Cycloastragenol Inhibits Experimental Abdominal Aortic Aneurysm Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Sample Size Calculation
2.4. Experimental Animals, Ethical Statement, Housing and Husbandry
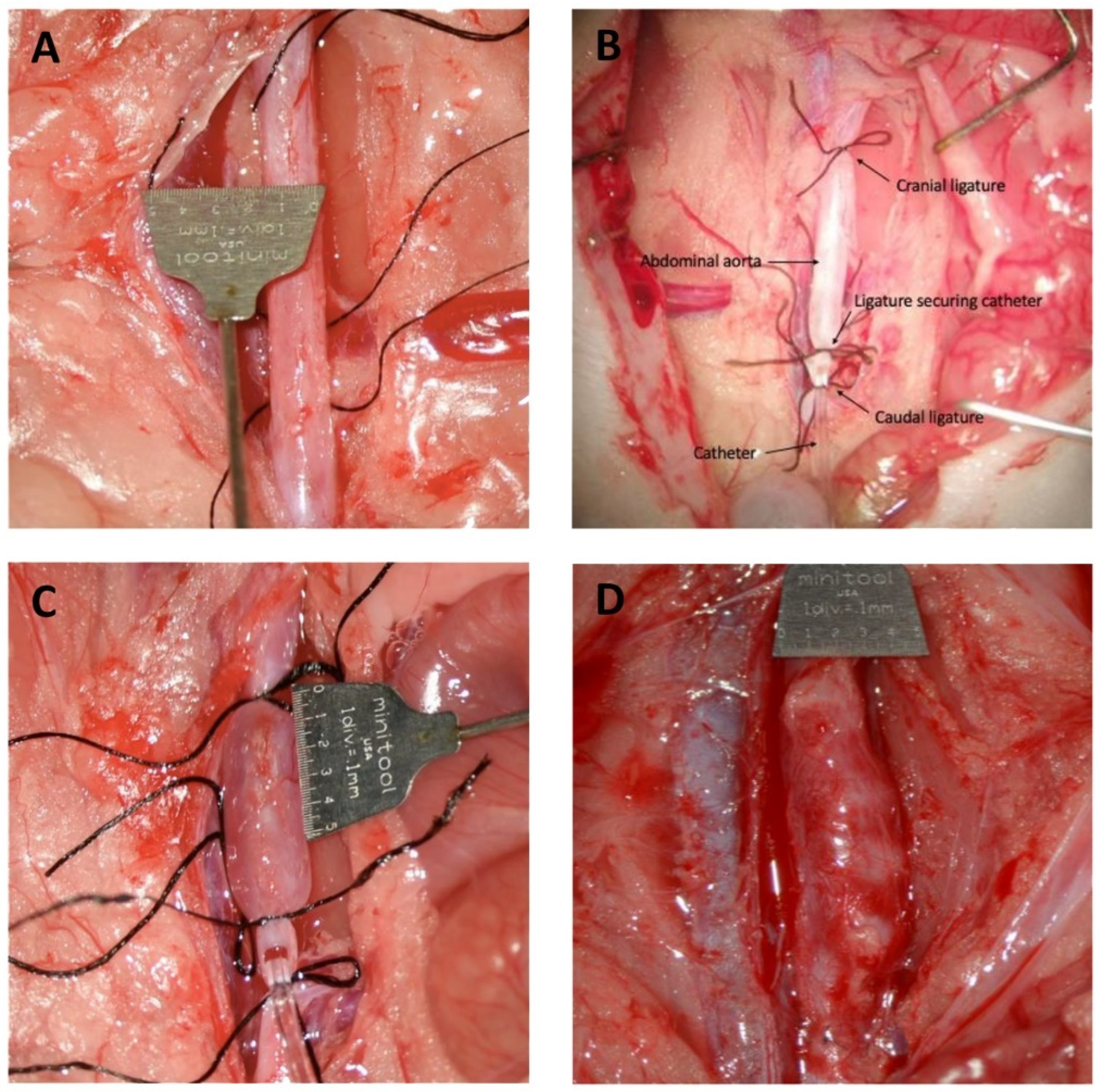
2.5. Induction of Abdominal Aortic Aneurysm by Perfusion of Pancreatic Porcine Elastase (PPE) in the Infrarenal Region of the Aorta
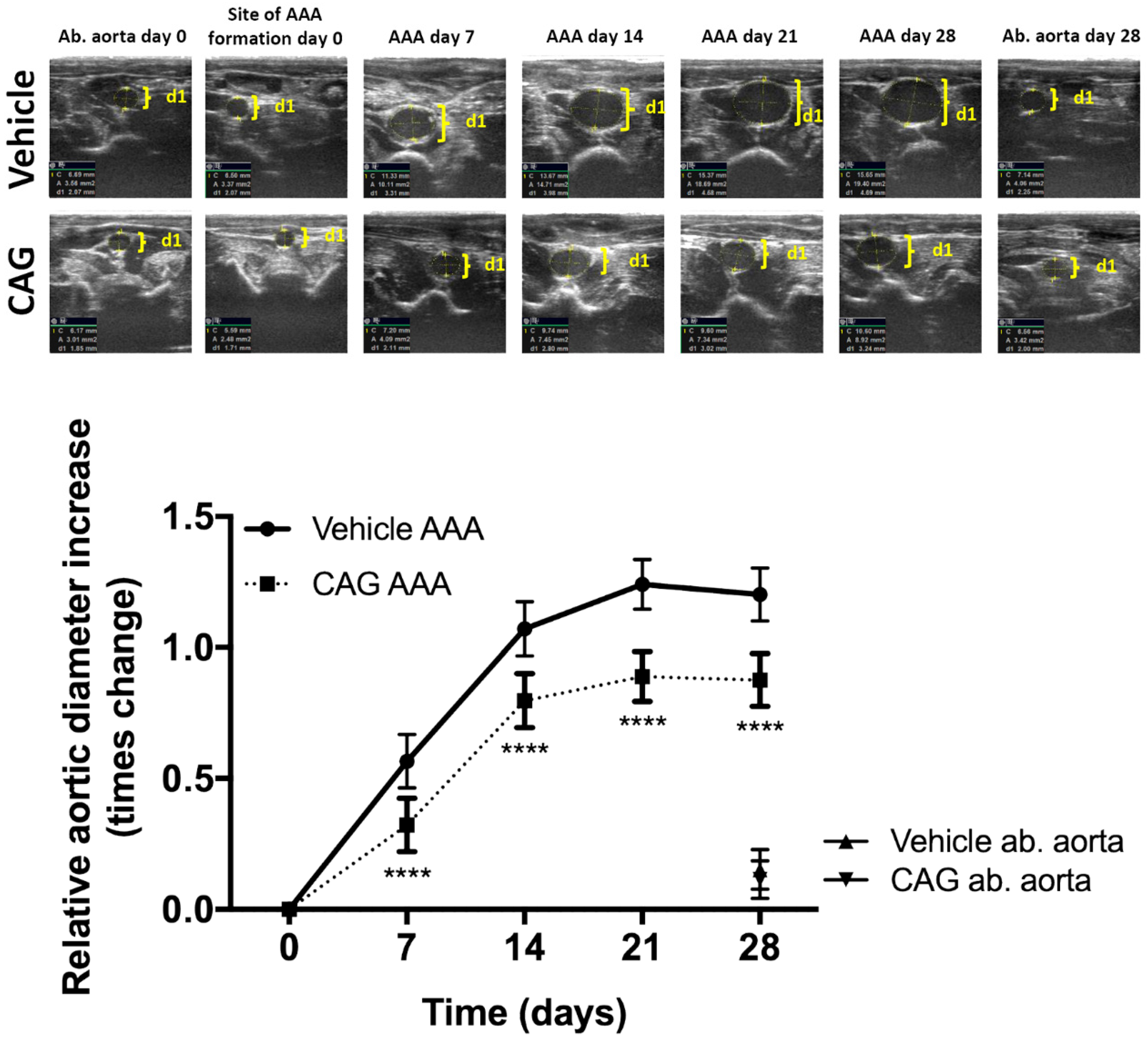
2.6. Ultrasound Measurement of Aneurysm Progression
2.7. At Termination
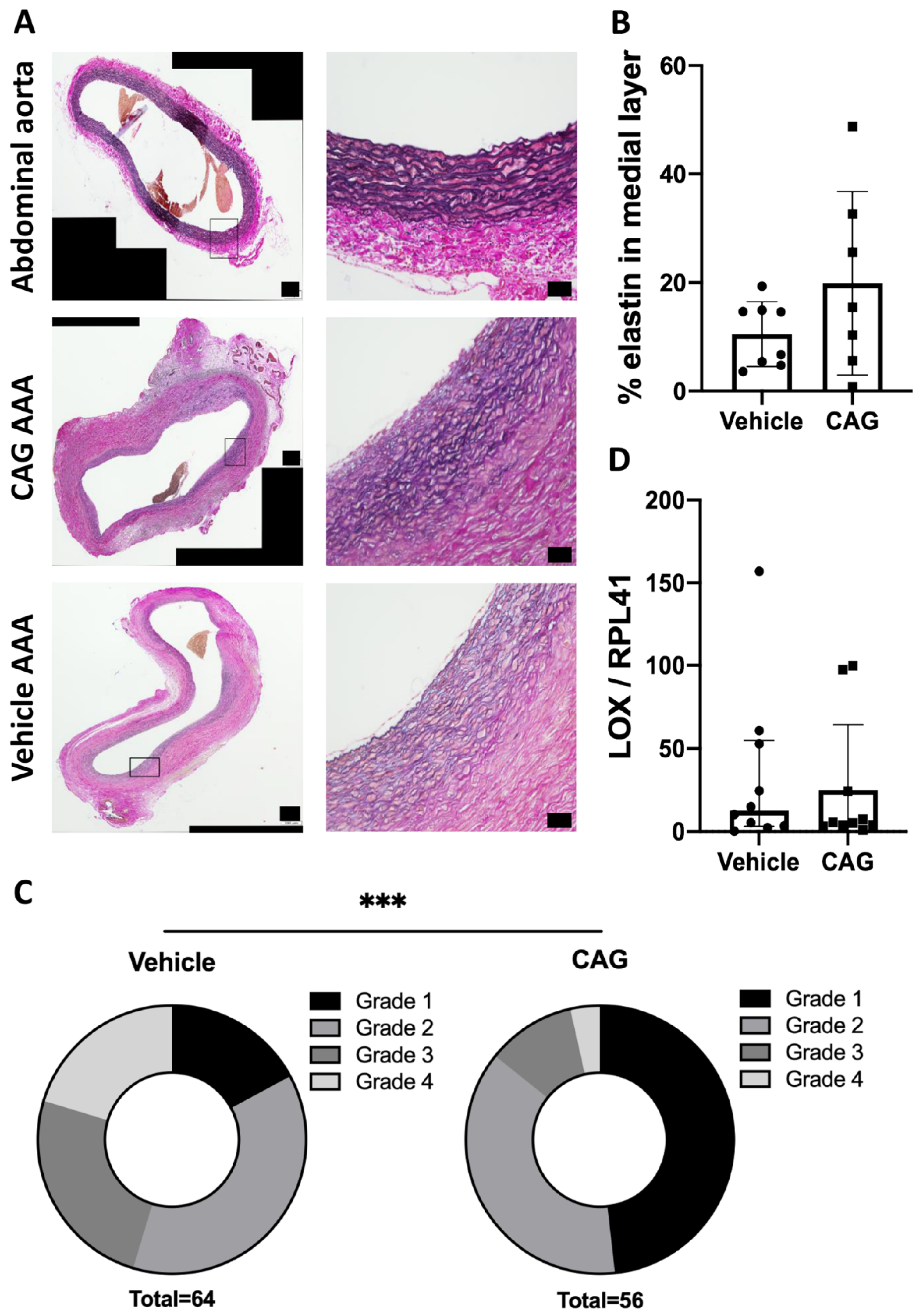
2.8. Miller’s Elastin and Calcium Von Kossa’s Staining
2.9. Immunohistochemistry
2.10. Elastin Content Analysis and Immunohistochemical Cell Count
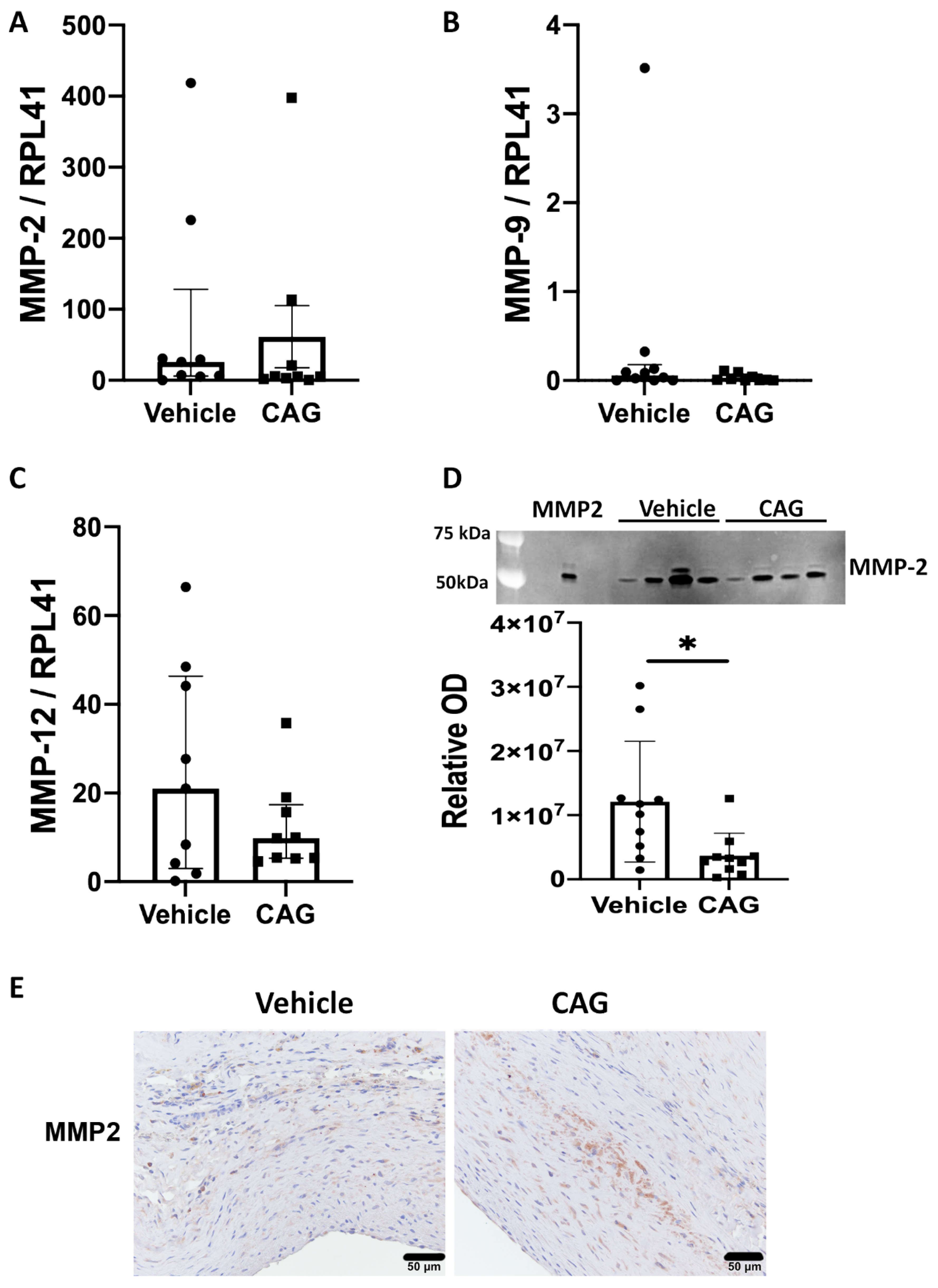
2.11. Zymography
2.12. Proteomic Analysis
2.13. Quantitative Polymerase Chain Reaction Measurements (qPCR)
2.14. Statistical Methods
3. Results
3.1. CAG Treatment Inhibited AAA Expansion
3.2. CAG Treatment Affects Elastin Integrity
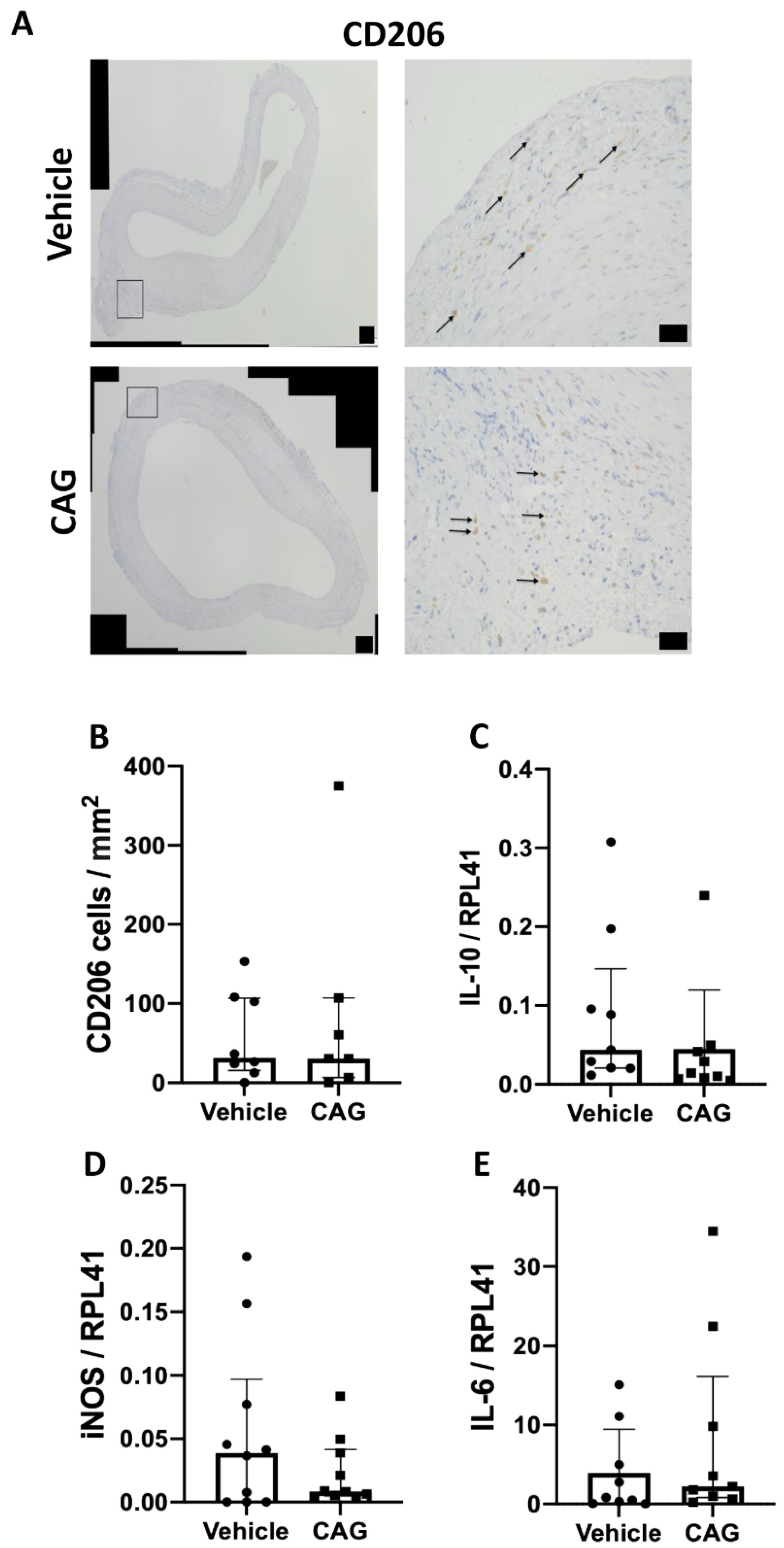
3.3. The Effect of CAG on Infiltration of Inflammatory Cells into the Aneurysm Wall
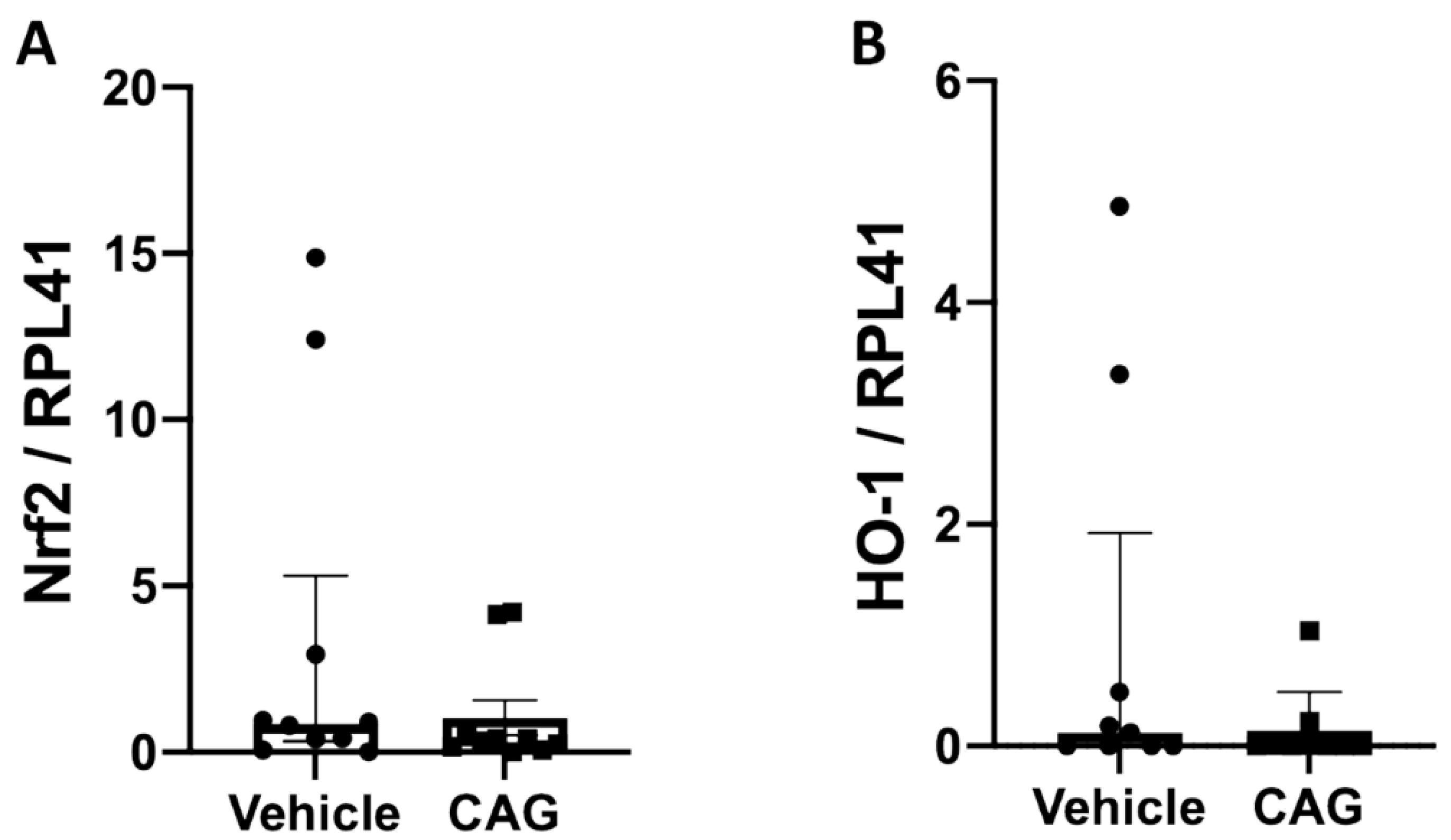
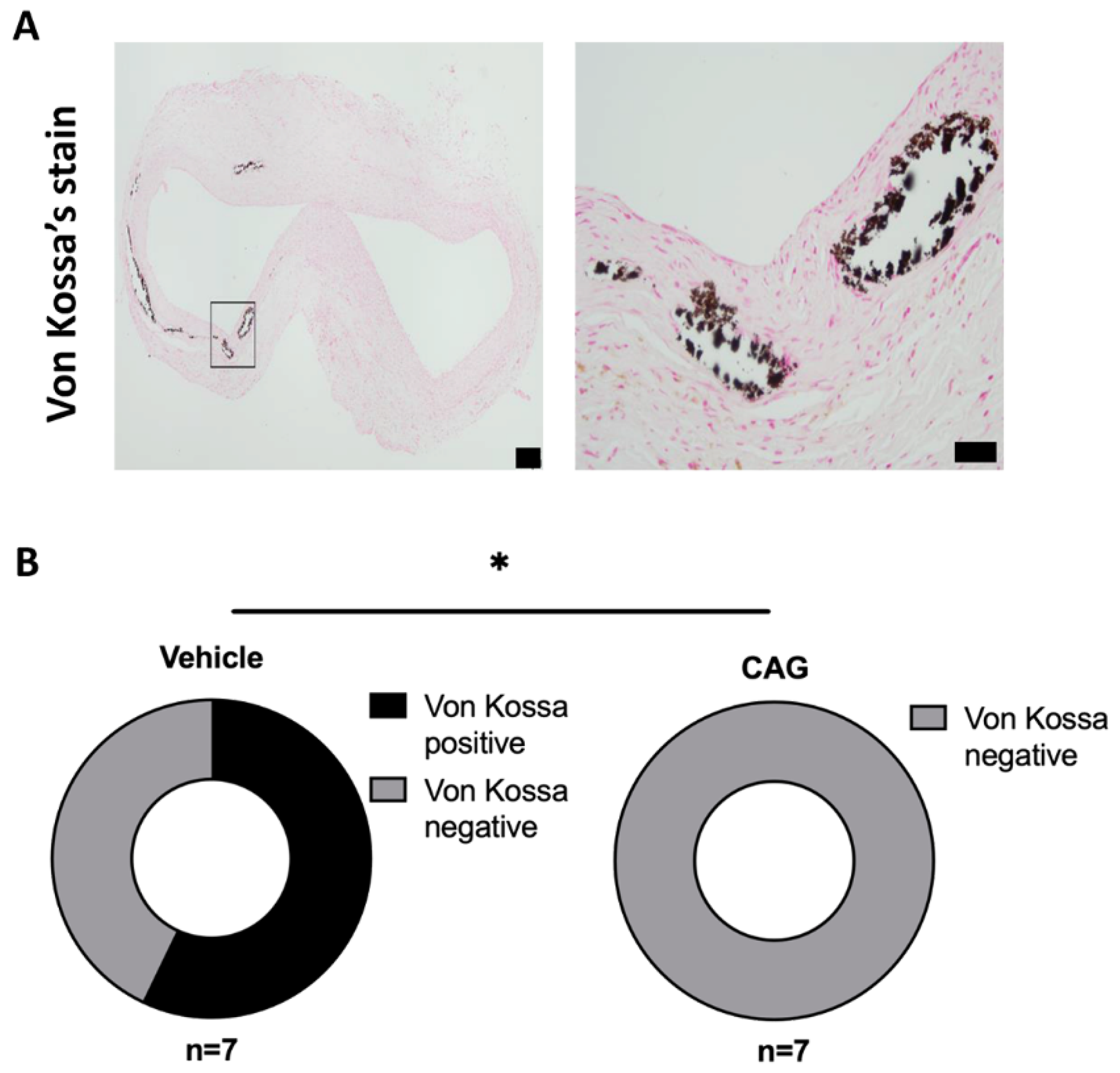
3.4. The Effect of CAG on Oxidative Stress and Calcification of the Aneurysm Wall
3.5. The Effect of CAG AAA Protein Composition Using Explorative Proteomics
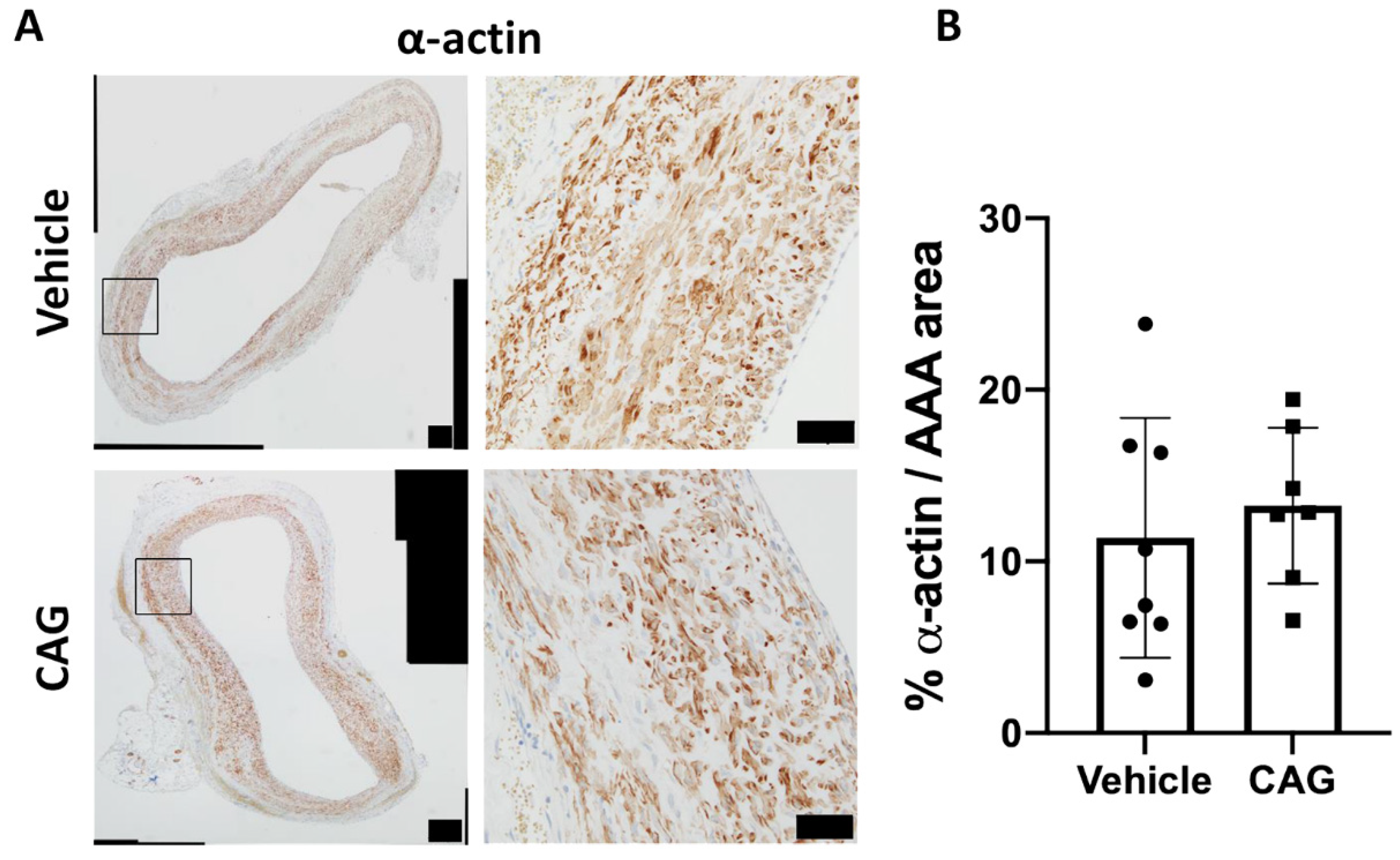
3.6. Effect of CAG on Vascular Smooth Muscle Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. Cardiovasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Handa, A.; Silver, L.E.; Rothwell, P.M. Population-Based Study of Incidence of Acute Abdominal Aortic Aneurysms With Projected Impact of Screening Strategy. J. Am. Heart Assoc. 2015, 4, e001926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filardo, G.; Powell, J.T.; Martinez, M.A.; Ballard, D.J. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015, 2015, Cd001835. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.C. Clinical practice. Abdominal aortic aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.; van Keulen, J.W.; Rantner, B.; et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41 (Suppl. 1), S1–S58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ailawadi, G.; Eliason, J.L.; Upchurch, G.R., Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J. Vasc. Surg. 2003, 38, 584–588. [Google Scholar] [CrossRef] [Green Version]
- Campa, J.S.; Greenhalgh, R.M.; Powell, J.T. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis 1987, 65, 13–21. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Schluterman, M.K.; Brekken, R.A. Fibulin-5, an integrin-binding matricellular protein: Its function in development and disease. J. Cell Commun. Signal. 2009, 3, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Dale, M.A.; Xiong, W.; Carson, J.S.; Suh, M.K.; Karpisek, A.D.; Meisinger, T.M.; Casale, G.P.; Baxter, B.T. Elastin-Derived Peptides Promote Abdominal Aortic Aneurysm Formation by Modulating M1/M2 Macrophage Polarization. J. Immunol. 2016, 196, 4536–4543. [Google Scholar] [CrossRef] [Green Version]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Dale, M.A.; Ruhlman, M.K.; Baxter, B.T. Inflammatory cell phenotypes in AAAs: Their role and potential as targets for therapy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1746–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, M.L.; Gavrila, D.; Weintraub, N.L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Zhou, L.; Yang, Y.; Liu, Y. Cycloastragenol: An exciting novel candidate for age-associated diseases. Exp. Ther. Med. 2018, 16, 2175–2182. [Google Scholar] [CrossRef]
- Sun, C.; Jiang, M.; Zhang, L.; Yang, J.; Zhang, G.; Du, B.; Ren, Y.; Li, X.; Yao, J. Cycloastragenol mediates activation and proliferation suppression in concanavalin A-induced mouse lymphocyte pan-activation model. Immunopharmacol. Immunotoxicol. 2017, 39, 131–139. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.L.; Cao, S.P.; Cai, H.; Zhao, Z.M.; Song, Y.H. Cycloastragenol ameliorates experimental heart damage in rats by promoting myocardial autophagy via inhibition of AKT1-RPS6KB1 signaling. Biomed. Pharmacother. 2018, 107, 1074–1081. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, S.; Zhao, Y.; Huang, J.; Wang, Y.; Li, Y.; Fan, S.; Yang, L.; Ji, G.; Tong, Q.; et al. Cycloastragenol improves hepatic steatosis by activating farnesoid X receptor signalling. Pharmacol. Res. 2017, 121, 22–32. [Google Scholar] [CrossRef]
- Deng, G.; Chen, W.; Wang, P.; Zhan, T.; Zheng, W.; Gu, Z.; Wang, X.; Ji, X.; Sun, Y. Inhibition of NLRP3 inflammasome-mediated pyroptosis in macrophage by cycloastragenol contributes to amelioration of imiquimod-induced psoriasis-like skin inflammation in mice. Int. Immunopharmacol. 2019, 74, 105682. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Wang, Q.; Cao, Y.; Xu, L.; Qi, R. Inhibitory effects of cycloastragenol on abdominal aortic aneurysm and its related mechanisms. Br. J. Pharmacol. 2019, 176, 282–296. [Google Scholar] [CrossRef] [Green Version]
- Anidjar, S.; Salzmann, J.L.; Gentric, D.; Lagneau, P.; Camilleri, J.P.; Michel, J.B. Elastase-induced experimental aneurysms in rats. Circulation 1990, 82, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Lysgaard Poulsen, J.; Stubbe, J.; Lindholt, J.S. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 487–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group NCRRGW. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.F.; Salmon, M.; Su, G.; Lu, G.; Ailawadi, G.; Upchurch, G.R., Jr. Aromatase is required for female abdominal aortic aneurysm protection. J. Vasc. Surg. 2015, 61, 1565–1574.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ailawadi, G.; Eliason, J.L.; Roelofs, K.J.; Sinha, I.; Hannawa, K.K.; Kaldjian, E.P.; Lu, G.; Henke, P.K.; Stanley, J.C.; Weiss, S.J.; et al. Gender differences in experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2116–2122. [Google Scholar] [CrossRef] [Green Version]
- Abelson, K.S.; Jacobsen, K.R.; Sundbom, R.; Kalliokoski, O.; Hau, J. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab. Anim. 2012, 46, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Schack, A.S.; Stubbe, J.; Steffensen, L.B.; Mahmoud, H.; Laursen, M.S.; Lindholt, J.S. Intraluminal infusion of Penta-Galloyl Glucose reduces abdominal aortic aneurysm development in the elastase rat model. PLoS ONE 2020, 15, e0234409. [Google Scholar] [CrossRef]
- Steffensen, L.B.; Stubbe, J.; Lindholt, J.S.; Beck, H.C.; Overgaard, M.; Bloksgaard, M.; Genovese, F.; Nielsen, S.H.; Tha, M.L.T.; Bang-Moeller, S.K.; et al. Basement membrane collagen IV deficiency promotes abdominal aortic aneurysm formation. Sci. Rep. 2021, 11, 12903. [Google Scholar] [CrossRef]
- Mulorz, J.; Spin, J.M.; Beck, H.C.; Tha Thi, M.L.; Wagenhäuser, M.U.; Rasmussen, L.M.; Lindholt, J.S.; Tsao, P.S.C.; Steffensen, L.B. Hyperlipidemia does not affect development of elastase-induced abdominal aortic aneurysm in mice. Atherosclerosis 2020, 311, 73–83. [Google Scholar] [CrossRef]
- Wintmo, P.; Johansen, S.H.; Hansen, P.B.L.; Lindholt, J.S.; Urbonavicius, S.; Rasmussen, L.M.; Bie, P.; Jensen, B.L.; Stubbe, J. The water channel AQP1 is expressed in human atherosclerotic vascular lesions and AQP1 deficiency augments angiotensin II-induced atherosclerosis in mice. Acta Physiol. 2017, 220, 446–460. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabkin, S.W. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog. Mol. Biol. Transl. Sci. 2017, 147, 239–265. [Google Scholar] [PubMed]
- Buijs, R.V.; Willems, T.P.; Tio, R.A.; Boersma, H.H.; Tielliu, I.F.; Slart, R.H.; Zeebregts, C.J. Calcification as a risk factor for rupture of abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 542–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncada, S.; Vane, J.R. The role of prostacyclin in vascular tissue. Fed. Proc. 1979, 38, 66–71. [Google Scholar]
- Wirka, R.C.; Wagh, D.; Paik, D.T.; Pjanic, M.; Nguyen, T.; Miller, C.L.; Kundu, R.; Nagao, M.; Coller, J.; Koyano, T.K.; et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by singlecell analysis. Nat. Med. 2019, 25, 1280–1289. [Google Scholar] [CrossRef]
- Li, P.F.; Dietz, R.; von Harsdorf, R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett. 1997, 404, 249–252. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, K.; Hasegawa, T.; Tanaka, A.; Wulan, B.; Yu, J.; Morimoto, N.; Okita, Y.; Okada, K. Free-radical scavenger edaravone inhibits both formation and development of abdominal aortic aneurysm in rats. J. Vasc. Surg. 2012, 55, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Peppin, G.J.; Weiss, S.J. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc. Natl. Acad. Sci. USA 1986, 83, 4322–4326. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [Green Version]
- Sénémaud, J.; Caligiuri, G.; Etienne, H.; Delbosc, S.; Michel, J.B.; Coscas, R. Translational Relevance and Recent Advances of Animal Models of Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhou, Y.; Wu, S.; Huang, K.; Thapa, S.; Tao, L.; Wang, J.; Shen, Y.; Wang, J.; Xue, Y.; et al. Astragaloside IV Attenuated 3,4-Benzopyrene-Induced Abdominal Aortic Aneurysm by Ameliorating Macrophage-Mediated Inflammation. Front. Pharmacol. 2018, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Aoki, H.; Ohno, S.; Furusho, A.; Hirakata, S.; Nishida, N.; Ito, S.; Hayashi, M.; Imaizumi, T.; Fukumoto, Y. The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS ONE 2017, 12, e0185923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhou, Y.Z.; Wu, Y.; Wu, Q.Y.; Liao, X.B.; Fu, X.M.; Zhou, X.M. Diverse roles of macrophage polarization in aortic aneurysm: Destruction and repair. J. Transl. Med. 2018, 16, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emeto, T.I.; Moxon, J.V.; Au, M.; Golledge, J. Oxidative stress and abdominal aortic aneurysm: Potential treatment targets. Clin. Sci. 2016, 130, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Usui, F.; Shirasuna, K.; Kimura, H.; Tatsumi, K.; Kawashima, A.; Karasawa, T.; Yoshimura, K.; Aoki, H.; Tsutsui, H.; Noda, T.; et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Ran, R.; Zhang, C.; Li, R.; Chen, B.; Zhang, W.; Zhao, Z.; Fu, Z.; Du, Z.; Du, X.; Yang, X.; et al. Evaluation and Comparison of the Inhibition Effect of Astragaloside IV and Aglycone Cycloastragenol on Various UDP-Glucuronosyltransferase (UGT) Isoforms. Molecules 2016, 21, 1616. [Google Scholar] [CrossRef] [Green Version]
- Azuma, J.; Wong, R.J.; Morisawa, T.; Hsu, M.; Maegdefessel, L.; Zhao, H.; Kalish, F.; Kayama, Y.; Wallenstein, M.B.; Deng, A.C.; et al. Heme Oxygenase-1 Expression Affects Murine Abdominal Aortic Aneurysm Progression. PLoS ONE 2016, 11, e0149288. [Google Scholar] [CrossRef]
- Schillinger, M.; Exner, M.; Mlekusch, W.; Domanovits, H.; Huber, K.; Mannhalter, C.; Wagner, O.; Minar, E. Heme oxygenase-1 gene promoter polymorphism is associated with abdominal aortic aneurysm. Thromb. Res. 2002, 106, 131–136. [Google Scholar] [CrossRef]
- Szabo, N.J. Dietary safety of cycloastragenol from Astragalus spp.: Subchronic toxicity and genotoxicity studies. Food Chem. Toxicol. 2014, 64, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Mion, C.; Arujo, A.; N’Guyen, Q.V.; Paleyrac, G.; Hemmendinger, S.; Cazenave, J.P. Prostacyclin (epoprostenol) as the sole antithrombotic agent in postdilutional hemofiltration. Nephron 1988, 48, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Stitham, J.; Midgett, C.; Martin, K.A.; Hwa, J. Prostacyclin: An inflammatory paradox. Front. Pharmacol. 2011, 2, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, Q.; Zhao, W.; Li, J.; Sun, Y.; Liu, K.; Liu, B.; Zhang, N. Astragaloside IV and cycloastragenol are equally effective in inhibition of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in the endothelium. J. Ethnopharmacol. 2015, 169, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, R.; Xing, B.; Zhou, L.; Zhang, P.; Song, L. Astragaloside IV ameliorates preeclampsia-induced oxidative stress through the Nrf2/HO-1 pathway in a rat model. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E904–E911. [Google Scholar] [CrossRef] [PubMed]
- Carsten, C.G., 3rd; Calton, W.C.; Johanning, J.M.; Armstrong, P.J.; Franklin, D.P.; Carey, D.J.; Elmore, J.R. Elastase is not sufficient to induce experimental abdominal aortic aneurysms. J. Vasc. Surg. 2001, 33, 1255–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]

| Target | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | R2-Value |
|---|---|---|---|
| Lysyl oxidase (LOX) | ACCTGGTACCCGATCCCTAC | AGTCTCTGACATCCGCCCTA | 0.99 |
| Inducible nitric oxidase synthase (iNOS) | AGGCAAGCCCTCACCTACTT | GATGGGAACTCTTCCAGCAC | 0.98 |
| Mature macrophages (F4/80) | TTTTGGCTGCTCCTCTTCTG | TGGCATAAGCTGGACAAGTG | 0.98 |
| Interleukin-6 (IL-6) | CAGAGTCATTCAGAGCAATAC | CTTTCAAGATGACTTGGATGG | 0.98 |
| Interleukin-10 (IL-10) | TCTCCCCTGTGAGAATAAAA | TAGACACCTTTGTCTTGGAG | 0.96 |
| Matrix Metalloprotease 2 (MMP-2) | GATCTTCTTCCTTCAAGGATCG | TACACGGCATCAATCTTTTC | 0.99 |
| Matrix Metalloprotease 9 (MMP-9) | TACTTTGGAAACGCAAATGG | GTGTAGGATTCTACTGGG | 0.99 |
| Matrix Metalloprotease 12 (MMP-12) | CAATATTGGAGGTACGATGTG | GTCATATTCCAATTGGTAGGC | 0.90 |
| Cluster of differentation 45 (CD45) | GCTATAAAAGACCCCTTCAG | CATTAGGCAAATAGAGACACTG | 0.99 |
| Heme oxygenase 1 (HO-1) | ACAGAAGAGGCTAAGACCG | CAGGCATCTCCTTCCATT | 0.99 |
| Nuclear factor erythroid-2-related factor (Nrf2) | CCATTTGTAGATGACCATGAG | CTATTAAGACACTGTAACTCGG | 0.95 |
| Ribsomal Protein L41 (RPL41) | TGGCGGAAGAAGAGAATGC | TGGACCTCTGCCTCATCTTT | 0.99 |
| Accession | Description | Fold Change | p-Value |
|---|---|---|---|
| O08658 | Nuclear pore complex protein | 1.17 | 0.001 |
| Q9Z1X1 | Extended synaptotagmin-1 | 1.15 | 0.003 |
| P61227 | Ras-related protein Rap-2b | 0.77 | 0.003 |
| P20171 | GTPase HRas OS = Rattus norvegicus | 0.79 | 0.004 |
| P53534 | Glycogen phosphorylase, brain form (Fragment) | 1.14 | 0.004 |
| P21263 | Nestin | 1.71 | 0.005 |
| Q62969 | Prostacyclin synthase | 1.25 | 0.007 |
| O35353 | Guanine nucleotide-binding protein subunit beta-4 | 1.22 | 0.010 |
| Q4V8H8 | EH domain-containing protein 2 | 1.16 | 0.012 |
| P09414 | Nuclear factor 1 A-type | 1.39 | 0.014 |
| O89043 | DNA polymerase alpha subunit B | 1.19 | 0.014 |
| Q8CF97 | Deubiquitinating protein VCIP135 | 0.80 | 0.014 |
| P63029 | Translationally-controlled tumor protein | 0.90 | 0.014 |
| P29975 | Aquaporin-1 | 1.25 | 0.016 |
| Q62745 | CD81 antigen | 1.07 | 0.016 |
| B2RYW9 | Fumarylacetoacetate hydrolase domain-containing protein 2 | 1.20 | 0.018 |
| Q7TQ16 | Cytochrome b-c1 complex subunit 8 | 1.18 | 0.018 |
| P60892 | Ribose-phosphate pyrophosphokinase 1 | 1.14 | 0.019 |
| Q9JLZ1 | Glutaredoxin-3 | 0.93 | 0.020 |
| Q9WVH8 | Fibulin-5 | 1.15 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melin, L.G.; Dall, J.H.; Lindholt, J.S.; Steffensen, L.B.; Beck, H.C.; Elkrog, S.L.; Clausen, P.D.; Rasmussen, L.M.; Stubbe, J. Cycloastragenol Inhibits Experimental Abdominal Aortic Aneurysm Progression. Biomedicines 2022, 10, 359. https://doi.org/10.3390/biomedicines10020359
Melin LG, Dall JH, Lindholt JS, Steffensen LB, Beck HC, Elkrog SL, Clausen PD, Rasmussen LM, Stubbe J. Cycloastragenol Inhibits Experimental Abdominal Aortic Aneurysm Progression. Biomedicines. 2022; 10(2):359. https://doi.org/10.3390/biomedicines10020359
Chicago/Turabian StyleMelin, Leander Gaarde, Julie Husted Dall, Jes S. Lindholt, Lasse B. Steffensen, Hans Christian Beck, Sophie L. Elkrog, Pernille D. Clausen, Lars Melholt Rasmussen, and Jane Stubbe. 2022. "Cycloastragenol Inhibits Experimental Abdominal Aortic Aneurysm Progression" Biomedicines 10, no. 2: 359. https://doi.org/10.3390/biomedicines10020359
APA StyleMelin, L. G., Dall, J. H., Lindholt, J. S., Steffensen, L. B., Beck, H. C., Elkrog, S. L., Clausen, P. D., Rasmussen, L. M., & Stubbe, J. (2022). Cycloastragenol Inhibits Experimental Abdominal Aortic Aneurysm Progression. Biomedicines, 10(2), 359. https://doi.org/10.3390/biomedicines10020359








