Somatostatin-Mediated Regulation of Retinoic Acid-Induced Differentiation of SH-SY5Y Cells: Neurotransmitters Phenotype Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. SH-SY5Y Cell Culture and Induction of Differentiation
2.2. Cell Morphology and Quantitative Analysis of Neurite Outgrowth
2.3. Immunofluorescence Immunocytochemistry
2.4. Western Blot Analysis
2.5. Semi-Quantitative Analysis of Neurotransmitter Phenotype in Relative Cell Numbers Showing Developmental Changes
2.6. Statistical Analysis
3. Results
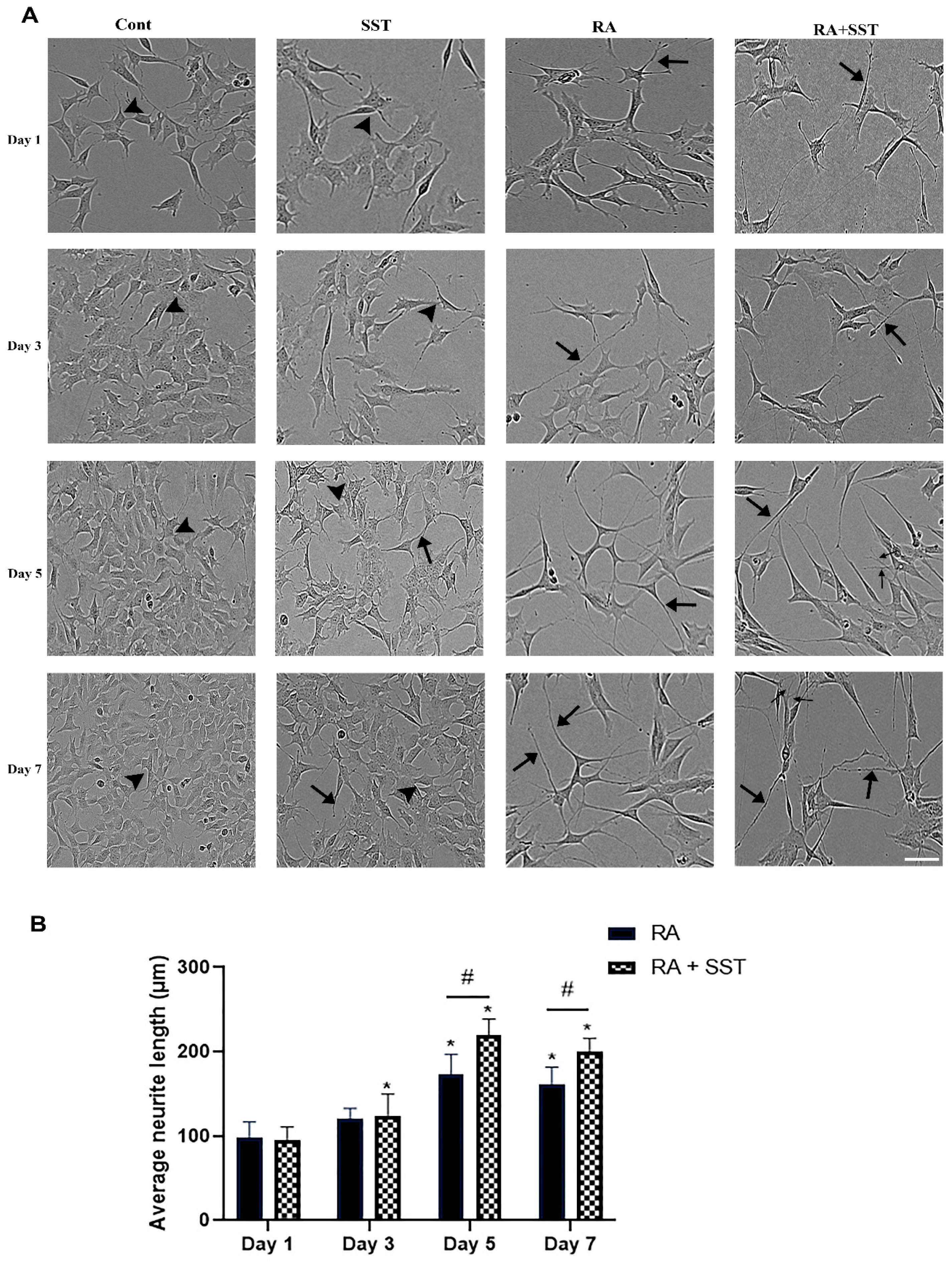
3.1. Retinoic Acid-Induced Morphological Changes, Neurite Formation, and Elongation of SH-SY5Y Cells
3.2. Developmental Changes in Synaptophysin in SH-SY5Y Cells
3.3. SST Promote Synaptophysin Translocation to Neurites Formation
3.4. Time-Dependent Changes in Subcellular Distribution and Expression of Somatostatin
3.5. Somatostatin Regulates Its Own Expression in SH-SY5Y Cells
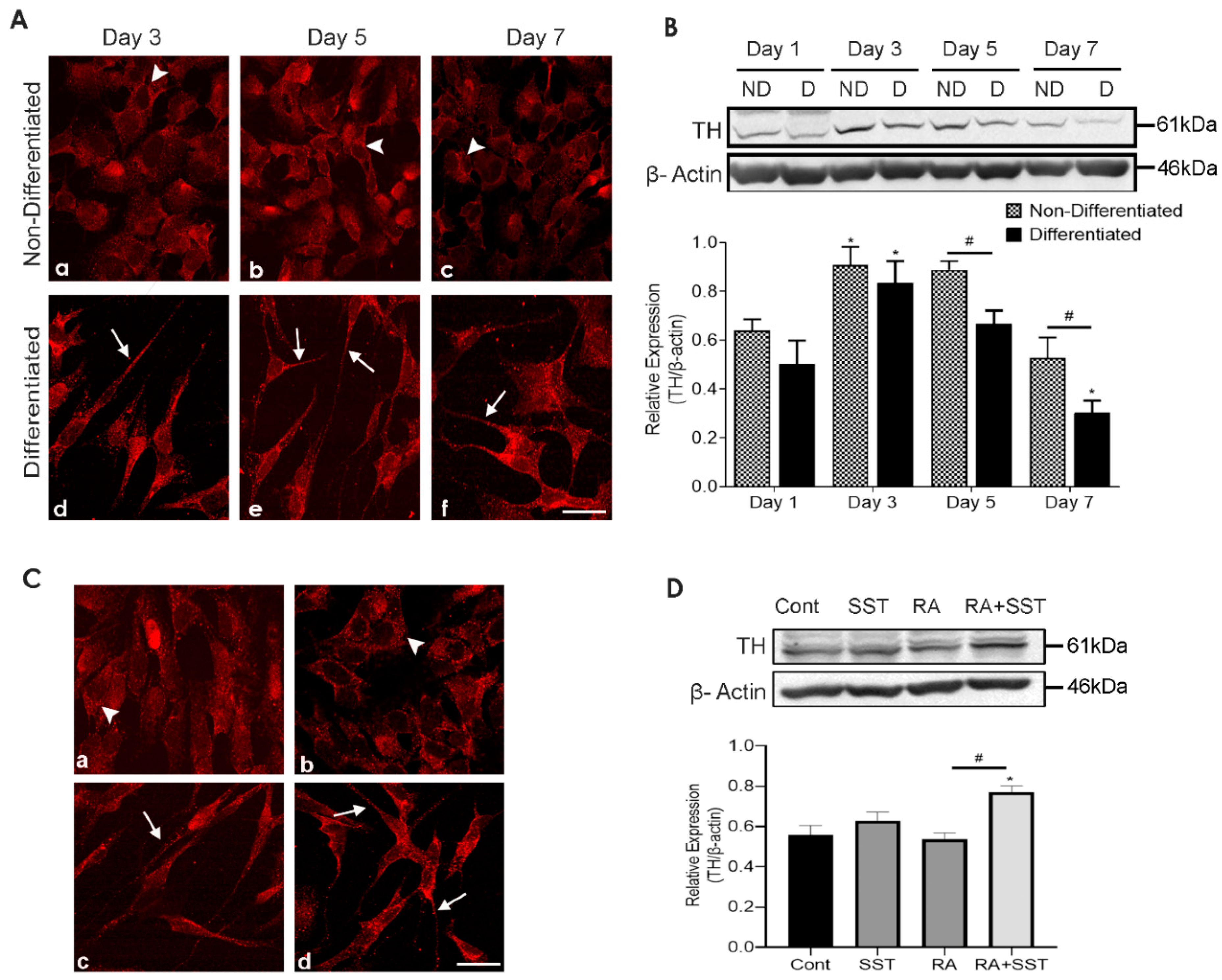
3.6. Time-Dependent Changes in Tyrosine Hydroxylase Immunoreactivity in Undifferentiated and Differentiating SH-SY5Y Cells
3.7. Somatostatin-Mediated Regulation of TH Expression in SH-SY5Y Cells
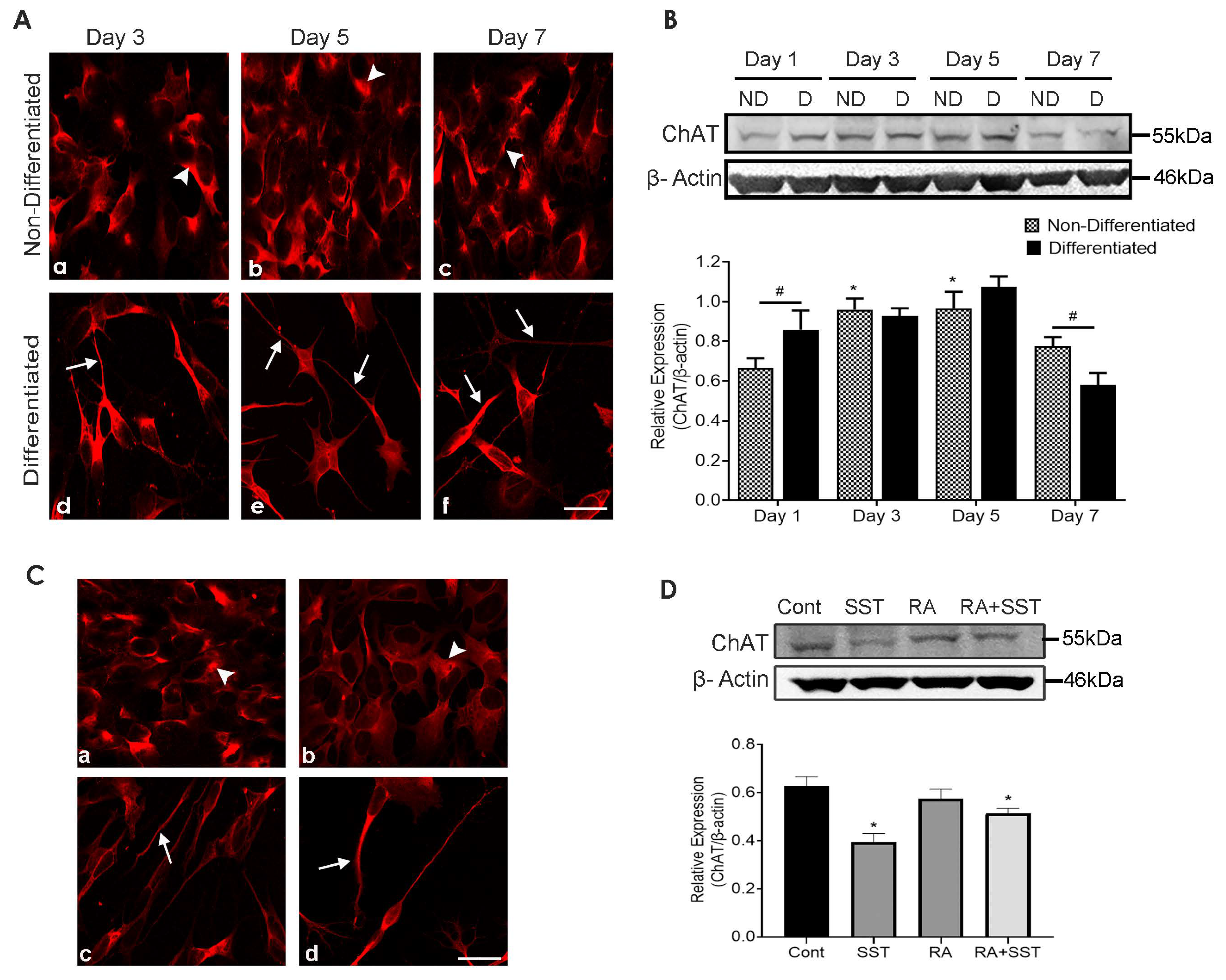
3.8. Developmental Changes in Choline Acetyltransferase in SH-SY5Y Cells
3.9. Somatostatin Decreases ChAT Expression in SH-SY5Y Cells
3.10. Time-Dependent Changes in Brain Nitric Oxide Synthase Immunoreactivity in SH-SY5Y Cells
3.11. Role of SST on Brain Nitric Oxide Synthase Expression in SH-SY5Y Cells
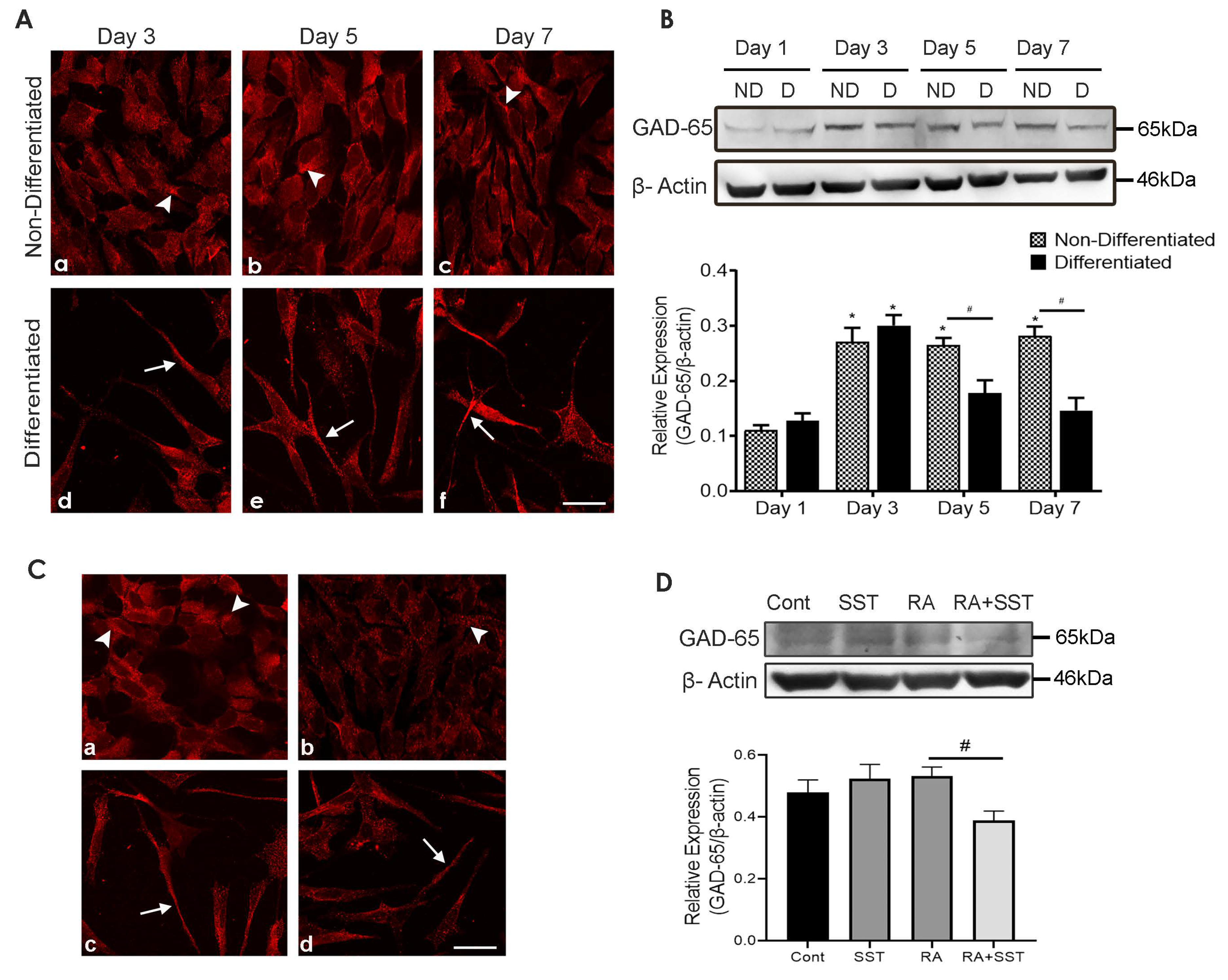
3.12. Developmental Changes in Glutamic Acid Decarboxylase-65 in SH-SY5Y Cells
3.13. SST Regulates Glutamic Acid Decarboxylase-65 in SH-SY5Y Cells
3.14. GABA Immunoreactivity Suppressed in SH-SY5Y Cells during Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shohayeb, B.; Diab, M.; Ahmed, M.; Ng, D.C.H. Factors that influence adult neurogenesis as potential therapy. Transl. Neurodegener. 2018, 7, 4. [Google Scholar] [CrossRef]
- Urban, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Gan, H.T.; Lam, C.S.; Poonepalli, A.; Ramasamy, S.; Tay, Y.; Tham, M.; Yu, Y.H. Transcription factors and neural stem cell self-renewal, growth and differentiation. Cell Adhes. Migr. 2009, 3, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Choe, Y.; Pleasure, S.J.; Mira, H. Control of Adult Neurogenesis by Short-Range Morphogenic-Signaling Molecules. Cold Spring Harb. Perspect. Biol. 2016, 8, a018887. [Google Scholar] [CrossRef] [Green Version]
- Cheung, Y.T.; Lau, W.K.W.; Yu, M.S.; Lai, C.S.W.; Yeung, S.C.; So, K.F.; Chang, R.C.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Ferlemann, F.C.; Menon, V.; Condurat, A.L.; Robbler, J.; Pruszak, J. Surface marker profiling of SH-SY5Y cells enables small molecule screens identifying BMP4 as a modulator of neuroblastoma differentiation. Sci. Rep. 2017, 7, 13612. [Google Scholar] [CrossRef] [Green Version]
- Forster, J.I.; Koglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M.A. Characterization of Differentiated SH-SY5Y as Neuronal Screening Model Reveals Increased Oxidative Vulnerability. J. Biomol. Screen 2016, 21, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Kito, K.; Ito, T.; Sakaki, Y. Fluorescent differential display analysis of gene expression in differentiating neuroblastoma cells. Gene 1997, 184, 73–81. [Google Scholar] [CrossRef]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef] [Green Version]
- Paik, S.; Somvanshi, R.K.; Kumar, U. Somatostatin-Mediated Changes in Microtubule-Associated Proteins and Retinoic Acid-Induced Neurite Outgrowth in SH-SY5Y Cells. J. Mol. Neurosci. 2019, 68, 120–134. [Google Scholar] [CrossRef]
- Presgraves, S.P.; Ahmed, T.; Borwege, S.; Joyce, J.N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 2004, 5, 579–598. [Google Scholar] [CrossRef]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar] [CrossRef]
- Agholme, L.; Lindstrom, T.; Kagedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimers Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [Green Version]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Clagett-Dame, M.; McNeill, E.M.; Muley, P.D. Role of all-Trans retinoic acid in neurite outgrowth and axonal elongation. J. Neurobiol. 2006, 66, 739–756. [Google Scholar] [CrossRef]
- Maden, M. Retinoic acid and limb regeneration—A personal view. Int. J. Dev. Biol. 2002, 46, 883–886. [Google Scholar]
- Constantinescu, R.; Constantinescu, A.T.; Reichmann, H.; Janetzky, B. Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. J. Neural Transm. Suppl. 2007, 72, 17–28. [Google Scholar] [CrossRef]
- Murillo, J.R.; Goto-Silva, L.; Sanchez, A.; Nogueira, F.C.S.; Domont, G.B.; Junqueira, M. Quantitative proteomic analysis identifies proteins and pathways related to neuronal development in differentiated SH-SY5Y neuroblastoma cells. EuPA Open Proteom. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Zimmermann, M.; Gardoni, F.; Marcello, E.; Colciaghi, F.; Borroni, B.; Padovani, A.; Cattabeni, F.; Di Luca, M. Acetylcholinesterase inhibitors increase ADAM10 activity by promoting its trafficking in neuroblastoma cell lines. J. Neurochem. 2004, 90, 1489–1499. [Google Scholar] [CrossRef]
- Cai, Y.; Xing, L.; Yang, T.; Chai, R.; Wang, J.; Bao, J.; Shen, W.; Ding, S.; Chen, G. The neurodevelopmental role of dopaminergic signaling in neurological disorders. Neurosci. Lett. 2021, 741, 135540. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.Q. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef]
- Gritti, A.; Parati, E.A.; Cova, L.; Frolichsthal, P.; Galli, R.; Wanke, E.; Faravelli, L.; Morassutti, D.J.; Roisen, F.; Nickel, D.D.; et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 1091–1100. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.; Palmer, T.D.; Gage, F.H. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J. Neurobiol. 1999, 38, 65–81. [Google Scholar] [CrossRef]
- Lopes, F.M.; Schroder, R.; da Frota, M.L.C.; Zanotto, A.; Muller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Cooper-Kuhn, C.M.; Winkler, J.; Kuhn, H.G. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J. Neurosci. Res. 2004, 77, 155–165. [Google Scholar] [CrossRef]
- Coronas, V.; Durand, M.; Chabot, J.G.; Jourdan, F.; Quirion, R. Acetylcholine induces neuritic outgrowth in rat primary olfactory bulb cultures. Neuroscience 2000, 98, 213–219. [Google Scholar] [CrossRef]
- Packer, M.A.; Stasiv, Y.; Benraiss, A.; Chmielnicki, E.; Grinberg, A.; Westphal, H.; Goldman, S.A.; Enikolopov, G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 9566–9571. [Google Scholar] [CrossRef] [Green Version]
- Schmid, L.C.; Mittag, M.; Poll, S.; Steffen, J.; Wagner, J.; Geis, H.R.; Schwarz, I.; Schmidt, B.; Schwarz, M.K.; Remy, S.; et al. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer’s Disease Model. Neuron 2016, 92, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Ballo, L.; Pietila, L.; Viesselmann, C.; Ballweg, J.; Lumbard, D.; Stevenson, M.; Merriam, E.; Dent, E.W. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 15597–15603. [Google Scholar] [CrossRef] [Green Version]
- War, S.A.; Kim, B.; Kumar, U. Human somatostatin receptor-3 distinctively induces apoptosis in MCF-7 and cell cycle arrest in MDA-MB-231 breast cancer cells. Mol. Cell. Endocrinol. 2015, 413, 129–144. [Google Scholar] [CrossRef]
- Paik, S.; Somvanshi, R.K.; Oliveira, H.A.; Zou, S.; Kumar, U. Somatostatin Ameliorates beta-Amyloid-Induced Cytotoxicity via the Regulation of CRMP2 Phosphorylation and Calcium Homeostasis in SH-SY5Y Cells. Biomedicines 2021, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Bodenant, C.; Laquerrière, A.; Paresy, M.; Hemet, J.; Vaudry, H.; Leroux, P. Somatostatin does not affect multiplication of granule cells in the rat cerebellum. Peptides 1997, 18, 257–262. [Google Scholar] [CrossRef]
- Kungel, M.; Piechotta, K.; Rietzel, H.J.; Friauf, E. Influence of the neuropeptide somatostatin on the development of dendritic morphology: A cysteamine-depletion study in the rat auditory brainstem. Dev. Brain Res. 1997, 101, 107–114. [Google Scholar] [CrossRef]
- Sanford, S.D.; Gatlin, J.C.; Hokfelt, T.; Pfenninger, K.H. Growth cone responses to growth and chemotropic factors. Eur. J. Neurosci. 2008, 28, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Grimm-Jorgensen, Y. Somatostatin and calcitonin stimulate neurite regeneration of molluscan neurons in vitro. Brain Res. 1987, 403, 121–126. [Google Scholar] [CrossRef]
- Kentroti, S.; Vernadakis, A. Survival and proliferation in developing neuroblasts in cultures derived from embryos treated with ethanol during early neuroembryogenesis: Effects attenuated by somatostatin. J. Neurosci. Res. 1991, 30, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Bulloch, A.G. Somatostatin enhances neurite outgrowth and electrical coupling of regenerating neurons in Helisoma. Brain Res. 1987, 412, 6–17. [Google Scholar] [CrossRef]
- Epelbaum, J.; Dournaud, P.; Fodor, M.; Viollet, C. The neurobiology of somatostatin. Crit. Rev. Neurobiol. 1994, 8, 25–44. [Google Scholar]
- Maubert, E.; Slama, A.; Ciofi, P.; Viollet, C.; Tramu, G.; Dupouy, J.P.; Epelbaum, J. Developmental patterns of somatostatin-receptors and somatostatin-immunoreactivity during early neurogenesis in the rat. Neuroscience 1994, 62, 317–325. [Google Scholar] [CrossRef]
- Taniwaki, T.; Schwartz, J.P. Somatostatin enhances neurofilament expression and neurite outgrowth in cultured rat cerebellar granule cells. Brain Res. Dev. Brain Res. 1995, 88, 109–116. [Google Scholar] [CrossRef]
- Viollet, C.; Lepousez, G.; Loudes, C.; Videau, C.; Simon, A.; Epelbaum, J. Somatostatinergic systems in brain: Networks and functions. Mol. Cell. Endocrinol. 2008, 286, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pool, M.; Thiemann, J.; Bar-Or, A.; Fournier, A.E. NeuriteTracer: A novel ImageJ plugin for automated quantification of neurite outgrowth. J. Neurosci. Methods 2008, 168, 134–139. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B. Tests for Normal Distribution. In Goodness-Of-Fit Techniques; Statistics: Textbooks and Monographs; Dekker: New York, NY, USA, 1986. [Google Scholar]
- Singh, S.; Somvanshi, R.K.; Panda, V.; Kumar, U. Comparative distribution of somatostatin and somatostatin receptors in PTU-induced hypothyroidism. Endocrine 2020, 70, 92–106. [Google Scholar] [CrossRef]
- Kumar, U.; Singh, S. Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. Int. J. Mol. Sci. 2020, 21, 2568. [Google Scholar] [CrossRef] [Green Version]
- Aguila, M.C. Somatostatin decreases somatostatin messenger ribonucleic acid levels in the rat periventricular nucleus. Peptides 1998, 19, 1573–1579. [Google Scholar] [CrossRef]
- Sarkanen, J.R.; Nykky, J.; Siikanen, J.; Selinummi, J.; Ylikomi, T.; Jalonen, T.O. Cholesterol supports the retinoic acid-induced synaptic vesicle formation in differentiating human SH-SY5Y neuroblastoma cells. J. Neurochem. 2007, 102, 1941–1952. [Google Scholar] [CrossRef]
- Tarsa, L.; Goda, Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 1012–1016. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, S.H.; Li, J.Y.; Ahlman, H.; Dahlstrom, A. SSR2(a) receptor expression and adrenergic/cholinergic characteristics in differentiated SH-SY5Y cells. Neurochem. Res. 2003, 28, 449–460. [Google Scholar] [CrossRef]
- Kang, J.X.; Bell, J.; Leaf, A.; Beard, R.L.; Chandraratna, R.A. Retinoic acid alters the intracellular trafficking of the mannose-6-phosphate/insulin-like growth factor II receptor and lysosomal enzymes. Proc. Natl. Acad. Sci. USA 1998, 95, 13687–13691. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, B.; Leroux, P.; Lamacz, M.; Bodenant, C.; Balazs, R.; Vaudry, H. Somatostatin receptors are expressed by immature cerebellar granule cells: Evidence for a direct inhibitory effect of somatostatin on neuroblast activity. Proc. Natl. Acad. Sci. USA 1992, 89, 9627–9631. [Google Scholar] [CrossRef] [Green Version]
- Ferriero, D.M.; Sheldon, R.A.; Messing, R.O. Somatostatin enhances nerve growth factor-induced neurite outgrowth in PC12 cells. Brain Res. Dev. Brain Res. 1994, 80, 13–18. [Google Scholar] [CrossRef]
- Papadopoulos, G.C.; Cavanagh, M.E.; Antonopoulos, J.; Michaloudi, H.; Parnavelas, J.G. Postnatal development of somatostatin-containing neurons in the visual cortex of normal and dark-reared rats. Exp. Brain Res. 1993, 92, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Bendotti, C.; Hohmann, C.; Forloni, G.; Reeves, R.; Coyle, J.T.; Oster-Granite, M.L. Developmental expression of somatostatin in mouse brain. II. In situ hybridization. Brain Res. Dev. Brain Res. 1990, 53, 26–39. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kurihara, K.; Matsuoka, I. Retinoic acid induces BDNF responsiveness of sympathetic neurons by alteration of Trk neurotrophin receptor expression. FEBS Lett. 1994, 356, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Munoz, I.; Sanchez-Franco, F.; Vallejo, M.; Fernandez, A.; Palacios, N.; Fernandez, M.; Sanchez-Grande, M.; Cacicedo, L. Regulation of somatostatin gene expression by brain derived neurotrophic factor in fetal rat cerebrocortical cells. Brain Res. 2011, 1375, 28–40. [Google Scholar] [CrossRef]
- Izquierdo-Claros, R.M.; Boyano-Adanez, M.C.; Larsson, C.; Gustavsson, L.; Arilla, E. Acute effects of D1- and D2-receptor agonist and antagonist drugs on somatostatin binding, inhibition of adenylyl cyclase activity and accumulation of inositol 1,4,5-trisphosphate in the rat striatum. Brain Res. Mol. Brain Res. 1997, 47, 99–107. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, M.N.; Puebla, L.; Lopez-Sanudo, S.; Rodriguez-Martin, E.; Martin-Espinosa, A.; Rodriguez-Pena, M.S.; Juarranz, M.G.; Arilla, E. Dopamine enhances somatostatin receptor-mediated inhibition of adenylate cyclase in rat striatum and hippocampus. J. Neurosci. Res. 1997, 48, 238–248. [Google Scholar] [CrossRef]
- Marazioti, A.; Pitychoutis, P.M.; Papadopoulou-Daifoti, Z.; Spyraki, C.; Thermos, K. Activation of somatostatin receptors in the globus pallidus increases rat locomotor activity and dopamine release in the striatum. Psychopharmacology 2008, 201, 413–422. [Google Scholar] [CrossRef]
- Ma, W.; Maric, D.; Li, B.S.; Hu, Q.; Andreadis, J.D.; Grant, G.M.; Liu, Q.Y.; Shaffer, K.M.; Chang, Y.H.; Zhang, L.; et al. Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur. J. Neurosci. 2000, 12, 1227–1240. [Google Scholar] [CrossRef]
- Kaneko, N.; Okano, H.; Sawamoto, K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells Devoted Mol. Cell. Mech. 2006, 11, 1145–1159. [Google Scholar] [CrossRef]
- Delfs, J.R. Somatostatin and Alzheimer’s Disease: Possible pathophysiological associations. In Senile Dementia of the Alzheimer Type; Alan R. Liss, Inc.: New York, NY, USA, 1985; Volume 32, pp. 67–72. [Google Scholar]
- Del Bel, E.A.; Guimaraes, F.S.; Bermudez-Echeverry, M.; Gomes, M.Z.; Schiaveto-de-souza, A.; Padovan-Neto, F.E.; Tumas, V.; Barion-Cavalcanti, A.P.; Lazzarini, M.; Nucci-da-Silva, L.P.; et al. Role of nitric oxide on motor behavior. Cell. Mol. Neurobiol. 2005, 25, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Del-Bel, E.; Padovan-Neto, F.E.; Raisman-Vozari, R.; Lazzarini, M. Role of nitric oxide in motor control: Implications for Parkinson’s disease pathophysiology and treatment. Curr. Pharm. Des. 2011, 17, 471–488. [Google Scholar] [CrossRef]
- Fujibayashi, T.; Kurauchi, Y.; Hisatsune, A.; Seki, T.; Shudo, K.; Katsuki, H. Mitogen-activated protein kinases regulate expression of neuronal nitric oxide synthase and neurite outgrowth via non-classical retinoic acid receptor signaling in human neuroblastoma SH-SY5Y cells. J. Pharmacol. Sci. 2015, 129, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Personett, D.; Fass, U.; Panickar, K.; McKinney, M. Retinoic acid-mediated enhancement of the cholinergic/neuronal nitric oxide synthase phenotype of the medial septal SN56 clone: Establishment of a nitric oxide-sensitive proapoptotic state. J. Neurochem. 2000, 74, 2412–2424. [Google Scholar] [CrossRef] [PubMed]
- Nagl, F.; Schonhofer, K.; Seidler, B.; Mages, J.; Allescher, H.D.; Schmid, R.M.; Schneider, G.; Saur, D. Retinoic acid-induced nNOS expression depends on a novel PI3K/Akt/DAX1 pathway in human TGW-nu-I neuroblastoma cells. Am. J. Physiol.-Cell Ph. 2009, 297, C1146–C1156. [Google Scholar] [CrossRef] [PubMed]
- Barnabe-Heider, F.; Miller, F.D. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J. Neurosci. 2003, 23, 5149–5160. [Google Scholar] [CrossRef] [Green Version]
- Phung, Y.T.; Bekker, J.M.; Hallmark, O.G.; Black, S.M. Both neuronal NO synthase and nitric oxide are required for PC12 cell differentiation: A cGMP independent pathway. Mol. Brain Res. 1999, 64, 165–178. [Google Scholar] [CrossRef]
- Gendron, L.; Cote, F.; Payet, M.D.; Gallo-Payet, N. Nitric oxide and cyclic GMP are involved in angiotensin II AT(2) receptor effects on neurite outgrowth in NG108-15 cells. Neuroendocrinology 2002, 75, 70–81. [Google Scholar] [CrossRef]
- Yamazaki, M.; Chiba, K.; Mohri, T. Fundamental role of nitric oxide in neuritogenesis of PC12h cells. Br. J. Pharmacol. 2005, 146, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Rajput, P.S.; Kharmate, G.; Norman, M.; Liu, S.H.; Sastry, B.R.; Brunicardi, C.F.; Kumar, U. Somatostatin receptor 1 and 5 double knockout mice mimic neurochemical changes of Huntington’s disease transgenic mice. PLoS ONE 2011, 6, e24467. [Google Scholar] [CrossRef]
- Inotsuka, R.; Uchimura, K.; Yamatsu, A.; Kim, M.; Katakura, Y. gamma-Aminobutyric acid (GABA) activates neuronal cells by inducing the secretion of exosomes from intestinal cells. Food Funct. 2020, 11, 9285–9290. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, Y.; Onoue, S.; Tashiro, K.; Sato, M.; Hasegawa, T.; Katakura, Y. Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS ONE 2019, 14, e0217394. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, T.; Zaborszky, L. Somatostatin presynaptically inhibits both GABA and glutamate release onto rat basal forebrain cholinergic neurons. J. Neurophysiol. 2006, 96, 686–694. [Google Scholar] [CrossRef] [PubMed]



| Markers | Conditions | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|
| bNOS | ND | +++ | +++ | ++ |
| D | +++ | ++ | + | |
| ChAT | ND | +++ | +++ | ++ |
| D | +++ | +++ | ++ | |
| GABA | ND | ++ | +++ | ++ |
| D | ++ | +++ | + | |
| GAD-65 | ND | ++ | +++ | ++ |
| D | ++ | ++ | + | |
| SST | ND | ++ | ++ | + |
| D | +++ | +++ | + | |
| SYP | ND | + | +++ | ++ |
| D | ++ | ++ | + | |
| TH | ND | +++ | ++ | + |
| D | ++ | ++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Somvanshi, R.K.; Kumar, U. Somatostatin-Mediated Regulation of Retinoic Acid-Induced Differentiation of SH-SY5Y Cells: Neurotransmitters Phenotype Characterization. Biomedicines 2022, 10, 337. https://doi.org/10.3390/biomedicines10020337
Singh S, Somvanshi RK, Kumar U. Somatostatin-Mediated Regulation of Retinoic Acid-Induced Differentiation of SH-SY5Y Cells: Neurotransmitters Phenotype Characterization. Biomedicines. 2022; 10(2):337. https://doi.org/10.3390/biomedicines10020337
Chicago/Turabian StyleSingh, Sneha, Rishi K. Somvanshi, and Ujendra Kumar. 2022. "Somatostatin-Mediated Regulation of Retinoic Acid-Induced Differentiation of SH-SY5Y Cells: Neurotransmitters Phenotype Characterization" Biomedicines 10, no. 2: 337. https://doi.org/10.3390/biomedicines10020337
APA StyleSingh, S., Somvanshi, R. K., & Kumar, U. (2022). Somatostatin-Mediated Regulation of Retinoic Acid-Induced Differentiation of SH-SY5Y Cells: Neurotransmitters Phenotype Characterization. Biomedicines, 10(2), 337. https://doi.org/10.3390/biomedicines10020337





