Mesenchymal Stem Cells Isolated from Paediatric Paravertebral Adipose Tissue Show Strong Osteogenic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. ADMSC Isolation and Culturing
2.1.1. PV-ADMSC
2.1.2. LA-ADMSC
2.1.3. PCS-500-011
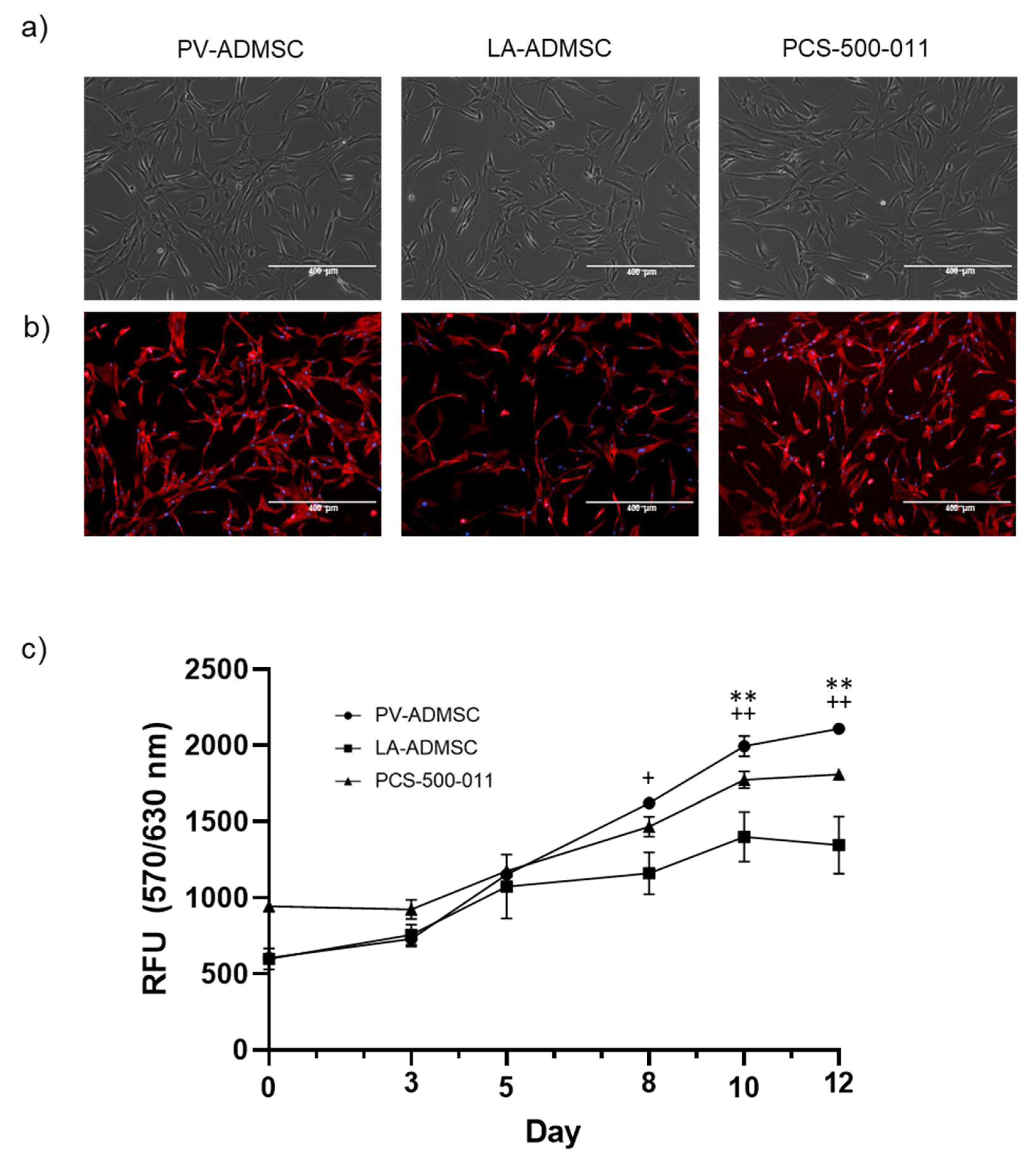
2.2. Cell Morphology
2.3. Metabolic Activity
2.4. RNA Isolation and RT-PCR
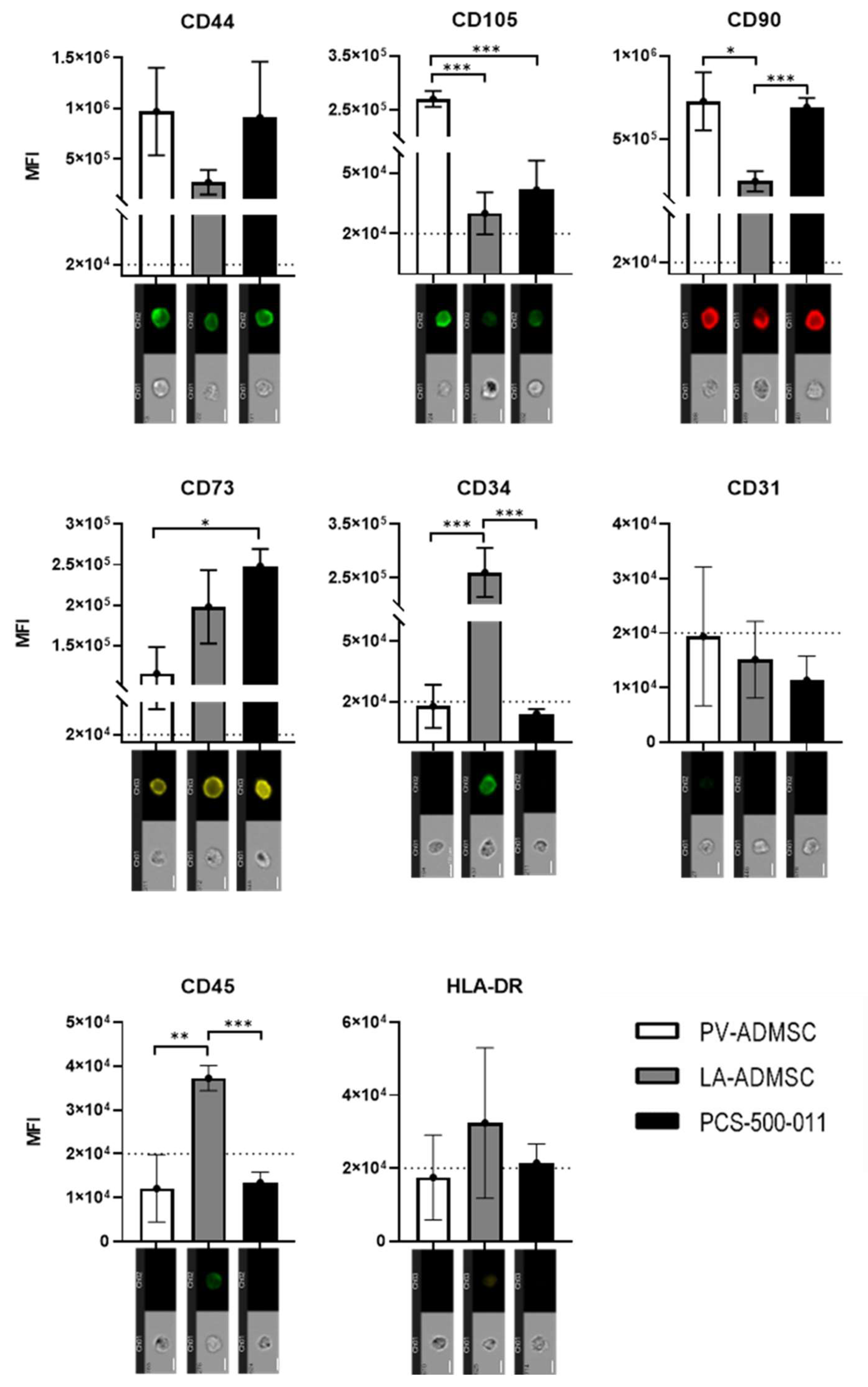
2.5. Cell Characterisation Using Imaging Flow Cytometry
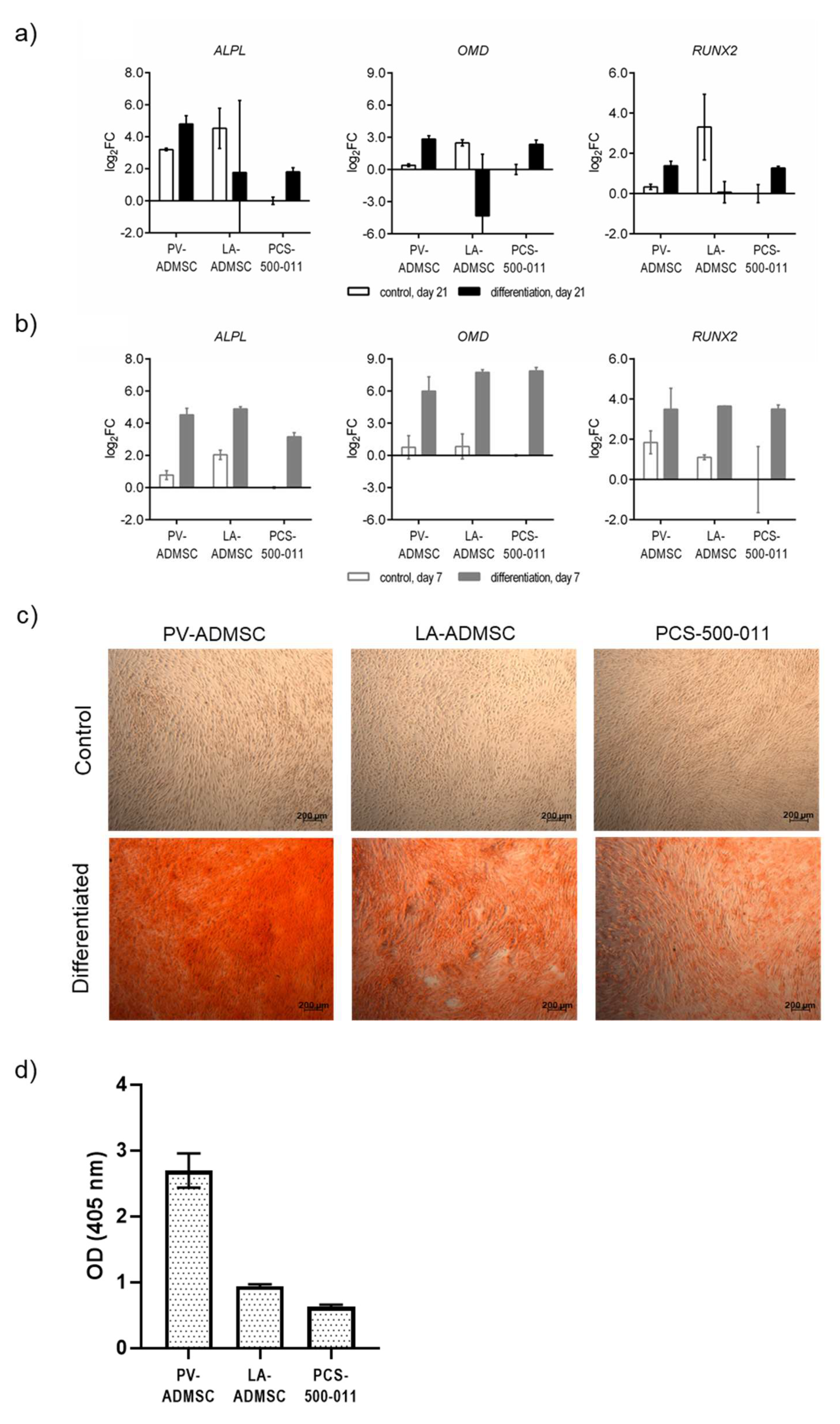
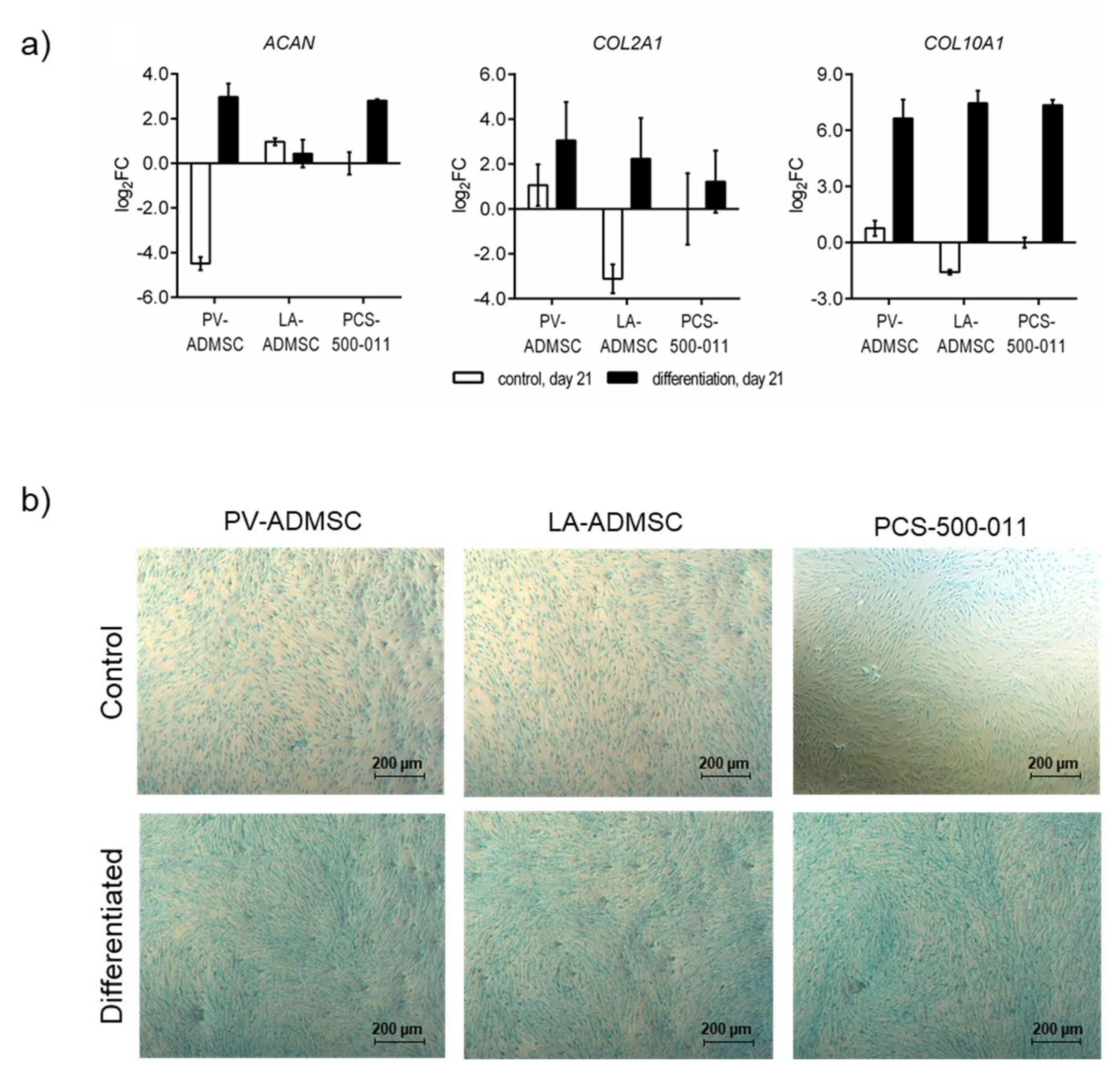
2.6. Induction of Cell Differentiation
2.7. Cell Encapsulation and Staining
2.8. Statistical Analysis
3. Results
3.1. Isolation of ADMSCs
3.2. Morphology and Growth Characteristics
3.3. Stem Marker Gene Expression
3.4. Stem Marker Protein Expression
3.5. Adipogenic Differentiation of MSCs
3.6. Osteogenic Differentiation of MSCs
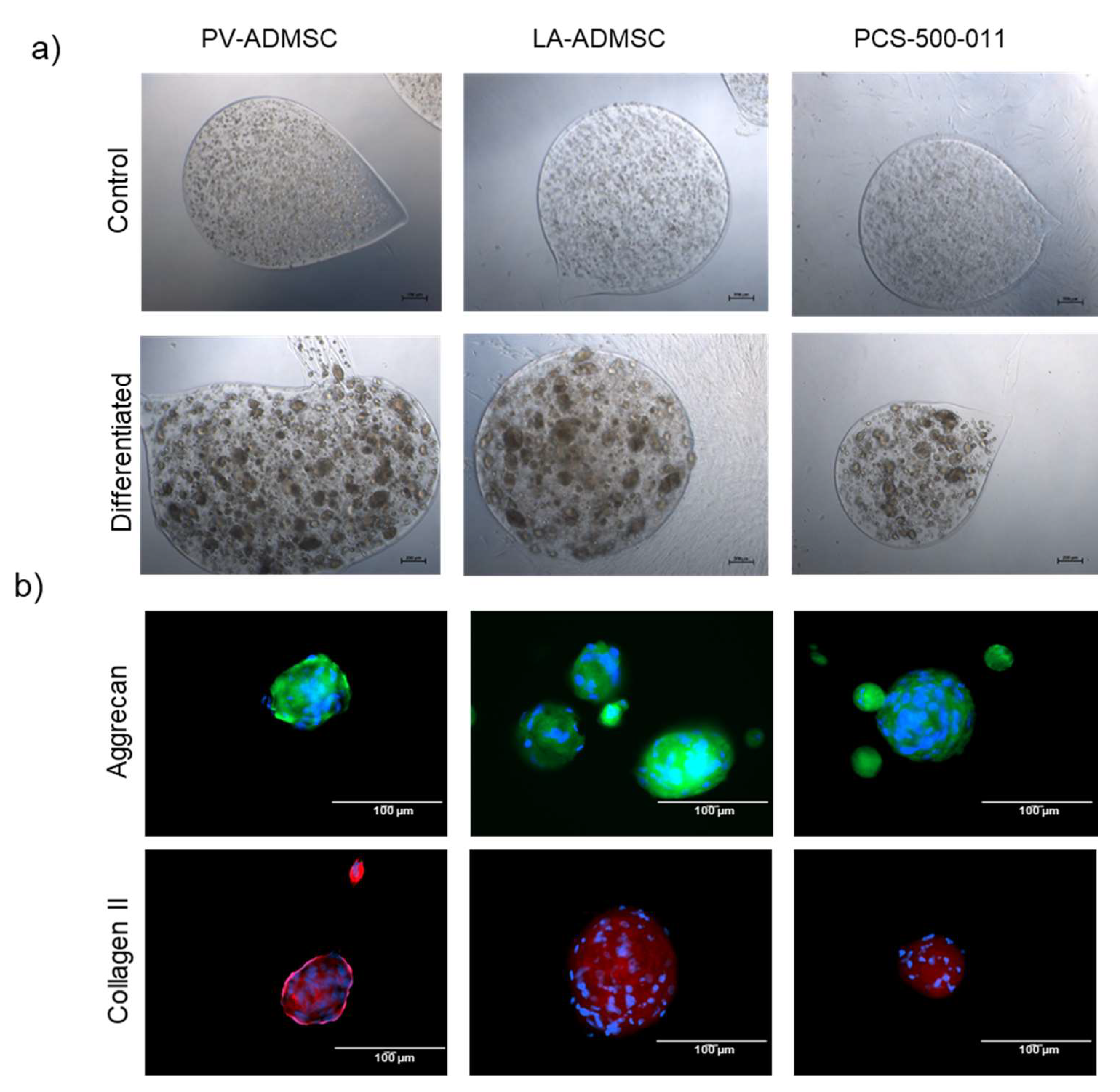
3.7. Chondrogenic Differentiation of MSCs
4. Discussion
5. Conclusions
- The regenerative power of the paediatric patient as the source of the ADMSCs cannot be overlooked and present an important advantage in potential clinical applications.
- Since the source sample was obtained in regular surgery, such procedures could be a yet unexploited future source of stem cells and lead to crucial new cell therapies in the future.
- Since our isolation relies on a waste product during surgery, it also does not pose any additional harm to the patient and is ethically acceptable.
- Using available separation technologies (e.g., MACS), this ADMSC source could be even directly applied after harvesting in patients, suitable for such cell therapy.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Han, I.; Kwon, B.-S.; Park, H.-K.; Kim, K.S. Differentiation Potential of Mesenchymal Stem Cells Is Related to Their Intrinsic Mechanical Properties. Int. Neurourol. J. 2017, 21, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, A.; Mitchell, K.; Soans, J.; Kim, L.; Zaidi, R. The use of mesenchymal stem cells for cartilage repair and regeneration: A systematic review. J. Orthop. Surg. Res. 2017, 12, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koźlik, M.; Wójcicki, P. The use of stem cells in plastic and reconstructive surgery. Adv. Clin. Exp. Med. 2014, 23, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimised Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 1465. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Palomares Cabeza, V.; Hoogduijn, M.J.; Kraaijeveld, R.; Franquesa, M.; Witte-Bouma, J.; Wolvius, E.B.; Farrell, E.; Brama, P.A.J. Pediatric Mesenchymal Stem Cells Exhibit Immunomodulatory Properties Toward Allogeneic T and B Cells under Inflammatory Conditions. Front. Bioeng. Biotechnol. 2019, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Balzanelli, M.G.; Distratis, P.; Catucci, O.; Cefalo, A.; Lazzaro, R.; Inchingolo, F.; Tomassone, D.; Aityan, S.K.; Ballini, A.; Nguyen, K.C.D.; et al. Mesenchymal Stem Cells: The Secret Children’s Weapons against the SARS-CoV-2 Lethal Infection. Appl. Sci. 2021, 11, 1696. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Bertana, S.; Parigi, G.P.; Giuntoli, M.; Pelagalli, M.; Battisti, C.; Bragheri, R.; Verga, L. Lipoblastoma and lipoblastomatosis in children. Minerva Pediatr. 1999, 51, 159–166. [Google Scholar] [PubMed]
- Lee, D.; Kuroki, T.; Nagai, T.; Kawano, K.; Higa, K.; Kurogi, S.; Hamanaka, H.; Chosa, E. Sarcopenia, Ectopic Fat Infiltration into the Lumbar Paravertebral Muscles, and Lumbo-Pelvic Deformity in Older Adults Undergoing Lumbar Surgery. Spine 2021, 47, E46–E57. [Google Scholar] [CrossRef] [PubMed]
- Eagles, M.E.; Gupta, N. Embryology of Spinal Dysraphism and its Relationship to Surgical Treatment. Can. J. Neurol. Sci. 2020, 47, 736–746. [Google Scholar] [CrossRef]
- Bakker-Niezen, S.H.; Walder, H.A.; Merx, J.L. The tethered spinal cord syndrome. Z. Kinderchir. 1984, 39 (Suppl. 2), 100–103. [Google Scholar] [CrossRef]
- Schneider, S.; Unger, M.; van Griensven, M.; Balmayor, E.R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med. Res. 2017, 22, 17. [Google Scholar] [CrossRef]
- Chang, J.; Liu, F.; Lee, M.; Wu, B.; Ting, K.; Zara, J.N.; Soo, C.; Al Hezaimi, K.; Zou, W.; Chen, X.; et al. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 9469–9474. [Google Scholar] [CrossRef] [Green Version]
- Naranda, J.; Sušec, M.; Maver, U.; Gradišnik, L.; Gorenjak, M.; Vukasović, A.; Ivković, A.; Rupnik, M.S.; Vogrin, M.; Krajnc, P. Polyester type polyHIPE scaffolds with an interconnected porous structure for cartilage regeneration. Sci. Rep. 2016, 6, 28695. [Google Scholar] [CrossRef]
- Huang, L.; Yi, L.; Zhang, C.; He, Y.; Zhou, L.; Liu, Y.; Qian, L.; Hou, S.; Weng, T. Synergistic Effects of FGF-18 and TGF-β3 on the Chondrogenesis of Human Adipose-Derived Mesenchymal Stem Cells in the Pellet Culture. Stem Cells Int. 2018, 2018, 7139485. [Google Scholar] [CrossRef] [Green Version]
- Stolke, D.; Zumkeller, M.; Seifert, V. Intraspinal lipomas in infancy and childhood causing a tethered cord syndrome. Neurosurg. Rev. 1988, 11, 59–65. [Google Scholar] [CrossRef]
- Sawadkar, P.; Mandakhbayar, N.; Patel, K.D.; Buitrago, J.O.; Kim, T.H.; Rajasekar, P.; Lali, F.; Kyriakidis, C.; Rahmani, B.; Mohanakrishnan, J.; et al. Three dimensional porous scaffolds derived from collagen, elastin and fibrin proteins orchestrate adipose tissue regeneration. J. Tissue Eng. 2021, 12, 20417314211019238. [Google Scholar] [CrossRef] [PubMed]
- Rožanc, J.; Žižek, M.; Milojević, M.; Maver, U.; Finšgar, M. Dexamethasone-Loaded Bioactive Coatings on Medical Grade Stainless Steel Promote Osteointegration. Pharmaceutics 2021, 13, 568. [Google Scholar] [CrossRef]
- Casanellas, I.; Lagunas, A.; Vida, Y.; Pérez-Inestrosa, E.; Andrades, J.A.; Becerra, J.; Samitier, J. The Janus Role of Adhesion in Chondrogenesis. Int. J. Mol. Sci. 2020, 21, 5269. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Wu, T.-Y.; Guan, Z.-Y.; Wang, P.-Y.; Yang, Y.-C.; Huang, C.-W.; Lin, T.-H.; Chen, H.-Y. Vapor-phased fabrication and modulation of cell-laden scaffolding materials. Nat. Commun. 2021, 12, 3413. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Ning, H.; Lin, G.; Lue, T.F. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 2012, 14, 1159–1163. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell. Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S.E. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 12097–12102. [Google Scholar] [CrossRef] [Green Version]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 5 November 2021).
- Knuth, C.A.; Kiernan, C.H.; Palomares Cabeza, V.; Lehmann, J.; Witte-Bouma, J.; Ten Berge, D.; Brama, P.A.; Wolvius, E.B.; Strabbing, E.M.; Koudstaal, M.J.; et al. Isolating Pediatric Mesenchymal Stem Cells with Enhanced Expansion and Differentiation Capabilities. Tissue Eng. Part C Methods 2018, 24, 313–321. [Google Scholar] [CrossRef]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.-J.; Zhou, G.-D.; Liu, Y.; Liu, X.; Chen, J.-N.; Luo, X.-S.; Cao, Y.-L. In vitro proliferation and differentiation of adipose-derived stem cells isolated using anti-CD105 magnetic beads. Int. J. Mol. Med. 2012, 30, 826–834. [Google Scholar] [CrossRef]
- Aslan, H.; Zilberman, Y.; Kandel, L.; Liebergall, M.; Oskouian, R.J.; Gazit, D.; Gazit, Z. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells 2006, 24, 1728–1737. [Google Scholar] [CrossRef]
- Arufe, M.C.; De la Fuente, A.; Fuentes-Boquete, I.; De Toro, F.J.; Blanco, F.J. Differentiation of synovial CD-105(+) human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J. Cell. Biochem. 2009, 108, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralisation. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Ruetze, M.; Richter, W. Adipose-derived stromal cells for osteoarticular repair: Trophic function versus stem cell activity. Expert Rev. Mol. Med. 2014, 16, e9. [Google Scholar] [CrossRef]
- Norambuena, G.A.; Khoury, M.; Jorgensen, C. Mesenchymal stem cells in osteoarticular pediatric diseases: An update. Pediatr. Res. 2012, 71, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Ahsan, M.; Saleem, Z.; Iqtedar, M.; Islam, M.; Danish, Z.; Khan, A.M. Mesenchymal stem cells (MSCs) as skeletal therapeutics–an update. J. Biomed. Sci. 2016, 23, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene Name | Gene ID | Function | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|---|---|
| CD44 | 960 | Mesenchymal stem cell markers | GAAGAAGGTGTGGGCAGAAG | TCTGCAGGTTCCTTGTCTCA |
| ENG | 2022 | GAGGCGGTGGTCAATATCCT | GGACACTCTGACCTGCACAA | |
| NT5E | 4907 | TCTCTCAAATCCAGGGACAAATT | GTCCACACCCCTCACTTTCT | |
| THY1 | 7070 | CTAACAGTCTTGCAGGTCTCC | CTTCTTTGTCTCACGGGTCAG | |
| CD34 | 947 | Haematopoietic stem cell marker | TCTTGGCCAACAGAACAGAAAT | ATAGCCAGTGATGCCCAAGA |
| PECAM1 | 5175 | Vascular endothelial marker | TGACCCTTCTGCTCTGTTCA | CTGAGGCTTGACGTGAGAGG |
| HLA-DRA | 3122 | Haematopoietic markers | CTCAAGCACTGGGAGTTTGA | CGTTCTGCTGCATTGCTTT |
| PTPRC | 5788 | ACTACTCCATCTAAGCCAACATG | CACCTCATTGTTTGTGCAAGT | |
| ADIPOQ | 9370 | Adipogenic markers | TGACATCAGGGCTCAGGAT | GGTGCCATCTCTGCCATCAC |
| FABP4 | 2167 | GCCAGGAATTTGACGAAGTCAC | TTCTGCACATGTACCAGGACAC | |
| PPARG | 5468 | GACCACTCCCACTCCTTTGA | TCCACTTTGATTGCACTTTGGTA | |
| ALPL [17] | 249 | Osteogenic markers | GACCTCCTCGGAAGACACTC | TGAAGGGCTTCTTGTCTGTG |
| OMD | 4958 | ACCCGAGTGTTTTCCAAGAAG | TGGTCATAGTCTTCATCCCACT | |
| RUNX2 | 860 | CAACTTCCTGTGCTCGGTG | CTCTTGCCTCGTCCACTCC | |
| ACAN [18] | 176 | Chondrogenic markers | TGAGGAGGGCTGGAACAAGTACC | GGAGGTGGTAATTGCAGGGAACA |
| COL2A1 [18] | 1280 | TTTCCCAGGTCAAGATGGTC | CTGCAGCACCTGTCTCACCA | |
| COL10A1 [19] | 1300 | GGCAACAGCATTATGACC | GATGATGGCACTCCCTGAA | |
| B2M | 567 | Internal control | TTCTGGCCTGGAGGCTATC | TCAGGAAATTTGACTTTCCATTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rožanc, J.; Gradišnik, L.; Velnar, T.; Gregorič, M.; Milojević, M.; Vihar, B.; Gole, B.; Maver, U. Mesenchymal Stem Cells Isolated from Paediatric Paravertebral Adipose Tissue Show Strong Osteogenic Potential. Biomedicines 2022, 10, 378. https://doi.org/10.3390/biomedicines10020378
Rožanc J, Gradišnik L, Velnar T, Gregorič M, Milojević M, Vihar B, Gole B, Maver U. Mesenchymal Stem Cells Isolated from Paediatric Paravertebral Adipose Tissue Show Strong Osteogenic Potential. Biomedicines. 2022; 10(2):378. https://doi.org/10.3390/biomedicines10020378
Chicago/Turabian StyleRožanc, Jan, Lidija Gradišnik, Tomaž Velnar, Minja Gregorič, Marko Milojević, Boštjan Vihar, Boris Gole, and Uroš Maver. 2022. "Mesenchymal Stem Cells Isolated from Paediatric Paravertebral Adipose Tissue Show Strong Osteogenic Potential" Biomedicines 10, no. 2: 378. https://doi.org/10.3390/biomedicines10020378
APA StyleRožanc, J., Gradišnik, L., Velnar, T., Gregorič, M., Milojević, M., Vihar, B., Gole, B., & Maver, U. (2022). Mesenchymal Stem Cells Isolated from Paediatric Paravertebral Adipose Tissue Show Strong Osteogenic Potential. Biomedicines, 10(2), 378. https://doi.org/10.3390/biomedicines10020378









