2021, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Shi, L.; Ito, F.; Ohara, Y.; Motooka, Y.; Tanaka, H.; Mizuno, M.; Hori, M.; Hirayama, T.; Hibi, H.; et al. Non-thermal plasma specifically kills oral squamous cell carcinoma cells in a catalytic Fe(II)-dependent manner. J. Clin. Biochem. Nutr. 2019, 65, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal Atmospheric Pressure Plasma Induces Apoptosis in Oral Cavity Squa-mous Cell Carcinoma: Involvement of DNA-damage-triggering sub-G1 Arrest via the ATM/p53 Pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Song, I.S.; Li, Y.; Kang, M.H.; Ryu, J.J.; Choi, E.H. Novel therapy for oral squamous cell carcinoma using non-thermal atmospheric pressure bio-compatible plasma activated water. Cancer Res. 2018, 78 (Suppl. S13), 2832. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, H.; Cheng, C.; Shen, J.; Bao, L.Z.; Han, W. Contribution of hydrogen peroxide to non-thermal atmospheric pressure plasma induced A549 lung cancer cell damage. Plasma Process Polym. 2017, 14, 1600162. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Ding, D.; Chen, Z.; Wang, Y.; Wang, S.; Zhang, W. Cold Atmospheric Plasma and Iron Oxide-Based Magnetic Nanoparticles for Synergetic Lung Cancer Therapy. Free Radic. Biol. Med. 2019, 130, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Joh, H.M.; Chung, T.H.; Chung, J.W. Anticancer Effects of Plasma-Activated Medium Produced by a Microwave-Excited At-mospheric Pressure Argon Plasma Jet. Oxid. Med. Cell. Longev. 2020, 30, 4205640. [Google Scholar] [CrossRef]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.-I.; Hori, M. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial–nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef]
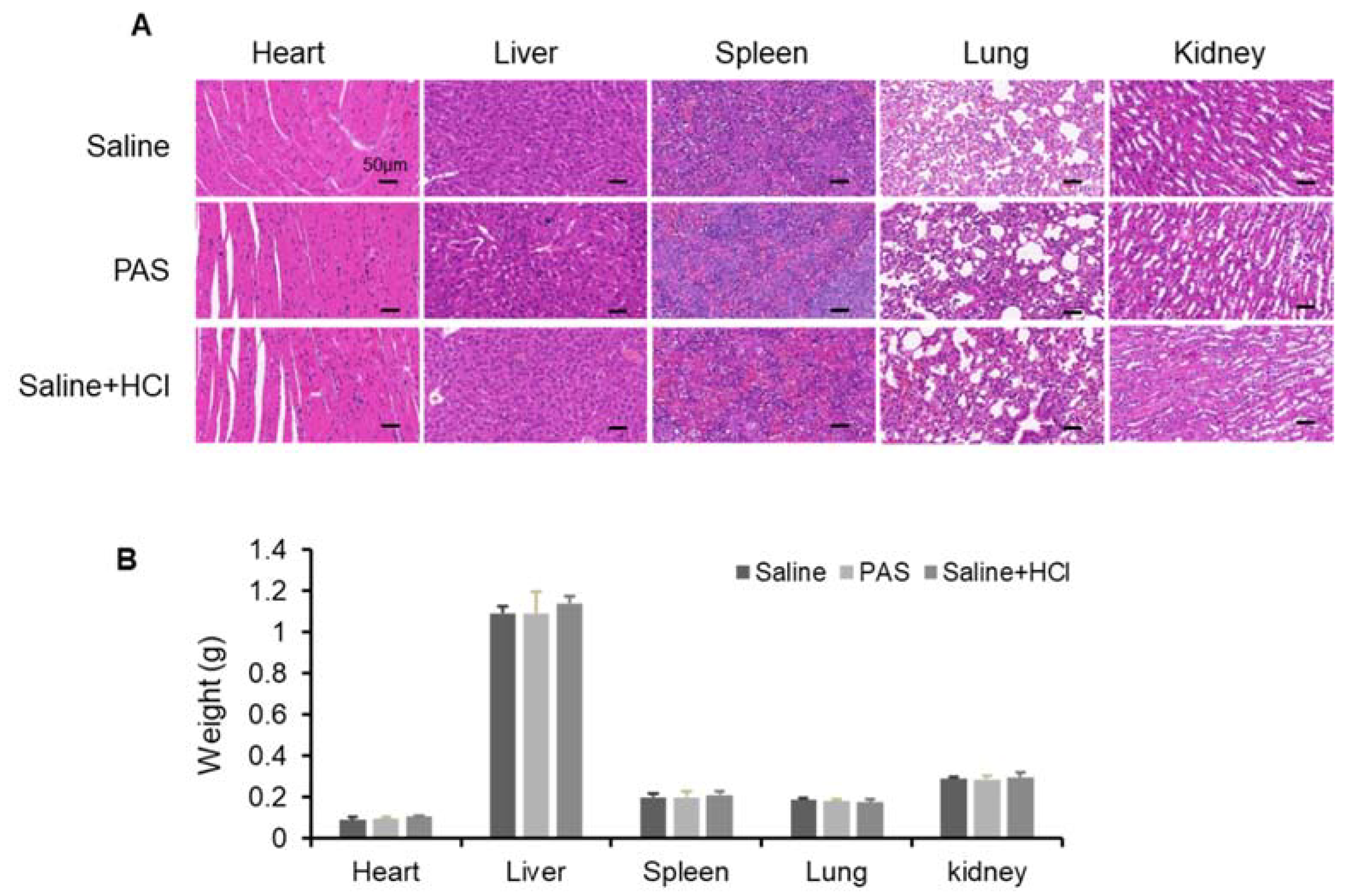
| ALT (U/L) | AST (U/L) | AKP (U/L) | |
|---|---|---|---|
| Saline | 101.44 ± 14.86 | 399.35 ± 53.87 | 95.01 ± 13.77 |
| PAS | 101.23 ± 5.93 | 403.62 ± 49.71 | 98.78 ± 7.59 |
| Saline + HCl | 99.58 ± 15.93 | 395.21 ± 68.85 | 95.04 ± 17.58 |
| BUN (mg/dL) | UA (μmol/L) | CR (μmol/L) | |
|---|---|---|---|
| Saline | 48.69 ± 5.66 | 244.71 ± 22.05 | 33.66 ± 4.42 |
| PAS | 49.37 ± 4.53 | 233.42 ± 20.97 | 30.29 ± 3.67 |
| Saline + HCl | 46.39 ± 6.72 | 235.07 ± 26.35 | 31.96 ± 4.12 |
| LDH (U/L) | LDH1 (U/L) | CK (U/L) | |
|---|---|---|---|
| Saline | 1438.18 ± 73.86 | 86.43 ± 12.01 | 2697.21 ± 155.35 |
| PAS | 1410.69 ± 59.76 | 84.63 ± 6.37 | 2538.49 ± 133.18 |
| Saline + HCl | 1470.50 ± 109.24 | 82.19 ± 16.52 | 2579.21 ± 157.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.; Xu, D.; Wang, S.; Li, B.; Peng, S.; Li, Q.; Zhang, H.; Fan, R.; Chen, H.; Kong, M.G. In Vivo Metabolic Analysis of the Anticancer Effects of Plasma-Activated Saline in Three Tumor Animal Models. Biomedicines 2022, 10, 528. https://doi.org/10.3390/biomedicines10030528
Qi M, Xu D, Wang S, Li B, Peng S, Li Q, Zhang H, Fan R, Chen H, Kong MG. In Vivo Metabolic Analysis of the Anticancer Effects of Plasma-Activated Saline in Three Tumor Animal Models. Biomedicines. 2022; 10(3):528. https://doi.org/10.3390/biomedicines10030528
Chicago/Turabian StyleQi, Miao, Dehui Xu, Shuai Wang, Bing Li, Sansan Peng, Qiaosong Li, Hao Zhang, Runze Fan, Hailan Chen, and Michael G. Kong. 2022. "In Vivo Metabolic Analysis of the Anticancer Effects of Plasma-Activated Saline in Three Tumor Animal Models" Biomedicines 10, no. 3: 528. https://doi.org/10.3390/biomedicines10030528
APA StyleQi, M., Xu, D., Wang, S., Li, B., Peng, S., Li, Q., Zhang, H., Fan, R., Chen, H., & Kong, M. G. (2022). In Vivo Metabolic Analysis of the Anticancer Effects of Plasma-Activated Saline in Three Tumor Animal Models. Biomedicines, 10(3), 528. https://doi.org/10.3390/biomedicines10030528







