The Spectrum of Acute Cerebrovascular Disease in Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
3. Results
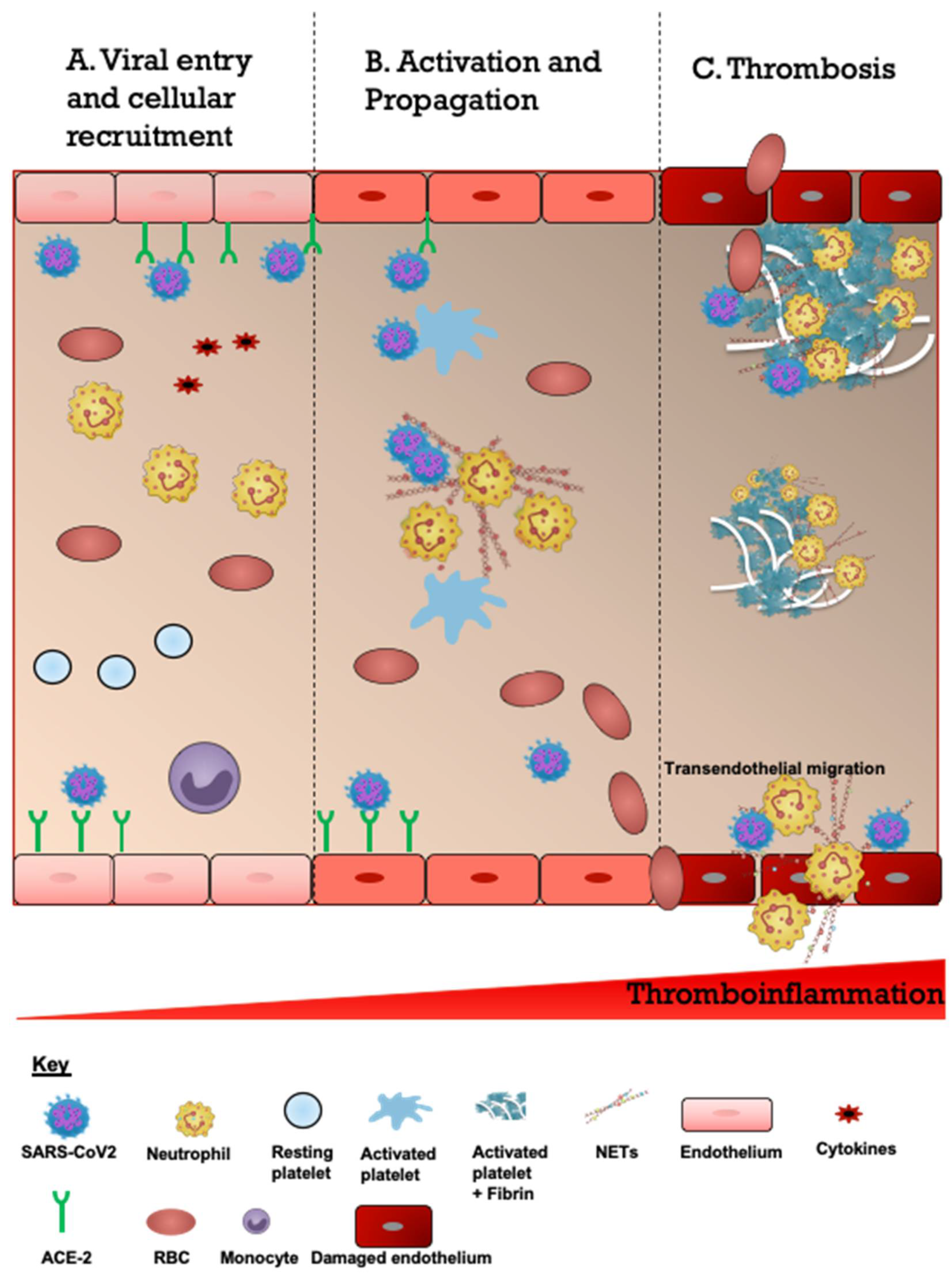
3.1. Etiology and Risk Factors of Acute CVD and COVID-19
3.2. Clinical Manifestations and Prognosis of Patients with Acute CVD and COVID-19
3.3. Laboratory Data of Patients with Acute CVD and COVID-19
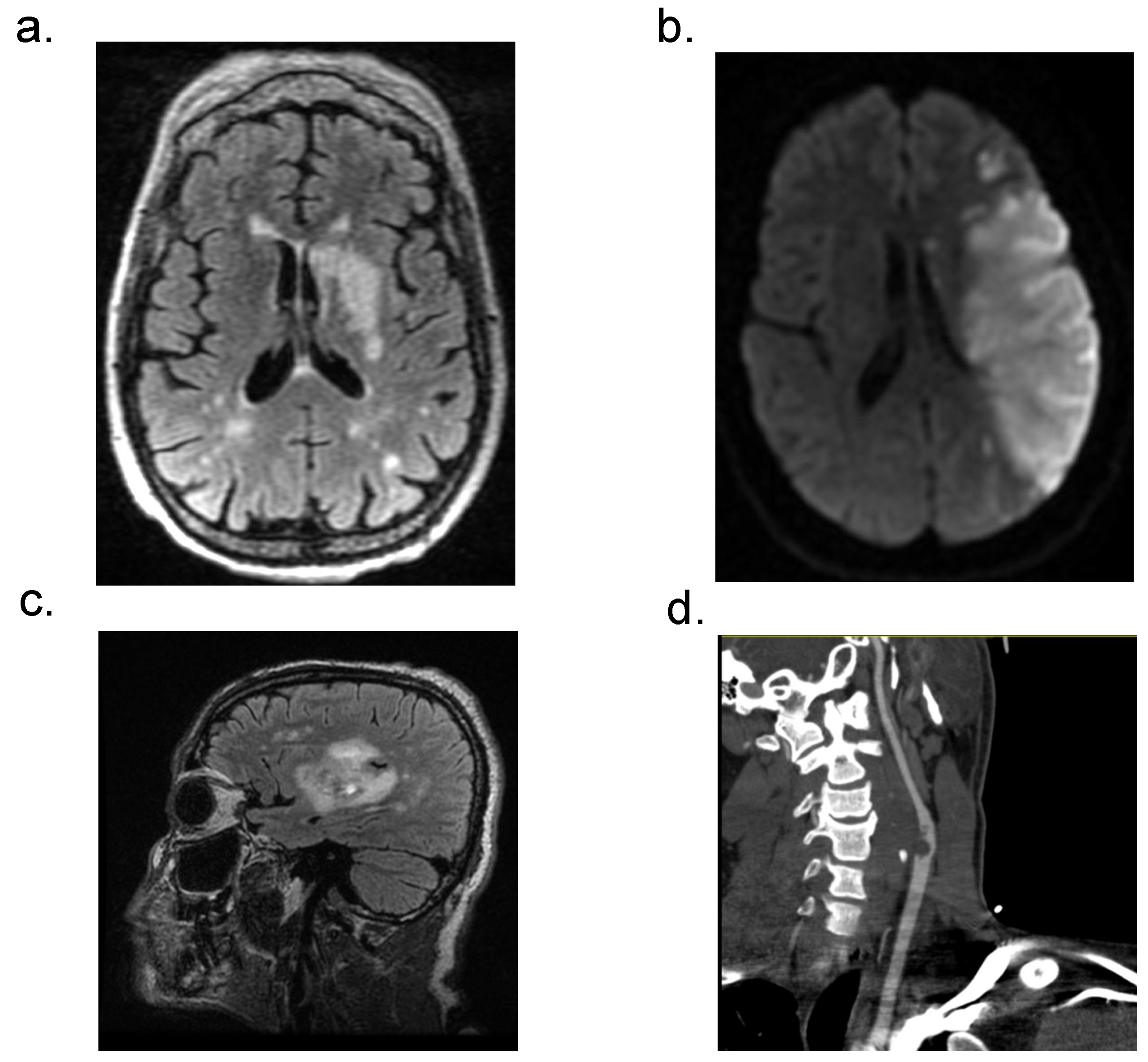
3.4. Neuroradiological Data of Patients with Acute CVD and COVID-19
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Lanza, G.; Falzone, L.; Fisicaro, F.; Ferri, R.; Bella, R. SARS-CoV-2 and the Nervous System: From Clinical Features to Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5475. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute Ischemic Stroke and COVID-19: An Analysis of 27 676 Patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Palaiodimou, L.; Zand, R.; Lioutas, V.A.; Krogias, C.; Katsanos, A.H.; Shoamanesh, A.; Sharma, V.K.; Shahjouei, S.; Baracchini, C.; et al. COVID-19 and cerebrovascular diseases: A comprehensive overview. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420978004. [Google Scholar] [CrossRef]
- Reddy, S.T.; Garg, T.; Shah, C.; Nascimento, F.A.; Imran, R.; Kan, P.; Bowry, R.; Gonzales, N.; Barreto, A.; Kumar, A.; et al. Cerebrovascular Disease in Patients with COVID-19: A Review of the Literature and Case Series. Case Rep. Neurol. 2020, 12, 199–209. [Google Scholar] [CrossRef]
- Nannoni, S.; de Groot, R.; Bell, S.; Markus, H.S. Stroke in COVID-19: A systematic review and meta-analysis. Int. J. Stroke 2021, 16, 137–149. [Google Scholar] [CrossRef]
- Ntaios, G.; Michel, P.; Georgiopoulos, G.; Guo, Y.; Li, W.; Xiong, J.; Calleja, P.; Ostos, F.; Gonzalez-Ortega, G.; Fuentes, B.; et al. Characteristics and Outcomes in Patients With COVID-19 and Acute Ischemic Stroke: The Global COVID-19 Stroke Registry. Stroke 2020, 51, e254–e258. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Beyrouti, R.; Adams, M.E.; Benjamin, L.; Cohen, H.; Farmer, S.F.; Goh, Y.Y.; Humphries, F.; Jager, H.R.; Losseff, N.A.; Perry, R.J.; et al. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 2020, 91, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Iffah, R.; Gavins, F.N.E. Thromboinflammation in coronavirus disease 2019: The clot thickens. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Talasaz, A.H.; Kakavand, H.; Van Tassell, B.; Aghakouchakzadeh, M.; Sadeghipour, P.; Dunn, S.; Geraiely, B. Cardiovascular Complications of COVID-19: Pharmacotherapy Perspective. Cardiovasc. Drugs Ther. 2021, 35, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, H.K.; Park, J.H.; Cho, S.J.; Kwon, M.; Jo, C.; Koh, Y.H. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem. Biophys. Res. Commun. 2020, 528, 413–419. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef]
- Harrison, S.L.; Buckley, B.J.R.; Rivera-Caravaca, J.M.; Zhang, J.; Lip, G.Y.H. Cardiovascular risk factors, cardiovascular disease, and COVID-19: An umbrella review of systematic reviews. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 330–339. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.H.; Beghi, E.; Helbok, R.; Moro, E.; Sampson, J.; Altamirano, V.; Mainali, S.; Bassetti, C.; Suarez, J.I.; McNett, M. Global Incidence of Neurological Manifestations Among Patients Hospitalized With COVID-19-A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw. Open 2021, 4, e2112131. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Liang, X.; Gao, B.; Liu, M.; Li, W.; Chen, Z.; Wang, Z. COVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke: Incidence, Potential Pathological Mechanism, and Management. Front. Neurol. 2020, 11, 1152. [Google Scholar] [CrossRef]
- Genovesi, S.; Rebora, P.; Occhino, G.; Rossi, E.; Maloberti, A.; Belli, M.; Bonfanti, P.; Giannattasio, C.; Rossetti, C.; Epis, O.M.; et al. Atrial Fibrillation and Clinical Outcomes in a Cohort of Hospitalized Patients with Sars-Cov-2 Infection and Chronic Kidney Disease. J. Clin. Med. 2021, 10, 4108. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, F.; Di Napoli, M.; Liberto, A.; Fanella, M.; Di Stasio, F.; Pennisi, M.; Bella, R.; Lanza, G.; Mansueto, G. Neurological Sequelae in Patients with COVID-19: A Histopathological Perspective. Int. J Environ. Res. Public Health 2021, 18, 1415. [Google Scholar] [CrossRef] [PubMed]
- Sweid, A.; Hammoud, B.; Weinberg, J.H.; Oneissi, M.; Raz, E.; Shapiro, M.; DePrince, M.; Tjoumakaris, S.; Gooch, M.R.; Herial, N.A.; et al. Letter: Thrombotic Neurovascular Disease in COVID-19 Patients. Neurosurgery 2020, 87, E400–E406. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Fernandez, F.; Sandoval Valencia, H.; Barbella-Aponte, R.A.; Collado-Jimenez, R.; Ayo-Martin, O.; Barrena, C.; Molina-Nuevo, J.D.; Garcia-Garcia, J.; Lozano-Setien, E.; Alcahut-Rodriguez, C.; et al. Cerebrovascular disease in patients with COVID-19: Neuroimaging, histological and clinical description. Brain 2020, 143, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza, H.F.; Lee, J.S.; Ray, P. Neutrophils and lymphopenia, an unknown axis in severe COVID-19 disease. PLoS Pathog. 2021, 17, e1009850. [Google Scholar] [CrossRef]
- Zakeri, A.; Jadhav, A.P.; Sullenger, B.A.; Nimjee, S.M. Ischemic stroke in COVID-19-positive patients: An overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J. Neurointerv. Surg. 2021, 13, 202–206. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Goldberg, M.F. Neuroradiologic manifestations of COVID-19: What the emergency radiologist needs to know. Emerg. Radiol. 2020, 27, 737–745. [Google Scholar] [CrossRef]
- Batlle, D.; Soler, M.J.; Sparks, M.A.; Hiremath, S.; South, A.M.; Welling, P.A.; Swaminathan, S. Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. J. Am. Soc. Nephrol. 2020, 31, 1380–1383. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, C.; Zheng, X.; Jin, Y.; Duan, G.; Chen, M.; Chen, S. Elevated Procalcitonin Is Positively Associated with the Severity of COVID-19: A Meta-Analysis Based on 10 Cohort Studies. Medicina 2021, 57, 594. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef] [PubMed]
- Venketasubramanian, N.; Anderson, C.; Ay, H.; Aybek, S.; Brinjikji, W.; de Freitas, G.R.; Del Brutto, O.H.; Fassbender, K.; Fujimura, M.; Goldstein, L.B.; et al. Stroke Care during the COVID-19 Pandemic: International Expert Panel Review. Cerebrovasc. Dis. 2021, 50, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Zhang, S.; Xian, Y.; Xu, H.; Rutan, C.; Alger, H.M.; Walchok, J.G.; Williams, J.H.; de Lemos, J.A.; Decker-Palmer, M.R.; et al. Treatment and Outcomes of Patients with Ischemic Stroke During COVID-19: An Analysis From Get With The Guidelines-Stroke. Stroke 2021, 52, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
| COVID (n = 32) | Non-COVID (n = 36) | p Value (Chi Square) | |
|---|---|---|---|
| Age | 63 | 64 | 0.68 |
| Sex | |||
| Female | 10 (32%) | 15 (42%) | 0.37 |
| Male | 22 (68%) | 21 (58%) | 0.37 |
| Race | |||
| African American/Black | 18 (56%) | 26 (72%) | 0.68 |
| Caucasian/White | 6 (24%) | 9 (25%) | 0.68 |
| Other | 2 (8%) | 1 (3%) | 0.68 |
| Comorbidities | |||
| Hypertension | 22 (69%) | 33 (92%) | <0.001 |
| Hyperlipidemia | 10 (33%) | 18 (50%) | <0.001 |
| Diabetes | 9 (28%) | 12(33%) | <0.001 |
| Atrial fibrillation | 8 (25%) | 9 (25%) | 1.000 |
| Coronary artery disease | 5 (16%) | 7 (19%) | <0.001 |
| Smoking | 10 (31%) | 17 (47%) | 0.07 |
| Obesity | 12 (37%) | 14 (38.9) | 0.05 |
| Chronic kidney disease | 4 (13%) | 3(8.3%) | <0.001 |
| Malignancy | 3 (9%) | 2 (6%) | <0.001 |
| Clinical Features | |||
| Weakness | 27 (84%) | 30 (83%) | 0.02 |
| Impaired Consciousness | 14 (44%) | 5 (14%) | 0.001 |
| Dysarthria | 20 (62%) | 23 (64%) | 0.13 |
| Aphasia | 17 (53%) | 15 (42%) | 0.05 |
| Vision Impairment | 5 (16%) | 6 (17%) | 0.05 |
| Dizziness | 4 (12%) | 3 (8.3%) | 0.004 |
| Delirium | 6 (19%) | 0 (0%) | 0.001 |
| Seizures | 5 (16%) | 1 (3%) | 0.02 |
| COVID (n = 32) | Non-COVID (n = 36) | p Value | |
|---|---|---|---|
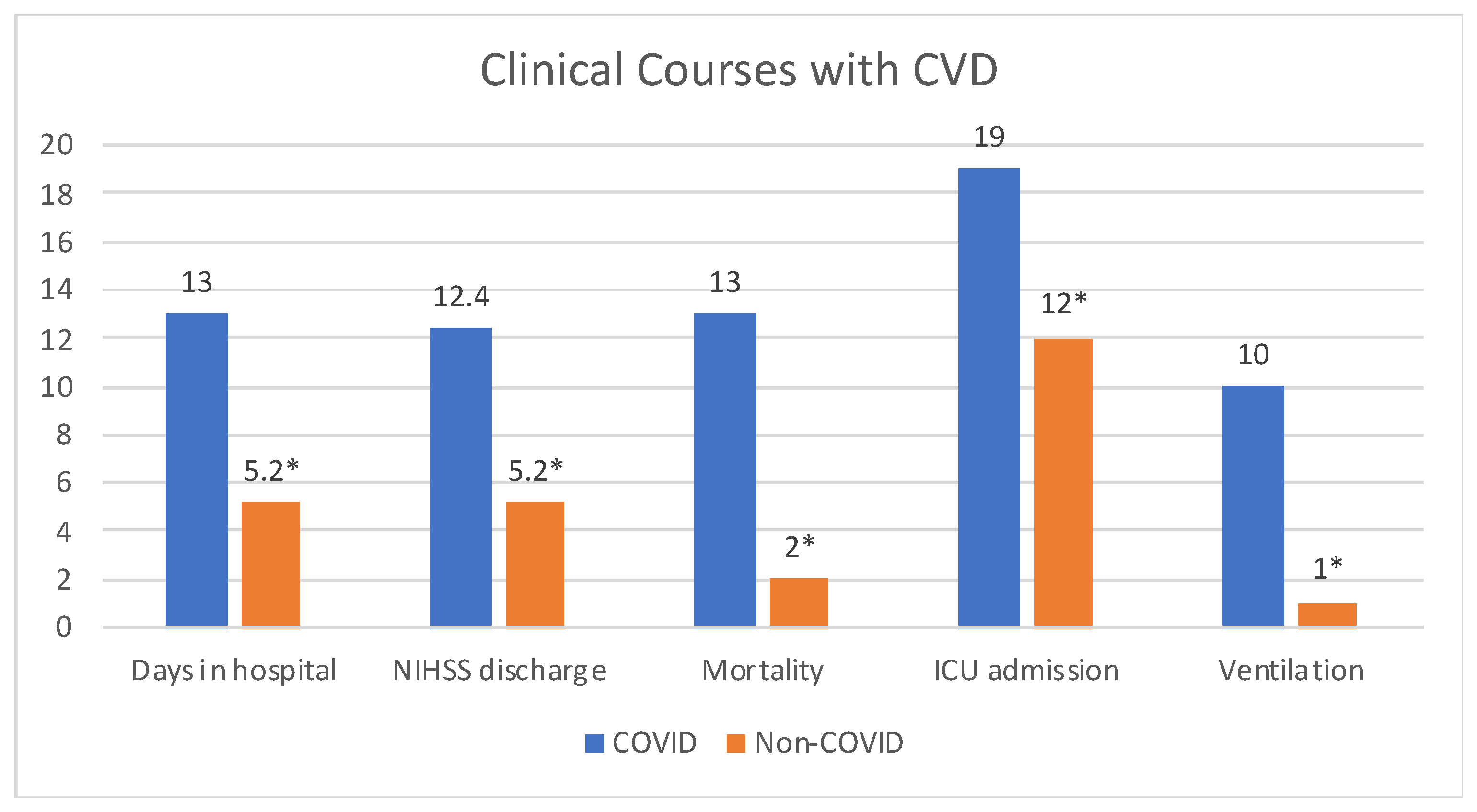
| NIHinitial (mean) | 11.7 | 8.2 | 0.08 |
| NIHdischarge (mean) | 12.4 | 5.2 | 0.004 |
| NIHd (mean) | 2.5 | −3.0 | 0.01 |
| Days in Hospital (mean) | 13 | 5.2 | 0.002 |
| ICU admission | 19 (59%) | 12 (33%) | 0.04 |
| Ventilation | 10 (31.3%) | 1 (3%) | <0.001 |
| Outcomes | |||
| Recovered | 19 (60%) | 34 (94%) | 0.001 |
| Death | 13 (40%) | 2 (6%) | 0.001 |
| COVID (n = 32) | Non-COVID (n = 36) | p Value (t-Test) | |
|---|---|---|---|
| WBC | 8.40 (±4.2) | 8.1 (±2.71) | 0.78 |
| ANC | 6.50 (±4.7 | 5.3 (±2.6) | 0.22 |
| ALC | 1.30 (±0.5) | 1.9 (±0.9) | 0.003 |
| NLR | 6.67 (±4.4) | 3.7 (±3.3) | 0.02 |
| Platelets | 260.5 (±106.2) | 258 (±78) | 0.91 |
| D-Dimer | 9792 (±19k) | NR | |
| CRP | 11.3 (±8.7) | NR | |
| Serum Creatinine | 2.3 (±2.3) | 1.1 (±0.5) | 0.006 |
| LDH | 460 (±232) | NR | |
| Ferritin | 2287 (±5244) | NR | |
| PT | 13.6 (±2.4) | 15 (±12) | 0.05 |
| INR | 1.20 (±0.21) | 1 (0.1) | 0.07 |
| APTT | 42 (±33) | 32 (±15) | 0.15 |
| TroponinMax | 0.08 (±0.16) | 0.02(±0.75) | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triay, R.; Buchhanolla, P.; Gaudet, A.; Winter, V.; Gaudet, A.; Faraji, M.; Gonzalez-Toledo, E.; Siddaiah, H.; Cuellar-Saenz, H.H.; Bailey, S.; et al. The Spectrum of Acute Cerebrovascular Disease in Patients with COVID-19. Biomedicines 2022, 10, 435. https://doi.org/10.3390/biomedicines10020435
Triay R, Buchhanolla P, Gaudet A, Winter V, Gaudet A, Faraji M, Gonzalez-Toledo E, Siddaiah H, Cuellar-Saenz HH, Bailey S, et al. The Spectrum of Acute Cerebrovascular Disease in Patients with COVID-19. Biomedicines. 2022; 10(2):435. https://doi.org/10.3390/biomedicines10020435
Chicago/Turabian StyleTriay, Rachel, Prabandh Buchhanolla, Alexas Gaudet, Victoria Winter, Alexandra Gaudet, Mehdi Faraji, Eduardo Gonzalez-Toledo, Harish Siddaiah, Hugo H. Cuellar-Saenz, Steven Bailey, and et al. 2022. "The Spectrum of Acute Cerebrovascular Disease in Patients with COVID-19" Biomedicines 10, no. 2: 435. https://doi.org/10.3390/biomedicines10020435
APA StyleTriay, R., Buchhanolla, P., Gaudet, A., Winter, V., Gaudet, A., Faraji, M., Gonzalez-Toledo, E., Siddaiah, H., Cuellar-Saenz, H. H., Bailey, S., Javalkar, V., Riel-Romero, R. M. S., Kelley, R. E., Gavins, F. N. E., & Ansari, J. (2022). The Spectrum of Acute Cerebrovascular Disease in Patients with COVID-19. Biomedicines, 10(2), 435. https://doi.org/10.3390/biomedicines10020435







