Comparison of Neutralizing Antibody Responses at 6 Months Post Vaccination with BNT162b2 and AZD1222
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Procedure
2.2. Bioanalysis
2.3. Data Analysis
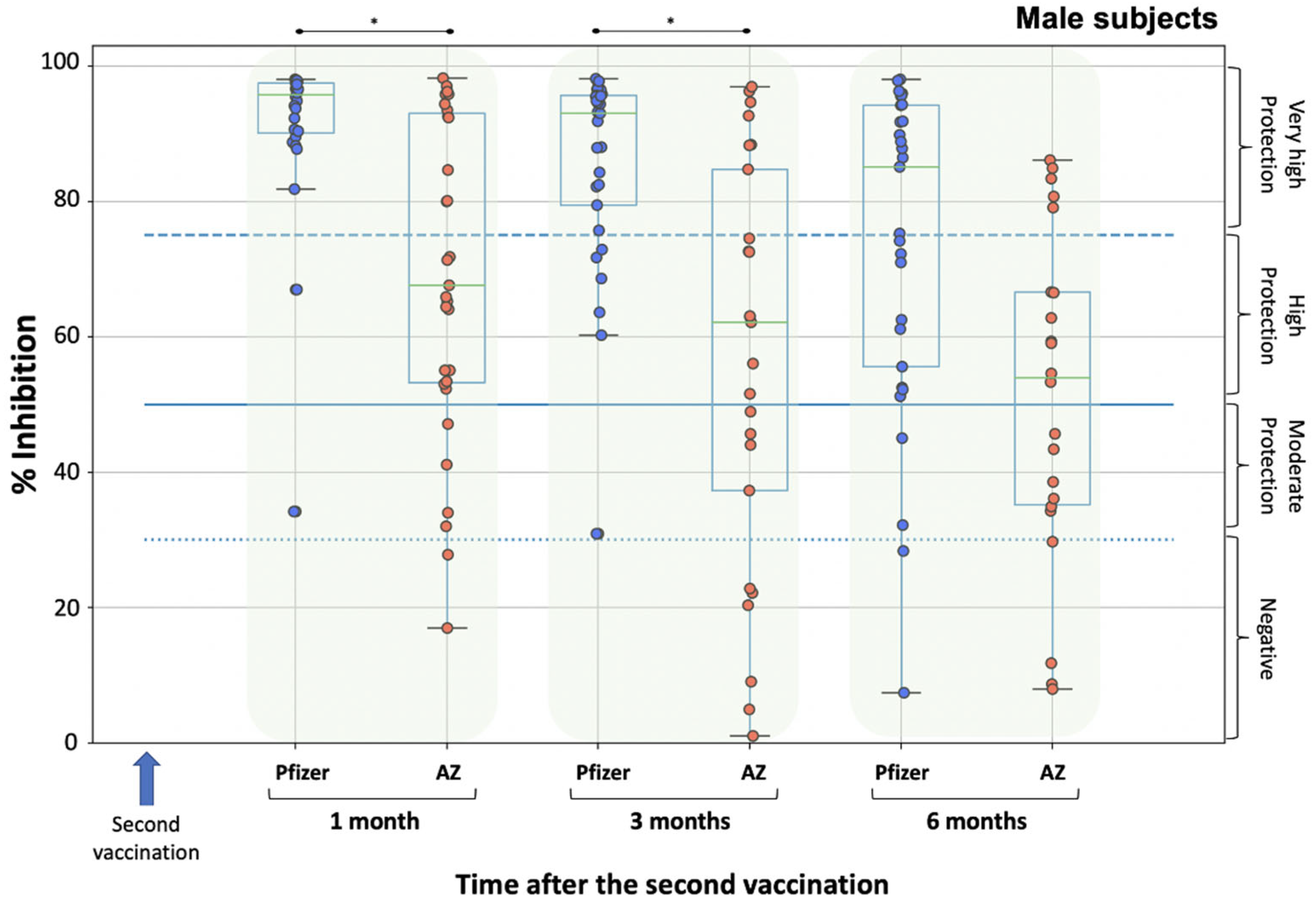
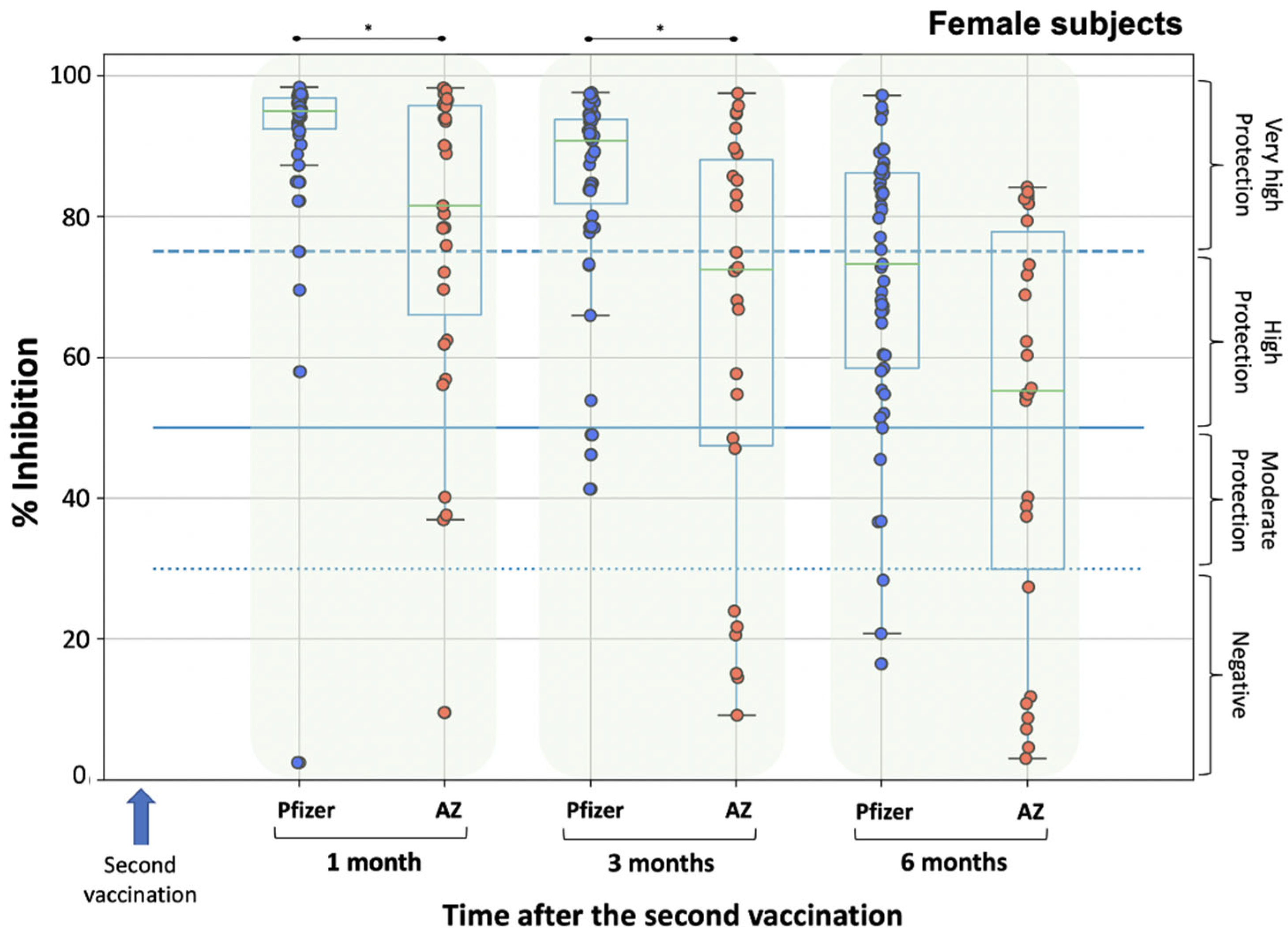
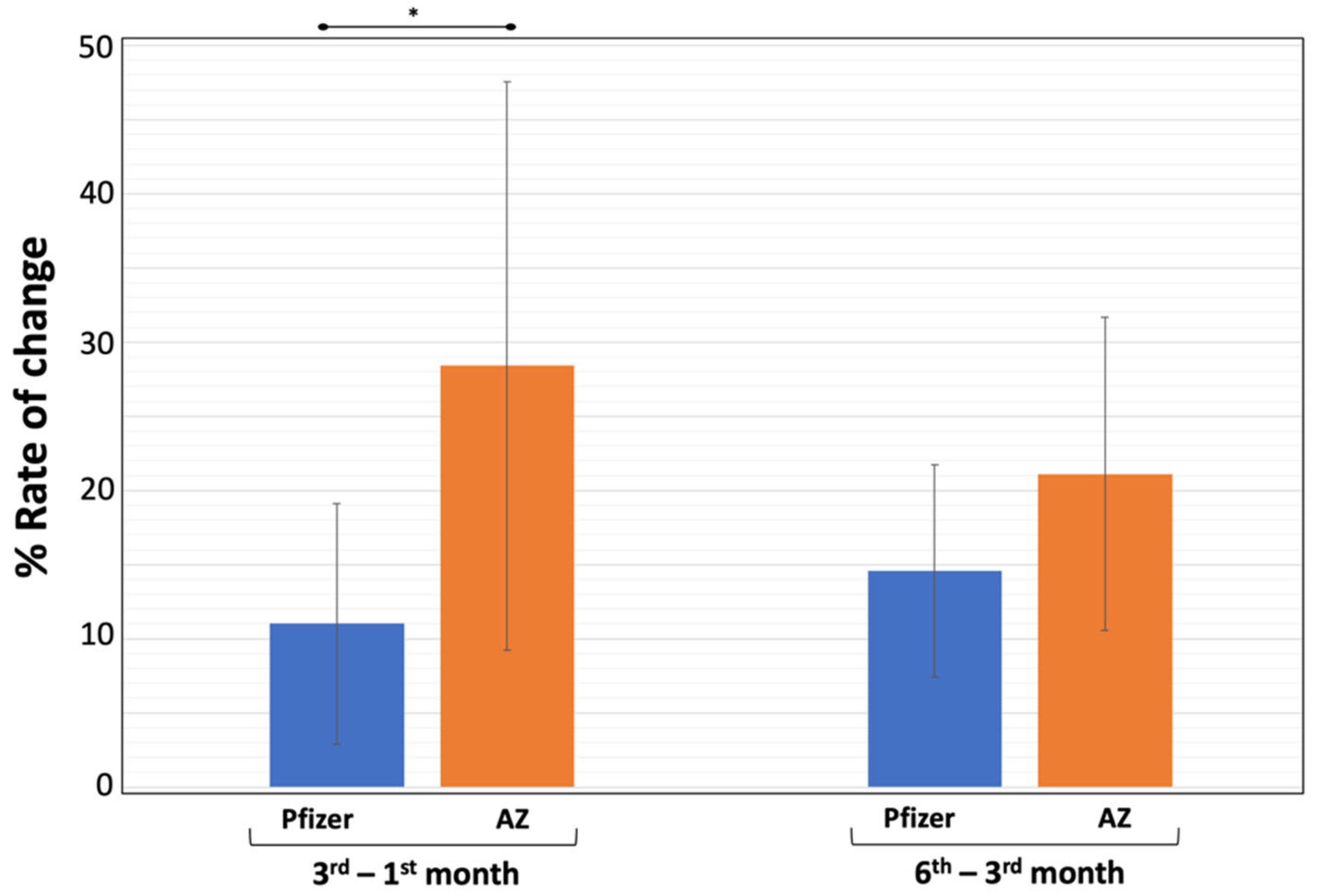
3. Results
3.1. Baseline Characteristics
3.2. Neutralizing Antibody Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Jalkanen, P.; Kolehmainen, P.; Hakkinen, H.K.; Huttunen, M.; Tahtinen, P.A.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S.H.; et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Trougakos, I.P.; Karalis, V.; Ntanasis-Stathopoulos, I.; Sklirou, A.D.; Bagratuni, T.; Papanagnou, E.D.; Patseas, D.; Gumeni, S.; Malandrakis, P.; et al. Comparison of neutralizing antibody responses against SARS-CoV-2 in healthy volunteers who received the BNT162b2 mRNA or the AZD1222 vaccine: Should the second AZD1222 vaccine dose be given earlier? Am. J. Hematol. 2021, 96, E321–E324. [Google Scholar] [CrossRef]
- Rosati, M.; Terpos, E.; Agarwal, M.; Karalis, V.; Bear, J.; Burns, R.; Hu, X.; Papademetriou, D.; Ntanasis-Stathopoulos, I.; Trougakos, I.P.; et al. Distinct neutralization profile of spike variants by antibodies induced upon SARS-CoV-2 infection or vaccination. Am. J. Hematol. 2022, 97, E3–E7. [Google Scholar] [CrossRef]
- The Lancet Infectious, D. COVID-19 vaccine equity and booster doses. Lancet Infect. Dis. 2021, 21, 1193. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising antibody activity against SARS-CoV-2 VOCs, B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Karalis, V.; Ntanasis-Stathopoulos, I.; Gumeni, S.; Apostolakou, F.; Sklirou, A.D.; Gavriatopoulou, M.; Skourti, S.; Kastritis, E.; et al. Kinetics of Anti-SARS-CoV-2 Antibody Responses 3 Months Post Complete Vaccination with BNT162b2: A Prospective Study in 283 Health Workers. Cells 2021, 10, 1942. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Karalis, V.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Gumeni, S.; Malandrakis, P.; Papanagnou, E.D.; Kastritis, E.; Trougakos, I.P.; Dimopoulos, M.A. Robust Neutralizing Antibody Responses 6 Months Post Vaccination with BNT162b2: A Prospective Study in 308 Healthy Individuals. Life 2021, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jurjenson, V.; Adamson, A.; Haljasmagi, L.; Rumm, A.P.; Maruste, R.; Karner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Bayart, J.L.; Douxfils, J.; Gillot, C.; David, C.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Gerin, V.; et al. Waning of IgG, Total and Neutralizing Antibodies 6 Months Post-Vaccination with BNT162b2 in Healthcare Workers. Vaccines 2021, 9, 92. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Joshi, G.; Borah, P.; Thakur, S.; Sharma, P.; Mayank; Poduri, R. Exploring the COVID-19 vaccine candidates against SARS-CoV-2 and its variants: Where do we stand and where do we go? Hum. Vaccin Immunother. 2021, 1–27. [Google Scholar] [CrossRef]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Perrin, S.; Hamonic, S.; Bourget, B.; Roue, C.; Brassard, O.; Tadie, E.; Gicquel, V.; Benezit, F.; Thibault, V.; et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: An observational study using surveillance data. Clin. Microbiol. Infect. 2021, 27, 1699.e5–1699.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kutateladze, T.G. Molecular structure analyses suggest strategies to therapeutically target SARS-CoV-2. Nat. Commun. 2020, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Iacobucci, G. COVID-19: Fourth vaccine doses-who needs them and why? BMJ 2022, 376, o30. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.; Rampling, T.; Venkatraman, N.; Bowyer, G.; Wright, D.; Lambe, T.; Imoukhuede, E.B.; Payne, R.; Fehling, S.K.; Strecker, T.; et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N. Engl. J. Med. 2016, 374, 1635–1646. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lim, S.Y.; Park, S.; Kwon, J.S.; Bae, S.; Park, J.Y.; Cha, H.H.; Seo, M.H.; Lee, H.J.; Lee, N.; et al. Immune responses to the ChAdOx1 nCoV-19 and BNT162b2 vaccines and to natural COVID-19 infections over a three-month period. J. Infect. Dis. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2021, 28, 202–221. [Google Scholar] [CrossRef]
- Kertes, J.; Gez, S.B.; Saciuk, Y.; Supino-Rosin, L.; Stein, N.S.; Mizrahi-Reuveni, M.; Zohar, A.E. Effectiveness of mRNA BNT162b2 Vaccine 6 Months after Vaccination among Patients in Large Health Maintenance Organization, Israel. Emerg. Infect. Dis. 2021, 28, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Salmeron Rios, S.; Cortes Zamora, E.B.; Avendano Cespedes, A.; Romero Rizos, L.; Sanchez-Jurado, P.M.; Sanchez-Nievas, G.; Mas Romero, M.; Tabernero Sahuquillo, M.T.; Blas Senalada, J.J.; Murillo Romero, A.; et al. Immunogenicity after 6 months of BNT162b2 vaccination in frail or disabled nursing home residents: The COVID-A Study. J. Am. Geriatr. Soc. 2021, 69, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.P.; Tafuri, S.; Migliore, G.; Vimercati, L.; Martinelli, A.; Lobifaro, A.; Diella, G.; Stefanizzi, P.; Group, O. BNT162b2 mRNA COVID-19 Vaccine Effectiveness in the Prevention of SARS-CoV-2 Infection and Symptomatic Disease in Five-Month Follow-Up: A Retrospective Cohort Study. Vaccines 2021, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernan, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to COVID-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | BNT162b2 | ChAdOx1 |
|---|---|---|
| Sample size | 83 | 199 |
| Gender | ||
| Men | 30 (36.1%) | 56 (47.1%) |
| Women | 53 (63.9%) | 63 (52.9%) |
| Age (median) | 61.0 | 61.5 |
| Body mass index (median) | 26.7 | 26.6 |
| Underweight (n, %) | 0 (%) | 0 (0%) |
| Normal weight (n, %) | 28 (33.7%) | 37 (31.1%) |
| Overweight (n, %) | 40 (48.2%) | 45 (37.8%) |
| Obese (n, %) | 15 (18.1%) | 37 (31.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpos, E.; Karalis, V.; Ntanasis-Stathopoulos, I.; Evangelakou, Z.; Gavriatopoulou, M.; Manola, M.S.; Malandrakis, P.; Gianniou, D.D.; Kastritis, E.; Trougakos, I.P.; et al. Comparison of Neutralizing Antibody Responses at 6 Months Post Vaccination with BNT162b2 and AZD1222. Biomedicines 2022, 10, 338. https://doi.org/10.3390/biomedicines10020338
Terpos E, Karalis V, Ntanasis-Stathopoulos I, Evangelakou Z, Gavriatopoulou M, Manola MS, Malandrakis P, Gianniou DD, Kastritis E, Trougakos IP, et al. Comparison of Neutralizing Antibody Responses at 6 Months Post Vaccination with BNT162b2 and AZD1222. Biomedicines. 2022; 10(2):338. https://doi.org/10.3390/biomedicines10020338
Chicago/Turabian StyleTerpos, Evangelos, Vangelis Karalis, Ioannis Ntanasis-Stathopoulos, Zoi Evangelakou, Maria Gavriatopoulou, Maria S. Manola, Panagiotis Malandrakis, Despoina D. Gianniou, Efstathios Kastritis, Ioannis P. Trougakos, and et al. 2022. "Comparison of Neutralizing Antibody Responses at 6 Months Post Vaccination with BNT162b2 and AZD1222" Biomedicines 10, no. 2: 338. https://doi.org/10.3390/biomedicines10020338
APA StyleTerpos, E., Karalis, V., Ntanasis-Stathopoulos, I., Evangelakou, Z., Gavriatopoulou, M., Manola, M. S., Malandrakis, P., Gianniou, D. D., Kastritis, E., Trougakos, I. P., & Dimopoulos, M. A. (2022). Comparison of Neutralizing Antibody Responses at 6 Months Post Vaccination with BNT162b2 and AZD1222. Biomedicines, 10(2), 338. https://doi.org/10.3390/biomedicines10020338











