Characterisation of Severe Traumatic Brain Injury Severity from Fresh Cerebral Biopsy of Living Patients: An Immunohistochemical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Tissue Collection and Processing
2.3. Immunohistochemistry
2.4. Data Analysis and Statistics
3. Results
3.1. Demographics of Patients
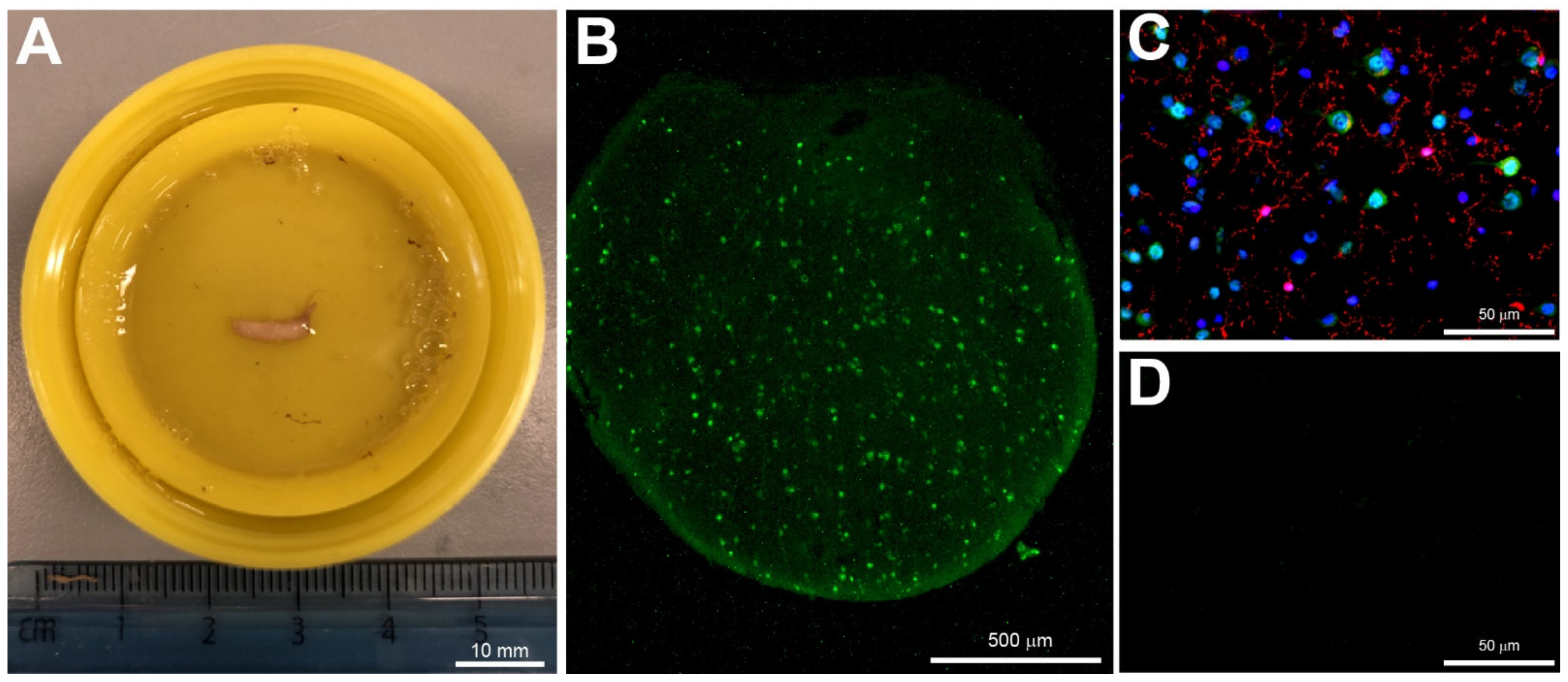
3.2. Biopsy Sampling
3.3. Immunohistochemistry
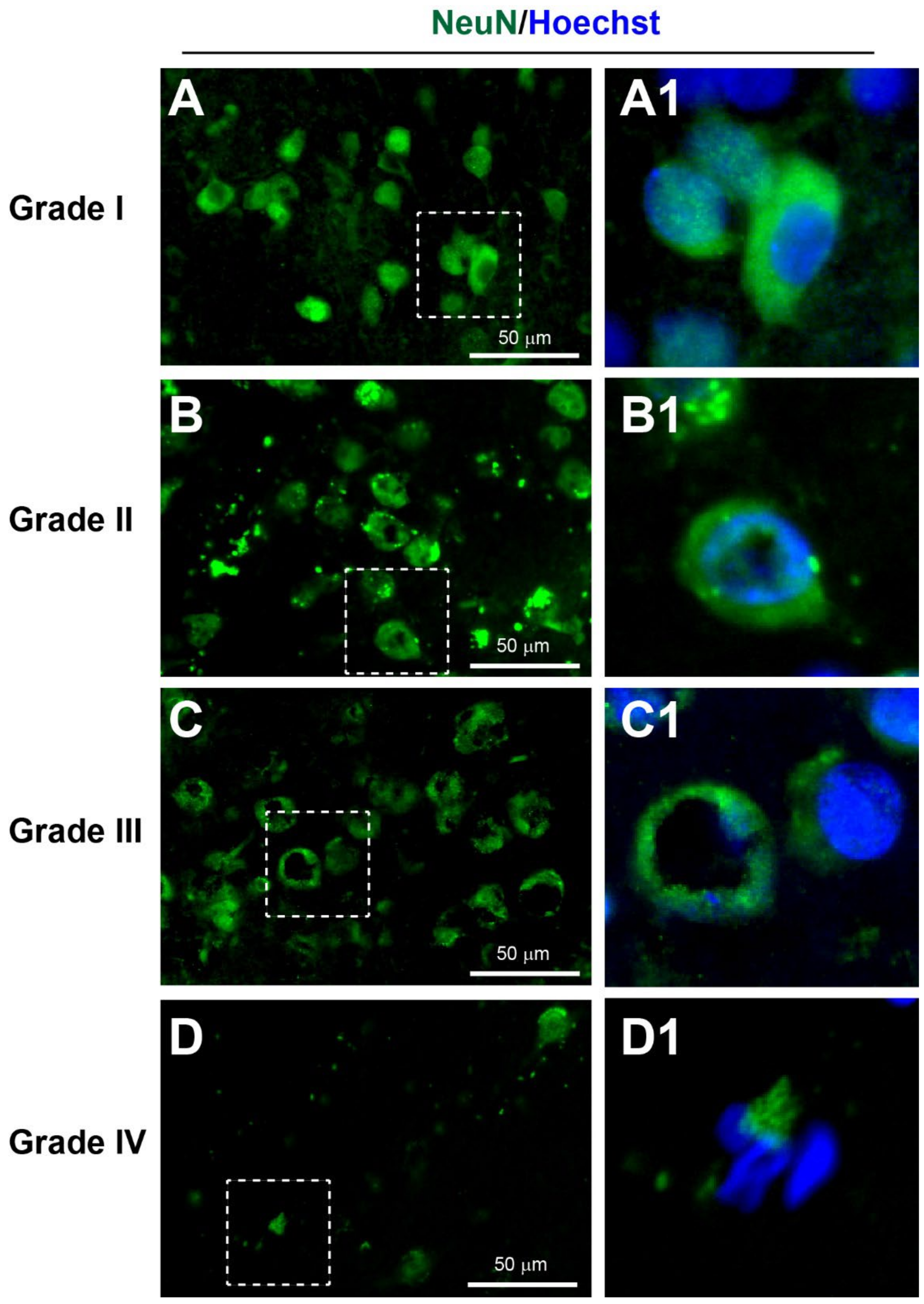
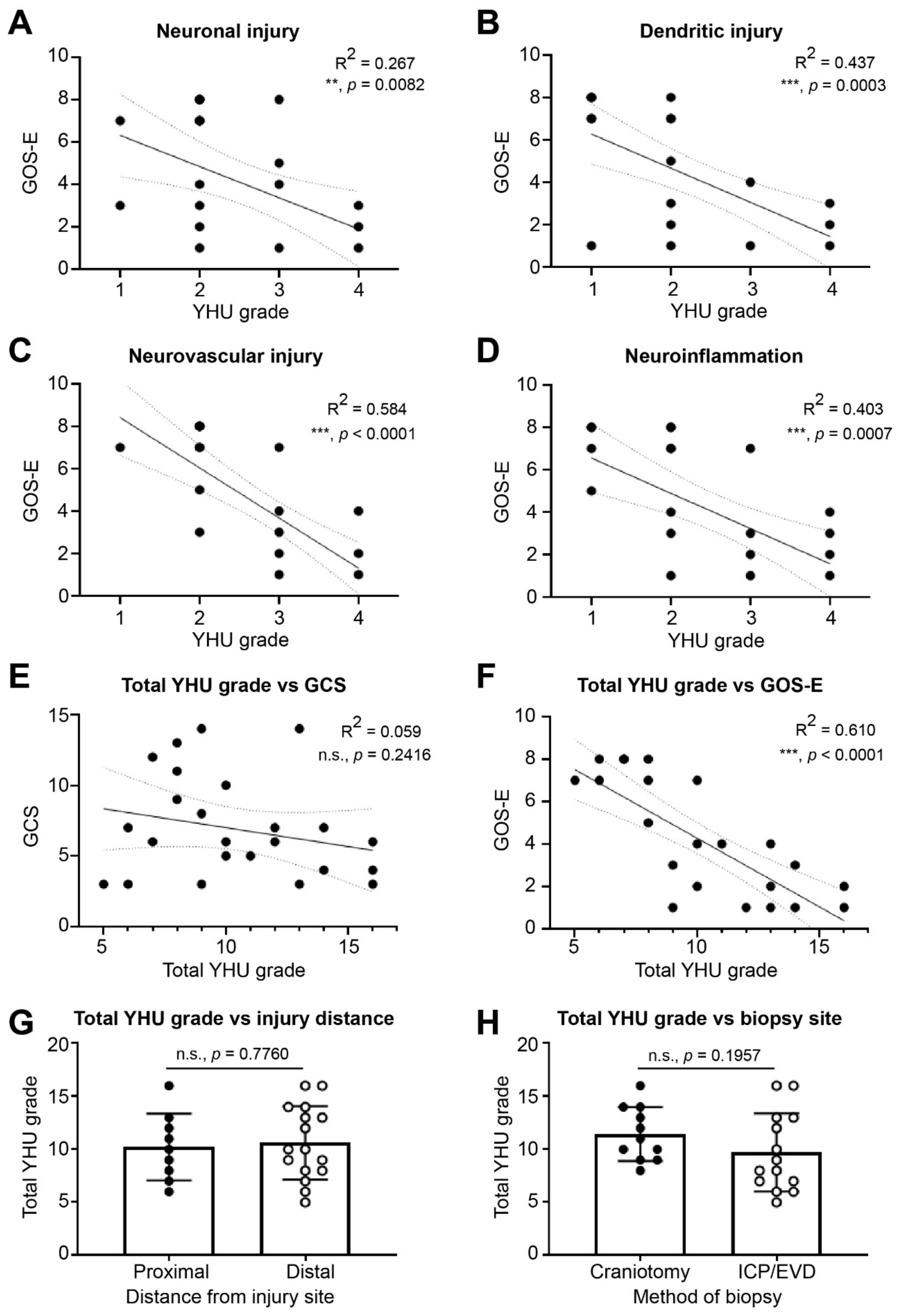
3.3.1. Neuronal Injury
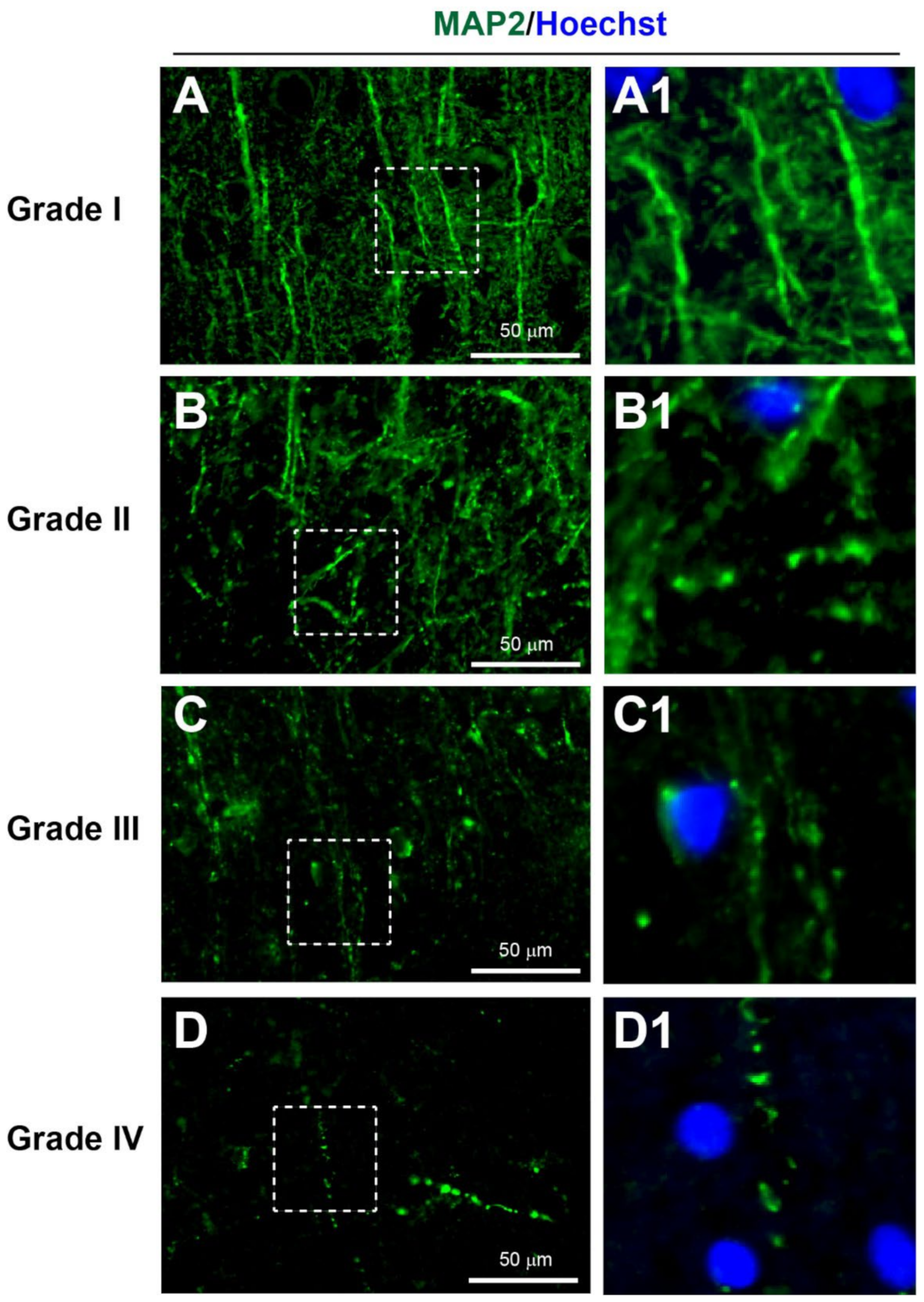
3.3.2. Dendritic Injury
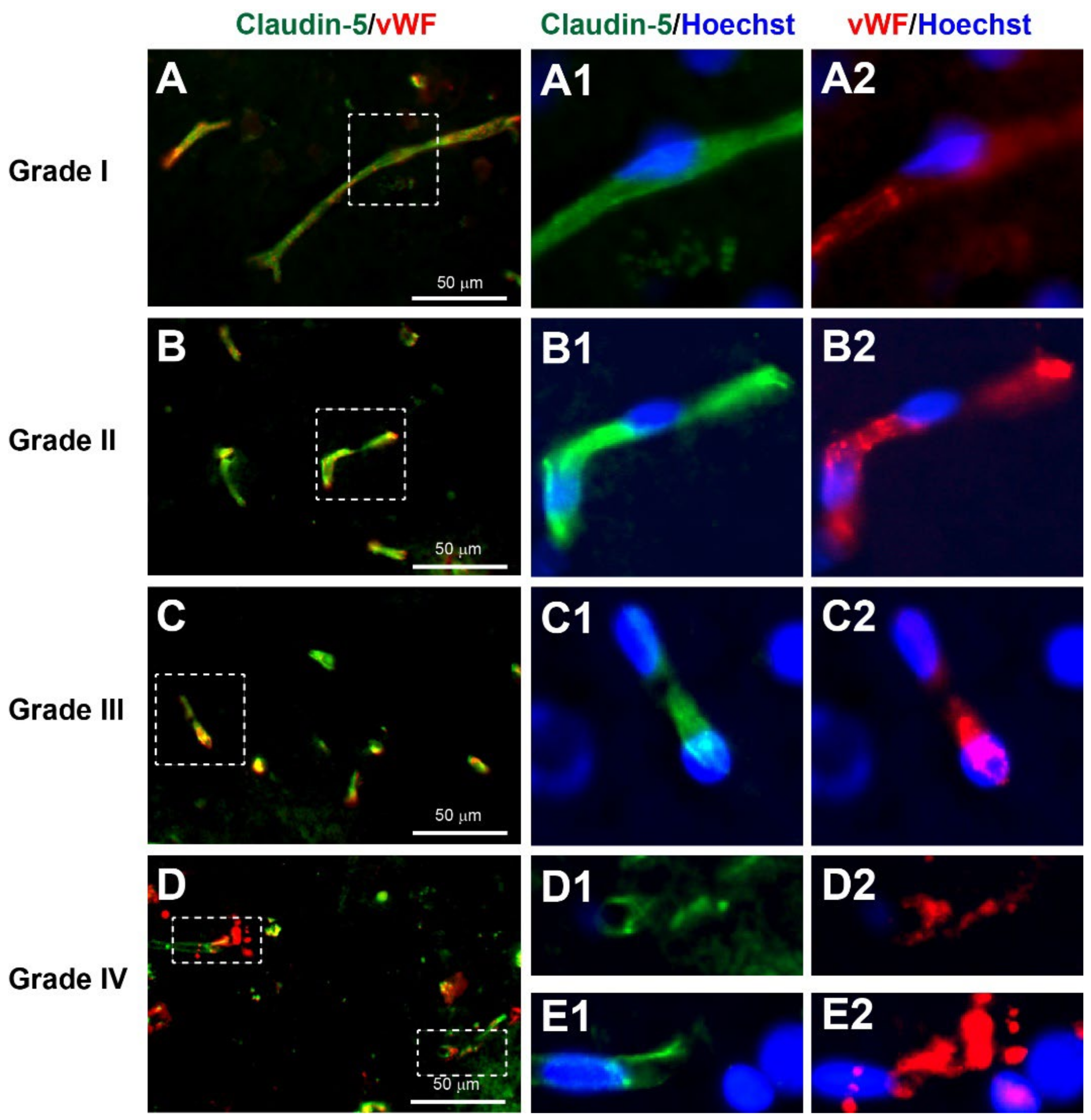
3.3.3. Neurovascular Injury
3.3.4. Neuroinflammation
3.4. Diagnosis and Prognosis
3.5. Further Characterisation of the Cellular and Molecular Expressions in the Brain Biopsies
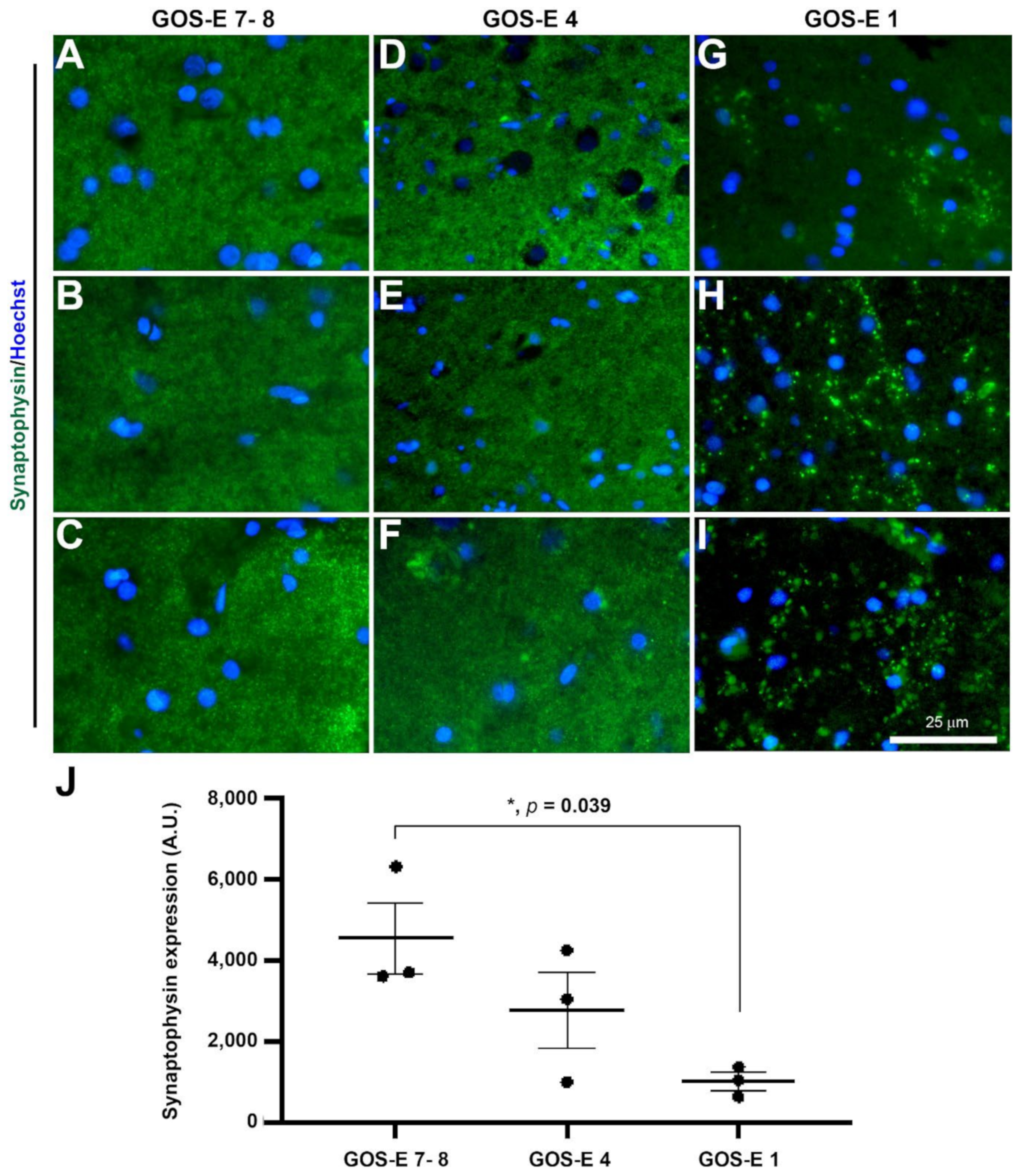
3.5.1. Reduced Synaptophysin Immunopositive Synapses in Severe TBI with Unfavourable Outcome
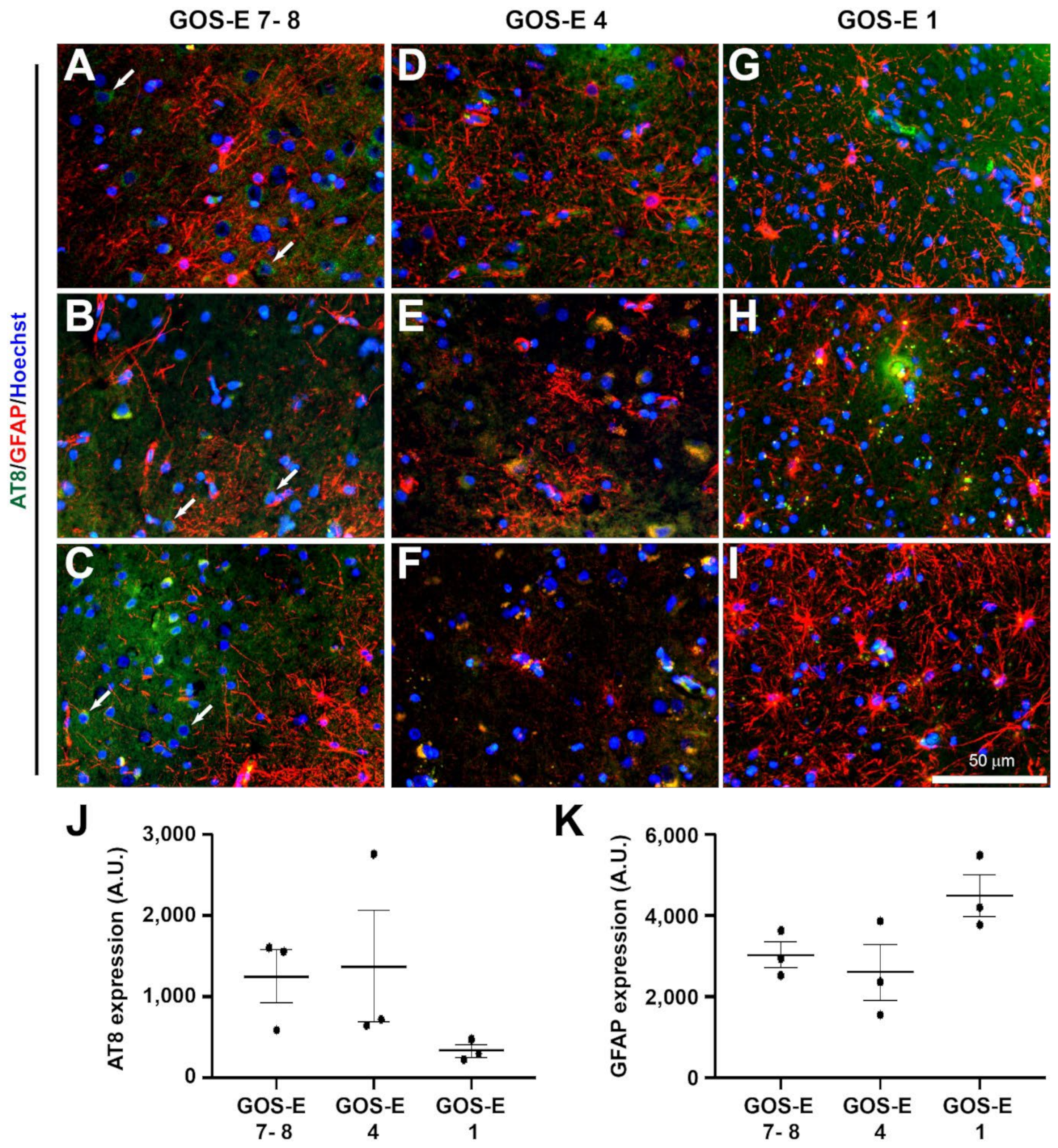
3.5.2. No Change in Tau Phosphorylation in Unfavourable Outcome after Severe TBI
3.5.3. No Change in Astrocytic Activation after TBI between Functional Outcomes
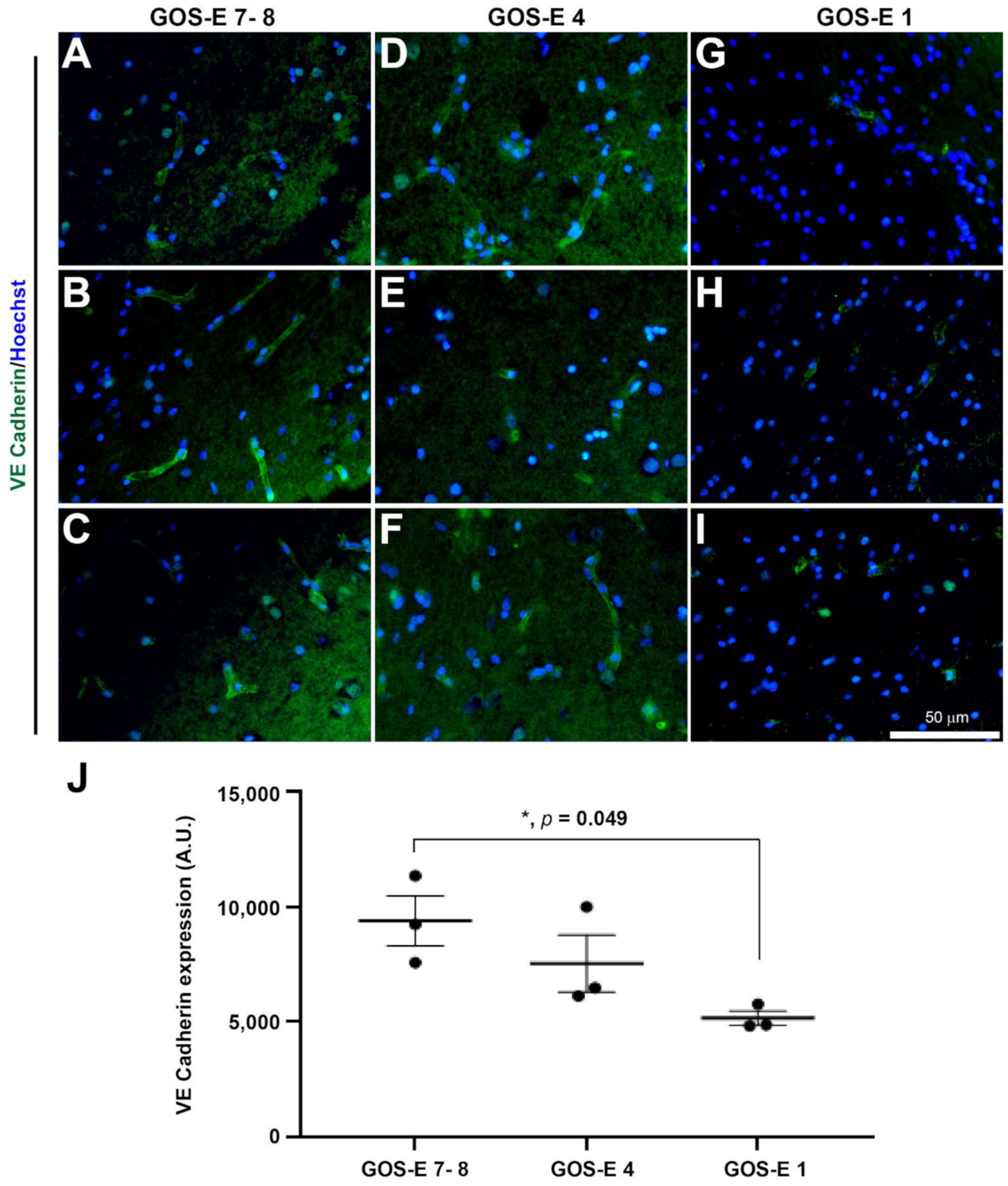
3.5.4. Reduction in Endothelial Adherens Junctions in Unfavourable Outcome after Severe TBI
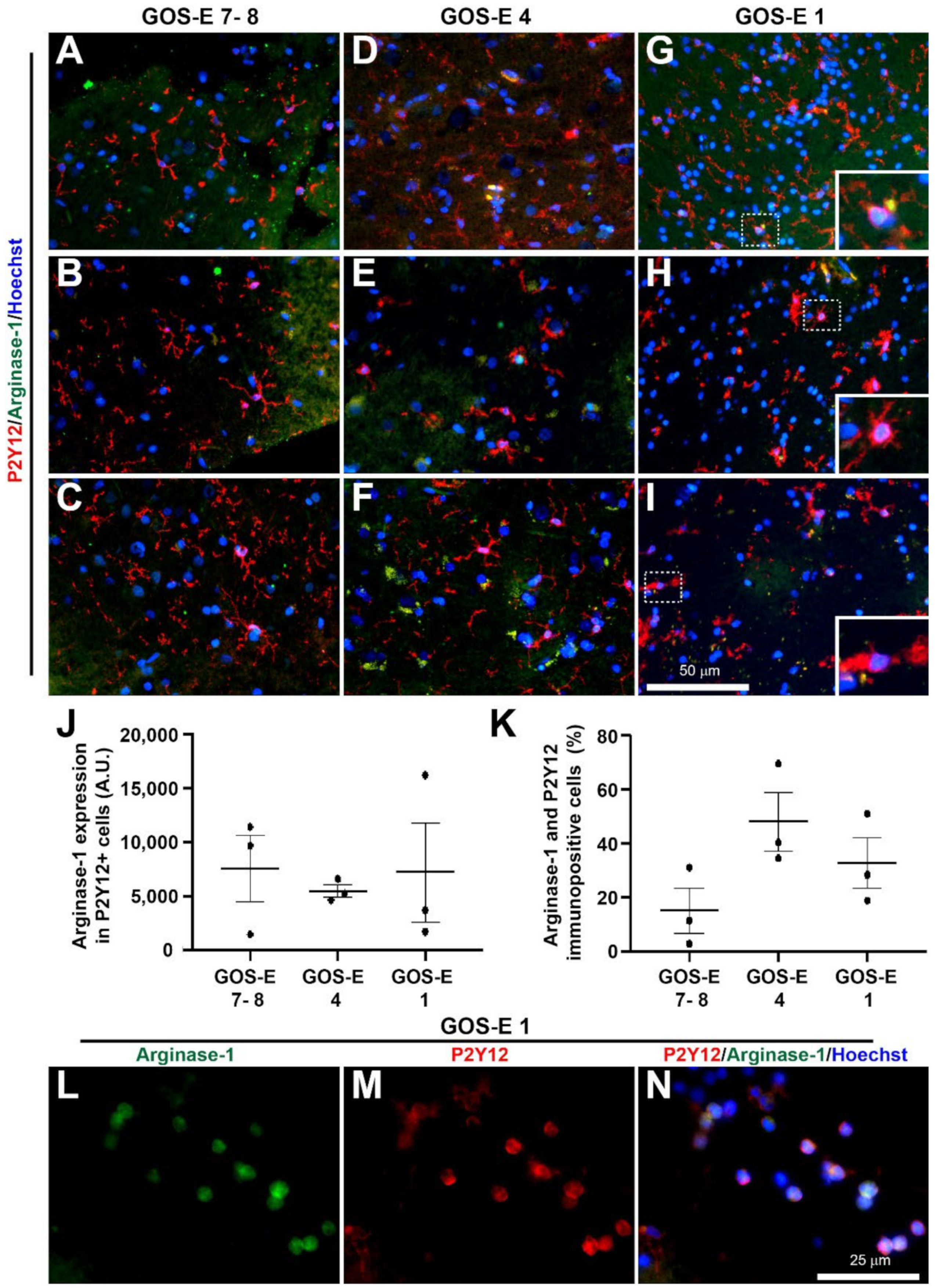
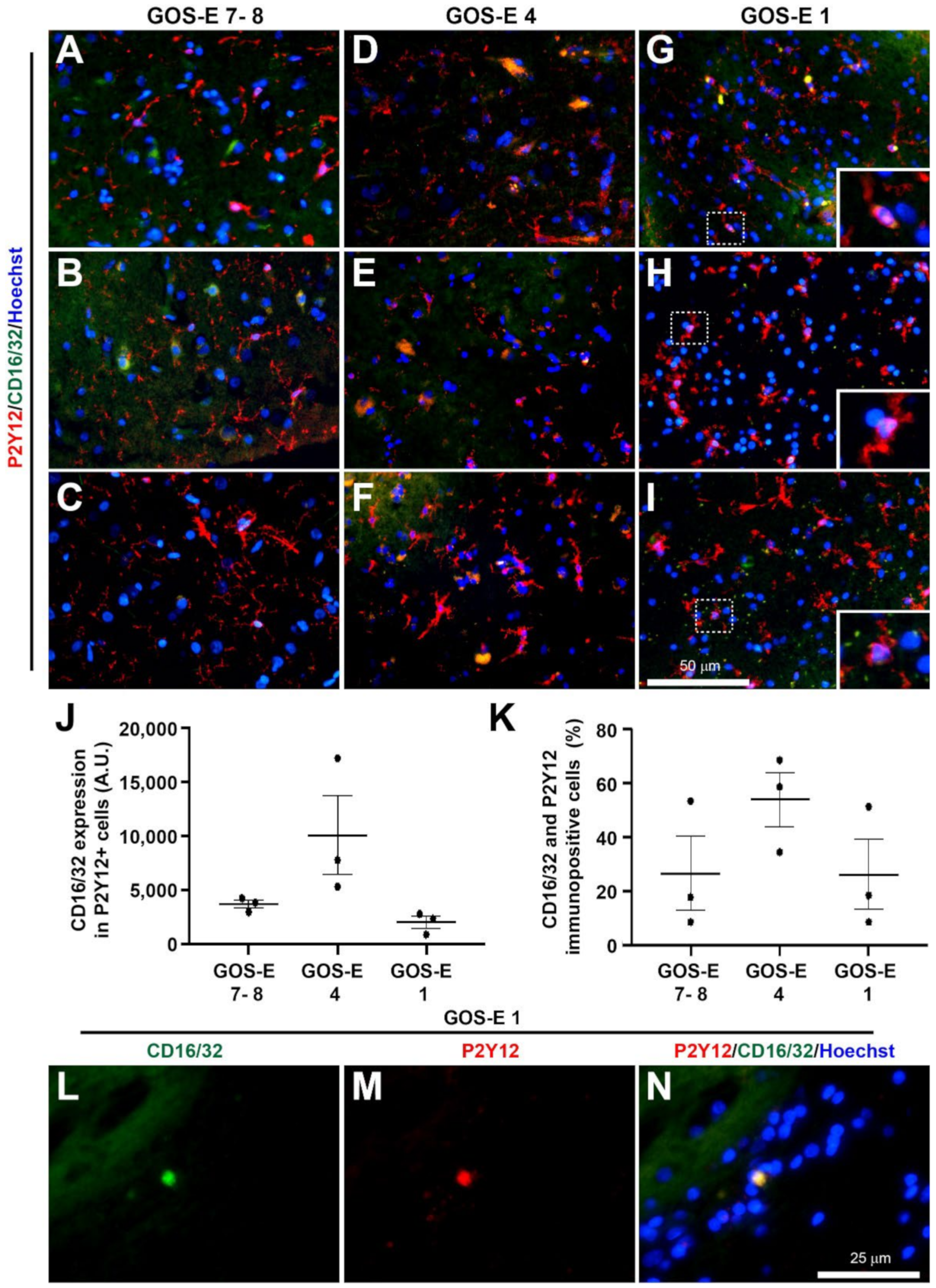
3.5.5. No Change in Microglia Activation State in Unfavourable Outcome after Severe TBI
Classical Anti-Inflammatory Microglia Activation
Classical Pro-Inflammatory Microglia Activation
4. Discussion
4.1. Injury Markers
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, J.; Tagliaferri, F.; Servadei, F. Cost of trauma in Europe. Eur. J. Neurol. 2005, 12 (Suppl. 1), 85–90. [Google Scholar] [CrossRef]
- Hardman, J.M.; Manoukian, A. Pathology of head trauma. Neuroimaging Clin. 2002, 12, 175–187. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, J. Factors contributing to outcome following traumatic brain injury. NeuroRehabilitation 2013, 32, 803–815. [Google Scholar] [CrossRef]
- Smith, M. Monitoring intracranial pressure in traumatic brain injury. Anesth. Analg. 2008, 106, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Samadani, U.; Daly, S.R. When will a clinical trial for trauamtic brain injury succeed? AANS Neurosurg. 2016, 25. [Google Scholar]
- Sherer, M.; Struchen, M.A.; Yablon, S.A.; Wang, Y.; Nick, T.G. Comparison of indices of traumatic brain injury severity: Glasgow Coma Scale, length of coma and post-traumatic amnesia. J. Neurol. Neurosurg. Psychiatry 2008, 79, 678–685. [Google Scholar] [CrossRef]
- Malec, J.F.; Brown, A.W.; Leibson, C.L.; Flaada, J.T.; Mandrekar, J.N.; Diehl, N.N.; Perkins, P.K. The mayo classification system for traumatic brain injury severity. J. Neurotrauma 2007, 24, 1417–1424. [Google Scholar] [CrossRef]
- Cole, J.H.; Leech, R.; Sharp, D.J. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015, 77, 571–581. [Google Scholar] [CrossRef]
- Tenovuo, O.; Diaz-Arrastia, R.; Goldstein, L.E.; Sharp, D.J.; van der Naalt, J.; Zasler, N.D. Assessing the Severity of Traumatic Brain Injury-Time for a Change? J. Clin. Med. 2021, 10, 148. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness: A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Menon, D.K.; Maas, A.I. Traumatic brain injury in 2014. Progress, failures and new approaches for TBI research. Nat. Rev. Neurol. 2015, 11, 71–72. [Google Scholar] [CrossRef]
- Murray, N.M.; Threlkeld, Z.D.; Hirsch, K.G. Will We Ever Make Headway in Severe Traumatic Brain Injury Treatment Trials? JAMA Neurol. 2020, 77, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 2011, 70, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Chen, N.Y.; Tu, P.H.; Wu, C.T.; Chiu, S.C.; Huang, Y.C.; Lim, S.N.; Yip, P.K. DHA Attenuates Cerebral Edema Following Traumatic Brain Injury via the Reduction in Blood-Brain Barrier Permeability. Int. J. Mol. Sci. 2020, 21, 6291. [Google Scholar] [CrossRef]
- Yip, P.K.; Carrillo-Jimenez, A.; King, P.; Vilalta, A.; Nomura, K.; Chau, C.C.; Egerton, A.M.; Liu, Z.H.; Shetty, A.J.; Tremoleda, J.L.; et al. Galectin-3 released in response to traumatic brain injury acts as an alarmin orchestrating brain immune response and promoting neurodegeneration. Sci. Rep. 2017, 7, 41689. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.L.; Chatlos, T.; Gorse, K.M.; Lafrenaye, A.D. Neuronal Membrane Disruption Occurs Late Following Diffuse Brain Trauma in Rats and Involves a Subpopulation of NeuN Negative Cortical Neurons. Front. Neurol. 2019, 10, 1238. [Google Scholar] [CrossRef]
- Posmantur, R.M.; Kampfl, A.; Taft, W.C.; Bhattacharjee, M.; Dixon, C.E.; Bao, J.; Hayes, R.L. Diminished microtubule-associated protein 2 (MAP2) immunoreactivity following cortical impact brain injury. J. Neurotrauma 1996, 13, 125–137. [Google Scholar] [CrossRef]
- Campbell, M.; Hanrahan, F.; Gobbo, O.L.; Kelly, M.E.; Kiang, A.S.; Humphries, M.M.; Nguyen, A.T.; Ozaki, E.; Keaney, J.; Blau, C.W.; et al. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat. Commun. 2012, 3, 849. [Google Scholar] [CrossRef]
- Yip, P.K.; Bowes, A.L.; Hall, J.C.E.; Burguillos, M.A.; Ip, T.H.R.; Baskerville, T.; Liu, Z.H.; Mohamed, M.; Getachew, F.; Lindsay, A.D.; et al. Docosahexaenoic acid reduces microglia phagocytic activity via miR-124 and induces neuroprotection in rodent models of spinal cord contusion injury. Hum. Mol. Genet. 2019, 28, 2427–2448. [Google Scholar] [CrossRef]
- Meshkini, A.; Shahzadi, S.; Alikhah, H.; Naghavi-Behzad, M. Role of stereotactic biopsy in histological diagnosis of multiple brain lesions. Asian J. Neurosurg. 2013, 8, 69–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waltregny, A.; Maula, A.A.; Brucher, J.M. Contribution of stereotactic brain biopsies to the diagnosis of presenile dementia. Stereotact. Funct. Neurosurg. 1990, 54–55, 409–412. [Google Scholar] [CrossRef]
- Warren, J.D.; Schott, J.M.; Fox, N.C.; Thom, M.; Revesz, T.; Holton, J.L.; Scaravilli, F.; Thomas, D.G.; Plant, G.T.; Rudge, P.; et al. Brain biopsy in dementia. Brain 2005, 128, 2016–2025. [Google Scholar] [CrossRef]
- Elobeid, A.; Laurell, K.; Cesarini, K.G.; Alafuzoff, I. Correlations between mini-mental state examination score, cerebrospinal fluid biomarkers, and pathology observed in brain biopsies of patients with normal-pressure hydrocephalus. J. Neuropathol. Exp. Neurol. 2015, 74, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Abu Hamdeh, S.; Shevchenko, G.; Mi, J.; Musunuri, S.; Bergquist, J.; Marklund, N. Proteomic differences between focal and diffuse traumatic brain injury in human brain tissue. Sci. Rep. 2018, 8, 6807. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Hasan, S.; Chari, A.; Ganau, M.; Uff, C. Defining New Research Questions and Protocols in the Field of Traumatic Brain Injury through Public Engagement: Preliminary Results and Review of the Literature. Emerg. Med. Int. 2019, 2019, 9101235. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.K.; Wong, L.F.; Pattinson, D.; Battaglia, A.; Grist, J.; Bradbury, E.J.; Maden, M.; McMahon, S.B.; Mazarakis, N.D. Lentiviral vector expressing retinoic acid receptor beta2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord. Hum. Mol. Genet. 2006, 15, 3107–3118. [Google Scholar] [CrossRef][Green Version]
- Yip, P.K.; Wong, L.F.; Sears, T.A.; Yanez-Munoz, R.J.; McMahon, S.B. Cortical overexpression of neuronal calcium sensor-1 induces functional plasticity in spinal cord following unilateral pyramidal tract injury in rat. PLoS Biol. 2010, 8, e1000399. [Google Scholar] [CrossRef]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- Weir, J.; Steyerberg, E.W.; Butcher, I.; Lu, J.; Lingsma, H.F.; McHugh, G.S.; Roozenbeek, B.; Maas, A.I.; Murray, G.D. Does the extended Glasgow Outcome Scale add value to the conventional Glasgow Outcome Scale? J. Neurotrauma 2012, 29, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Lecca, D.; Yang, L.Y.; Luo, W.; Scerba, M.T.; Tweedie, D.; Huang, P.S.; Jung, Y.J.; Kim, D.S.; Yang, C.H.; et al. 3,6’-dithiopomalidomide reduces neural loss, inflammation, behavioral deficits in brain injury and microglial activation. Elife 2020, 9, e54726. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.K.; Buslei, R.; Schmidt-Kastner, R.; Schmidt-Kastner, P.K.; Pietsch, T.; Wiestler, O.D.; Blumcke, I. NeuN: A useful neuronal marker for diagnostic histopathology. J. Histochem. Cytochem. 1996, 44, 1167–1171. [Google Scholar] [CrossRef]
- Little, J.R.; Sundt, T.M., Jr.; Kerr, F.W. Neuronal alterations in developing cortical infarction: An experimental study in monkeys. J. Neurosurg. 1974, 40, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Meissner, C.; Oehmichen, M. Microtubule-associated protein 2 (MAP2)—A promising approach to diagnosis of forensic types of hypoxia-ischemia. Acta Neuropathol. 2005, 110, 579–586. [Google Scholar] [CrossRef]
- Monnerie, H.; Tang-Schomer, M.D.; Iwata, A.; Smith, D.H.; Kim, H.A.; Le Roux, P.D. Dendritic alterations after dynamic axonal stretch injury in vitro. Exp. Neurol. 2010, 224, 415–423. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ramirez, S.H.; Sato, S.; Kiyota, T.; Cerny, R.L.; Kaibuchi, K.; Persidsky, Y.; Ikezu, T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am. J. Pathol. 2008, 172, 521–533. [Google Scholar] [CrossRef]
- Mbagwu, S.I.; Filgueira, L. Differential Expression of CD31 and Von Willebrand Factor on Endothelial Cells in Different Regions of the Human Brain: Potential Implications for Cerebral Malaria Pathogenesis. Brain Sci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Siddiq, I.; Park, E.; Liu, E.; Spratt, S.K.; Surosky, R.; Lee, G.; Ando, D.; Giedlin, M.; Hare, G.M.; Fehlings, M.G.; et al. Treatment of traumatic brain injury using zinc-finger protein gene therapy targeting VEGF-A. J. Neurotrauma 2012, 29, 2647–2659. [Google Scholar] [CrossRef]
- Carrillo-Jimenez, A.; Deniz, O.; Niklison-Chirou, M.V.; Ruiz, R.; Bezerra-Salomao, K.; Stratoulias, V.; Amouroux, R.; Yip, P.K.; Vilalta, A.; Cheray, M.; et al. TET2 Regulates the Neuroinflammatory Response in Microglia. Cell Rep. 2019, 29, 697–713. [Google Scholar] [CrossRef]
- Yip, P.K.; Kaan, T.K.; Fenesan, D.; Malcangio, M. Rapid isolation and culture of primary microglia from adult mouse spinal cord. J. Neurosci. Methods 2009, 183, 223–237. [Google Scholar] [CrossRef]
- Streit, W.J.; Xue, Q.S.; Tischer, J.; Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2014, 2, 142. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yip, P.K.; Adams, L.; Davies, M.; Lee, J.W.; Michael, G.J.; Priestley, J.V.; Michael-Titus, A.T. A Single Bolus of Docosahexaenoic Acid Promotes Neuroplastic Changes in the Innervation of Spinal Cord Interneurons and Motor Neurons and Improves Functional Recovery after Spinal Cord Injury. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 12733–12752. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Prokop, S.; Gorion, K.M.; Kim, J.D.; Sorrentino, Z.A.; Bell, B.M.; Manaois, A.N.; Chakrabarty, P.; Davies, P.; Giasson, B.I. Tau Ser208 phosphorylation promotes aggregation and reveals neuropathologic diversity in Alzheimer’s disease and other tauopathies. Acta Neuropathol. Commun. 2020, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Stern, R.A.; Nowinski, C.J.; Stein, T.D.; Alvarez, V.E.; Daneshvar, D.H.; Lee, H.S.; Wojtowicz, S.M.; Hall, G.; Baugh, C.M.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136, 43–64. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Lucke-Wold, B.P.; Turner, R.C.; Huber, J.D.; Rosen, C.L.; Simpkins, J.W. Role of Microvascular Disruption in Brain Damage from Traumatic Brain Injury. Compr. Physiol. 2015, 5, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef]
- Castejon, O.J.; Valero, C.; Diaz, M. Light and electron microscope study of nerve cells in traumatic oedematous human cerebral cortex. Brain Inj. 1997, 11, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Magaki, S.; Gardner, T.; Khanlou, N.; Yong, W.H.; Salamon, N.; Vinters, H.V. Brain biopsy in neurologic decline of unknown etiology. Hum. Pathol. 2015, 46, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Brandner, S.; von Deimling, A. Diagnostic, prognostic and predictive relevance of molecular markers in gliomas. Neuropathol. Appl. Neurobiol. 2015, 41, 694–720. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Vazquez-Rosa, E.; Koh, Y.; Dhar, M.; Chaubey, K.; Cintron-Perez, C.J.; Barker, S.; Miller, E.; Franke, K.; Noterman, M.F.; et al. Reducing acetylated tau is neuroprotective in brain injury. Cell 2021, 184, 2715–2732. [Google Scholar] [CrossRef] [PubMed]
- Hasbani, M.J.; Hyrc, K.L.; Faddis, B.T.; Romano, C.; Goldberg, M.P. Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp. Neurol. 1998, 154, 241–258. [Google Scholar] [CrossRef]
- Park, J.S.; Bateman, M.C.; Goldberg, M.P. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol. Dis. 1996, 3, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Zador, A. The function of dendritic spines: Devices subserving biochemical rather than electrical compartmentalization. J. Neurosci. 1993, 13, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deng, P.; Xu, Z.C.; Chen, J. Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS ONE 2011, 6, e24566. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G., III; Zhao, J.; Dash, P.K.; Soto, C.; Moreno-Gonzalez, I. Traumatic Brain Injury Induces Tau Aggregation and Spreading. J. Neurotrauma 2020, 37, 80–92. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Zhou, Y.; Hilton, T.; Zhao, Z.; Liu, W.; Wang, M.; Yeon, J.; Houck, K.; Thiagarajan, P.; et al. von Willebrand factor enhances microvesicle-induced vascular leakage and coagulopathy in mice with traumatic brain injury. Blood 2018, 132, 1075–1084. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Kumar, A.; Alvarez-Croda, D.M.; Stoica, B.A.; Faden, A.I.; Loane, D.J. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1732–1750. [Google Scholar] [CrossRef] [PubMed]
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; Hurlbert, R.J. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012, 135, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Mahmud, M.; Owen, D.R.; Johnson, M.R. Microglial positron emission tomography (PET) imaging in epilepsy: Applications, opportunities and pitfalls. Seizure 2017, 44, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Thau-Zuchman, O.; Ingram, R.; Harvey, G.G.; Cooke, T.; Palmas, F.; Pallier, P.N.; Brook, J.; Priestley, J.V.; Dalli, J.; Tremoleda, J.L.; et al. A Single Injection of Docosahexaenoic Acid Induces a Pro-Resolving Lipid Mediator Profile in the Injured Tissue and a Long-Lasting Reduction in Neurological Deficit after Traumatic Brain Injury in Mice. J. Neurotrauma 2020, 37, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.E.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The role of microglia in the healthy brain. J. Neurosci. 2011, 31, 16064–16069. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Gao, G.; Feng, J.; Jin, Y.; Wang, C.; Mao, Q.; Jiang, J. Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: A prospective cohort study. Crit. Care 2015, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- Luoto, T.M.; Raj, R.; Posti, J.P.; Gardner, A.J.; Panenka, W.J.; Iverson, G.L. A Systematic Review of the Usefulness of Glial Fibrillary Acidic Protein for Predicting Acute Intracranial Lesions following Head Trauma. Front. Neurol. 2017, 8, 652. [Google Scholar] [CrossRef]
- Okonkwo, D.O.; Puffer, R.C.; Puccio, A.M.; Yuh, E.L.; Yue, J.K.; Diaz-Arrastia, R.; Korley, F.K.; Wang, K.K.W.; Sun, X.; Taylor, S.R.; et al. Point-of-Care Platform Blood Biomarker Testing of Glial Fibrillary Acidic Protein versus S100 Calcium-Binding Protein B for Prediction of Traumatic Brain Injuries: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury Study. J. Neurotrauma 2020, 37, 2460–2467. [Google Scholar] [CrossRef]
- Cooper, D.J.; Rosenfeld, J.V.; Murray, L.; Arabi, Y.M.; Davies, A.R.; D’Urso, P.; Kossmann, T.; Ponsford, J.; Seppelt, I.; Reilly, P.; et al. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 2011, 364, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Kolias, A.G.; Tajsic, T.; Adeleye, A.; Aklilu, A.T.; Apriawan, T.; Bajamal, A.H.; Barthélemy, E.J.; Devi, B.I.; Bhat, D.; et al. Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury: Consensus statement. Acta Neurochir. 2019, 161, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Cammermeyer, J. The post-mortem origin and mechanism of neuronal hyperchromatosis and nuclear pyknosis. Exp. Neurol. 1960, 2, 379–405. [Google Scholar] [CrossRef]
- Li, X.; Pierre, K.; Yang, Z.; Nguyen, L.; Johnson, G.; Venetucci, J.; Torres, I.; Lucke-Wold, B.; Shi, Y.; Boutte, A.; et al. Blood-Based Brain and Global Biomarker Changes after Combined Hypoxemia and Hemorrhagic Shock in a Rat Model of Penetrating Ballistic-Like Brain Injury. Neurotrauma Rep. 2021, 2, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Lucke-Wold, B.P.; Robson, M.J.; Omalu, B.I.; Petraglia, A.L.; Bailes, J.E. Repetitive traumatic brain injury and development of chronic traumatic encephalopathy: A potential role for biomarkers in diagnosis, prognosis, and treatment? Front. Neurol. 2012, 3, 186. [Google Scholar] [CrossRef] [PubMed]

| SHIBA Code | TBI Type | Age Group (Years) | Gender | Computerized Tomography Imaging | Biopsy Time Post Injury (h) | Trauma Site | Biopsy Site | Biopsy Type | E | V | M | GCS | GOS-E (6 Months) | Injury to Death Duration (Day) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RTC (motorcycle) | 21–25 | Male | Shallow ASDH/ICH/Contusion/tSAH/IVH | 146 | LF | LF | Craniotomy | 1 | 1 | 3 | 5 | 4 | |
| 2 | RTC (motorcycle) | 21–25 | Male | Small EDH/Contusion/DAI | 5 | RF | RF | ICP | 1 | 1 | 5 | 7 | 7 | |
| 3 | GSW | 16–20 | Male | Small ASDH/Contusion/tSAH | 1 | LT | RF | ICP | 2 | 2 | 5 | 9 | 5 | |
| 4 | Assault | 40–45 | Male | Small contusions | 7 | LF | RF | ICP | 3 | 4 | 5 | 12 | 8 | |
| 5 | Fall | 16–20 | Male | Left ASDH | 1 | L | RF | ICP | 1 | 1 | 1 | 3 | 7 | |
| 6 | RTC (cyclist) | 36–40 | Male | Left ASDH/Diffuse contusions/Tight brain | 60 | LP | RF | ICP | 1 | 1 | 5 | 7 | 1 | 12 |
| 7 | Fall | 61–65 | Male | Left ASDH/LT contusions/All contrecoup | 8 | RP | LF | Craniotomy | 1 | 1 | 5 | 7 | 3 | |
| 8 | Fall | 46–50 | Female | EDH and contusions | 1 | LP | RF | ICP | 1 | 1 | 1 | 3 | 8 | |
| 9 | Fall | 16–20 | Male | DAI | 1 | RF | RF | ICP | 1 | 1 | 1 | 3 | 1 | 8 |
| 10 | Fall | 51–55 | Male | tSAH and contusions | 6 | OCC | RF | EVD | 3 | 2 | 5 | 10 | 7 | |
| 11 | Assault | 31–35 | Male | tSAH | 3 | RF | RF | ICP | 1 | 1 | 4 | 6 | 8 | |
| 12 | RTC (Pedestrian) | 56–60 | Male | ASDH and DAI | 3 | RP | LF | Craniotomy | 1 | 2 | 3 | 6 | 2 | |
| 13 | Fall | 46–50 | Female | ASDH | 48 | RF | RF | Craniotomy | 4 | 2 | 5 | 11 | 7 | |
| 14 | GSW | 21–25 | Male | GSW | 20 | LF | LF | Craniotomy | 4 | 4 | 6 | 14 | 3 | |
| 15 | Fall | 76–80 | Male | IVH and contusions | 1 | OCC | RF | EVD | 1 | 2 | 5 | 8 | 1 | 5 |
| 16 | Fall | 31–35 | Male | tSAH and contusions | 4 | LP | RF | ICP | 3 | 4 | 6 | 13 | 3 | |
| 17 | Fall | 56–60 | Male | ASDH | 4 | RP | LF | Craniotomy | 4 | 4 | 6 | 14 | 4 | |
| 18 | Fall | 26–30 | Male | ASDH | 2 | BLF | RF | Craniotomy | 1 | 1 | 1 | 3 | 3 | |
| 19 | RTC (Pedestrian) | 36–40 | Male | ASDH, contusions | 1 | LP | RF | Craniotomy | 1 | 1 | 2 | 4 | 1 | 5 |
| 20 | RTC (Pedestrian) | 31–35 | Male | Contusions and DAI | 3 | RF | RF | ICP | 1 | 1 | 2 | 4 | 2 | |
| 21 | Assault | 51–55 | Female | Open depressed skull fracture | 1 | LF | LF | Craniotomy | 1 | 1 | 4 | 6 | 1 | 33 |
| 22 | Train accident | 56–60 | Male | DAI | 18 | LP | RF | ICP | 1 | 1 | 1 | 3 | 2 | |
| 23 | RTC (Pedestrian) | 51–55 | Male | Contusions and DAI | 1 | OCC | RF | ICP | 1 | 2 | 3 | 6 | 1 | 2 |
| 24 | Fall | 56–60 | Male | ASDH/CSDH | 5 | RF | RF | Craniotomy | 1 | 1 | 3 | 5 | 4 | |
| 25 | RTC (Pedestrian) | 46–50 | Male | ASDH, grade 3 DAI | 2 | RP | LF | Craniotomy | 1 | 1 | 1 | 3 | 1 | 4 |
| YHU Grading Score | Neuronal (NeuN) | Dendritic (MAP2) | Neurovascular (Claudin-5/vWF) | Neuroinflammation (Iba1/P2Y12) |
|---|---|---|---|---|
| I | Many large round or polygonal cell bodies with uniform cytoplasm ± proximal processes | Many long, smooth and continuous processes surrounded with many thin processes | Several long tubular-shaped microvessels | Small cell bodies with long and thin processes |
| II | A few large round or polygonal cell bodies with small vacuoles in cytoplasm | Several long processes with some beading surrounded with some thin beaded processes | Several short tubular-shaped microvessels | Small cell bodies with short and thick processes |
| III | Extensive dysmorphic cell bodies with large vacuoles in cytoplasm | Limited long processes with extensive beading surrounded with limited thin beaded processes | Several extremely short microvessels | Enlarged cell bodies with very short processes |
| IV | Limited cell bodies with atrophic appearance | Limited beaded processes without any thin processes | Limited short and irregular fragments of microvessels | Limited atrophic cell bodies with cellular fragmentation |
| SHIBA Patient No. | Neuronal Injury | Dendritic Injury | Neurovascular Injury | Neuro Inflammation | Total YHU Grade | GOS-E |
|---|---|---|---|---|---|---|
| 1 | II | III | III | II | 10 | 4 |
| 2 | I | I | II | II | 6 | 7 |
| 3 | III | II | II | I | 8 | 5 |
| 4 | III | I | II | I | 7 | 8 |
| 5 | II | I | I | I | 5 | 7 |
| 6 | IV | III | III | II | 12 | 1 |
| 7 | IV | IV | III | III | 14 | 3 |
| 8 | II | I | II | I | 6 | 8 |
| 9 | III | IV | IV | II | 13 | 1 |
| 10 | II | II | III | III | 10 | 7 |
| 11 | II | I | II | II | 7 | 8 |
| 12 | II | II | III | III | 10 | 2 |
| 13 | II | II | II | II | 8 | 7 |
| 14 | I | II | II | IV | 9 | 3 |
| 15 | II | I | III | III | 9 | 1 |
| 16 | II | II | II | II | 8 | 8 |
| 17 | II | III | IV | IV | 13 | 4 |
| 18 | II | II | III | II | 9 | 3 |
| 19 | IV | III | IV | III | 14 | 1 |
| 20 | IV | IV | IV | IV | 16 | 2 |
| 21 | III | II | III | IV | 12 | 1 |
| 22 | II | IV | IV | III | 13 | 2 |
| 23 | IV | IV | IV | IV | 16 | 1 |
| 24 | III | III | III | II | 11 | 4 |
| 25 | IV | IV | IV | IV | 16 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yip, P.K.; Hasan, S.; Liu, Z.-H.; Uff, C.E.G. Characterisation of Severe Traumatic Brain Injury Severity from Fresh Cerebral Biopsy of Living Patients: An Immunohistochemical Study. Biomedicines 2022, 10, 518. https://doi.org/10.3390/biomedicines10030518
Yip PK, Hasan S, Liu Z-H, Uff CEG. Characterisation of Severe Traumatic Brain Injury Severity from Fresh Cerebral Biopsy of Living Patients: An Immunohistochemical Study. Biomedicines. 2022; 10(3):518. https://doi.org/10.3390/biomedicines10030518
Chicago/Turabian StyleYip, Ping K., Shumaila Hasan, Zhuo-Hao Liu, and Christopher E. G. Uff. 2022. "Characterisation of Severe Traumatic Brain Injury Severity from Fresh Cerebral Biopsy of Living Patients: An Immunohistochemical Study" Biomedicines 10, no. 3: 518. https://doi.org/10.3390/biomedicines10030518
APA StyleYip, P. K., Hasan, S., Liu, Z.-H., & Uff, C. E. G. (2022). Characterisation of Severe Traumatic Brain Injury Severity from Fresh Cerebral Biopsy of Living Patients: An Immunohistochemical Study. Biomedicines, 10(3), 518. https://doi.org/10.3390/biomedicines10030518







