Abstract
Cytokine profiles are often perturbed after infections of medical implants. With a non-invasive in vivo imaging system, we report in a mouse model that interferon expression after infection of subcutaneous implants with Streptococcus oralis, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Treponema denticola (alone or as a combination) was species-specific, persisted longer in the presence of implants, and notably decreased upon dual species infections. This type I interferon expression disappeared within two weeks; however, histology of implant–tissue interface indicated high recruitment of immune cells even after three weeks. This was suggestive that biomaterial-associated infections could have prolonged effects, including the systemic stimulation of inflammatory cytokines. The present study investigated the systemic impact of this chronic peri-implant inflammation on the systemic expression of inflammatory cytokines (23) using a multiplex assay. Initially, the cytokine measurement in murine fibroblasts exposed to periodontal pathogens remained limited to the expression of five cytokines, namely, IL-6, G-CSF, CXCL-1/KC, MCP-1 (MCAF), and IL-12 (p40). The systemic determination of cytokines in mice increased to 19 cytokines (IL-1α, IL-2, IL-3, IL-5, IL-6, IL-9, IL-12 (p40), IL-12 (p70), IL-13, IL-17A, CCL-11/Eotaxin, G-CSF, IFN-γ, CXCL1/KC, MCP-1 (MCAF), MIP-1α/CCL3, MIP-1β/CCL4, CCL5/RANTES, and TNF-α). Systemic induction of cytokines was species-specific in the mouse model. The cytokine induction from infected implants differed significantly from sole tissue infections and sterile implants. Notably, systemic cytokine induction decreased after infections with dual species compared to single species infections. These findings describe the systemic effect of chronic peri-implant inflammation on the systemic induction of inflammatory cytokines, and this effect was strongly correlated to the type and composition of initial infection. Systemic modulations in cytokine expression upon dual species infections exhibit an exciting pattern that might explain the complications associated with biomaterial-related infection in patients. Moreover, these findings validate the requirement of multispecies infections for pre-clinical studies involving animal models.
1. Introduction
Dental implants are used to restore masticatory function in partially or fully edentulous patients with a high success rate []. In diseased situations, implants can be colonized by infectious bacterial species and subsequent formation of recalcitrant biofilms can occur []. The pathogenesis of peri-implant diseases largely attributes to high inflammation resulting from interactions between tissue, implant material, and bacterial factors []. In the course of these inflammatory events, cytokines play critical role, and an imbalanced cytokine secretion may incur tissue destruction at the inflamed implant site and disease progression [,]. Stimulation in the secretion of inflammatory cytokines in the host was strongly correlated to the type of pathogen []. Recent studies have indicated a strong association of chronic inflammation to the type of bacteria related to peri-implant diseases [,,]. Some inflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, were frequently measured during biomaterial-related infections []. The secretion of pro-inflammatory cytokines was shown to be critical in the pathogenesis of implant-related infections. For example, human and experimental animal models studies reported a critical role of inflammatory cytokine expressions and their receptors in alveolar bone loss []. The biofilm formation around dental implants includes diverse bacterial species comprising commensal streptococci as initial colonizers [,,]. This initial accumulation by streptococci triggers the host for paracrine and autocrine secretion of chemical cytokines and growth factors at implantation sites in a homeostatic balance with the host immune system []. Towards diseased phase, a secondary accumulation of virulent species such as P. gingivalis or T. denticola increases and triggers high inflammation with uncontrolled secretion of inflammatory cytokines [].
Biomaterial-associated infections show high infiltration and prolonged persistence of host immune cells, suggesting that these infections may be a more important inducer of inflammatory reactions [,]. Elevated secretions of inflammatory cytokines such as IL-1, IL-6, and IL-10 remain essential markers of implant-associated inflammatory events []. These dysregulations in the inflammatory cytokine expressions could be influenced by various factors such as the type of pathogens, inter-bacterial interactions, and the presence of implant material. In the latest study, a non-invasive in vivo imaging method was established in transgenic mice to monitor the status of type I interferon-β after infection of the tissue and subcutaneous implants with different periodontal pathogens []. The interferon expression kinetics was bacterial species-specific, decreased for dual species infections, and remained prolonged around infected tissue in the presence of implants compared to tissue infections without implants. The imaging of type I interferon expression was possible for two weeks, and then it disappeared completely. The histology of infected peri-implant tissue indicated high recruitment of immune cells at the tissue–implant interface. This recruitment of inflammatory cells was mild around sterile implants or infected tissues without implants. It was therefore hypothesized that this intense tissue inflammation could modulate the expression of inflammatory cytokines. Serum was collected from mice to satisfy this hypothesis, and multiplex cytokine analysis was performed to measure 23 inflammatory cytokines. Since fibroblasts are the first cells to contact immediately with implanted materials or injected pathogens [], cells were exposed directly to four periodontal pathogens as single or as multispecies to monitor the sensitivity of cells and the induction of inflammatory cytokines.
2. Materials and Methods
2.1. Bacterial Cultivation
Streptococcus oralis (ATCC 9811, American Type Culture Collection, Manassas, VA, USA), Aggregatibacter actinomycetemcomitans (DSM 11123, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), and Porphyromonas gingivalis (DSM 20709) were cultured on tryptone soy agar (TSA) or fastidious anaerobe agar (FAA) plates supplemented with 5% sheep blood at 37 °C under anaerobic conditions (80% N2, 10% H2, 10% CO2). Few colonies of S. oralis were inoculated into tryptone soy broth (TSB) (Oxoid Limited, Hamsphire, United Kingdom) supplemented with 10% yeast extract (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) and 50 mM glucose (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) at 37 °C under constant shaking. A few colonies of A. actinomycetemcomitans or P. gingivalis were inoculated overnight into brain heart infusion medium (BHI; Oxoid, Wesl, Germany) supplemented with 10 μg/mL vitamin K (Roth, Karlsruhe, Germany) under anaerobic conditions []. Treponema denticola (DSM 14222) cultures were prepared in new oral spirochete (NOS) medium at 37 °C for 72 h under static anaerobic conditions [].
2.2. Co-Cultivation of Murine Fibroblasts with Bacteria
Murine fibroblasts (NIH3T3) were cultured in DMEM supplemented with 10% FCS under standard cell culture conditions []. Confluent cells were washed with PBS, suspended in DMEM without antibiotics, and then seeded on glass coverslips 106 cells per specimen (three biological replicates, each biological replicate was run in three technical replicates). Cells were incubated for 24 h under standard cell culture conditions and then exposed individually to 105 colony-forming units of S. oralis, A. actinomycetemcomitans, P. gingivalis, or T. denticola. Challenge of fibroblasts with a mixture of all species was done by 105 colony-forming units of each species. Infected cells were incubated for 24 h under standard cell culture conditions. After 24 h, supernatants were collected, centrifuged, and stored at −80 °C for multiplex assay. For confocal microscopy, adherent cells were treated for 30 min with SYTO®9 and propidium iodide (1:1000 dilutions in PBS) (LIVE/DEAD®BacklightTM Bacterial Viability Kit, Life Technologies, Carlsbad, CA, USA) [,]. Stained cells were imaged with confocal laser scanning microscope (CLSM) (SP-8, Leica Microsystems, Wetzlar, Germany). SYTO®9 signals were measured with multi-wavelength argon laser (excitation wavelength 488 nm) and an emission range of 500–550 nm.
2.3. Preparation of Titanium Implants
Implantable cylinders with 4.5 mm diameter were fabricated from grade 4 titanium (L.Klein SA, Biel, Switzerland). These implants with 7 mm length, 3.3 mm diameter, and 24 pores (0.5 mm each) were manufactured at Central Research Devices Service Unit, Hannover Medical School, Germany (Figure 1a) []. Such porous cylinders were expected to provide safe niches to the injected bacteria from the invasion of host immune cells (Figure 1b). These cylinders were implanted subcutaneously into mice (Figure 1c).
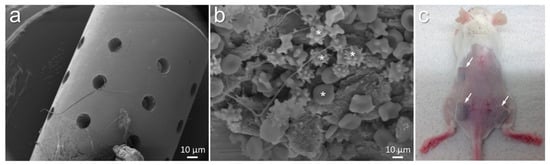
Figure 1.
Morphology of titanium cylinders designed for implantation into mice (a). Implants allowed accumulation of various host cells visible after 21 days of implantation ((b), white asterisks). Mouse showing the impression of subcutaneously implanted titanium cylinders ((c), white arrows indicate implanted titanium).
2.4. Subcutaneous Implantations and Infections in Small Animal Model
Animal experiments were performed on female 8–12-week-old transgenic mice (C.Balb/c1-Ifnb1tm1.2Lien, bred at the Central Animal Facility, Hannover Medical School, Hannover, Germany) []. All animals were kept inside individually ventilated cages with optimum provision of food and water. For each infection (alone or as combination), three animals were used. All animals were first anesthetized under a sterile bench with intraperitoneal injection of 10 mg/kg ketamine (Albrecht, Germany) and 4 mg/kg xylazine (Rompun, Bayer, Leverkusen, Germany). The fur from the sites of implantations was removed with a hair trimmer (Aesculap Suhl, GmbH, Germany) and then disinfected with 70% ethanol. Three incisions (1 cm each) were created with surgical scissor and tissue forceps (Fine Science Tools, GmbH, Heidelberg, Germany). Subcutaneous pouches were created from these incisions. Cylindrical titanium implants were placed into these pouches. Wounds were closed with simple interrupted sutures (Ethicon Vicryl, Johnson & Johnson Medical GmbH). Within 30 min after the closure of surgical wounds with or without implantation, infections of 5 μL per implant with bacterial suspension (as single or dual species from 1 × 108 CFU/mL) were done. Animals were then regularly observed, and blood was collected three weeks after implantation. All animal experiments were performed at Institute for Laboratory Animal Science and Central Animal facility, Hannover Medical School, according to the permission from the Lower Saxony State Office for Consumer Protection and Food Safety, Germany, with number: 33.12-42502-04-17/2580.
2.5. Measurement of Cytokines in Serum
After three weeks of implantation, mice were euthanized with high doses of anesthesia, and blood was collected intracardially without any coagulant. Blood samples were incubated at room temperature for 30 min and then centrifuged at 1500× g for 10 min at 4 °C. After centrifugation, the serum was collected and stored at −80 °C until further processing. The levels of IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17A, CCL-11/Eotaxin, G-CSF, GM-CSF, IFN-γ, CXCL1/KC, MCP-1 (MCAF), MIP-1α/CCL3, MIP-1β/CCL4, CCL5/RANTES, and TNF-α in serum were determined by using Bio-Plex Pro Mouse Cytokine 23-Plex Assay (Bio-Rad, Munich, Germany).
2.6. Statistical Analysis
SPSS Statistics (IBM, v. 26) was used for statistical analysis. The Kruskal–Wallis H test, a rank-based nonparametric test, with Dunn’s post hoc method was applied to test the null hypothesis that the following group were significantly different from each other: (i) sterile implant vs. various infected implants, (ii) tissue infections vs. peri-implant tissue infections, (iii) single vs. dual species infections for both peri-implant and tissues alone. Significance values were adjusted by the Bonferroni correction for multiple tests. Symbols ***, **, *, and # indicated p < 0.001, p < 0.01, p < 0.05, and significant p-value prior to Bonferroni correction, respectively.
3. Results
3.1. Cytokines Expression in Murine Fibroblasts
Fibroblasts are key structural cells to contact directly with implants and invading pathogens. Multiplex cytokine analysis was performed on cell culture supernatants after exposure of murine fibroblasts to commensal S. oralis or pathogenic A. actinomycetemcomitans, P. gingivalis, or T. denticola or a mixture of these species. Fibroblasts without infections showed homogenous cell layer seemingly adhering well with underlying surfaces (Figure 2a). Fibroblasts exposed to S. oralis could not survive with faster bacterial growth (Figure 2b, white arrows). Fibroblasts co-cultured with A. actinomycetemcomitans, P. gingivalis, and T. denticola still proliferated (Figure 2c–e). Fibroblasts exposed to the mixture of four species could also not survive, whereas remaining bacterial biofilms were dominated mainly by S. oralis (Figure 2f). Cytokine 23-plex assay could determine the expression of only five cytokines (Figure 2g–i and Figure S2). Particularly, for fibroblasts exposed to S. oralis or mixed bacterial cultures, cytokine expressions were subsequently very low (Figure 2g–i and Figure S2). There seemed bacterial-specific stimulation of IL-6, IL-12 (p40), G-CSF, CXCL-1, and MCP-1 in fibroblasts exposed to A. actinomycetemcomitans, P. gingivalis, or T. denticola (Figure 2g–i and Figure S2). The expression of G-CSF and CXCL-1 decreased statistically non-significantly in cells exposed to T. denticola compared to uninfected cells (Figure 2g–i and Figure S2).
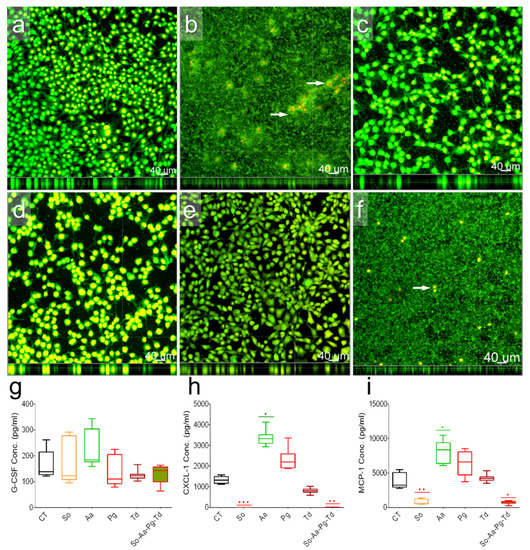
Figure 2.
Morphology and the expression of G-CSF, CXCL-1, and MCP1 in fibroblasts. Microscopic documentation of unstimulated fibroblasts (a) as well as those stimulated with S. oralis surrounding murine fibroblasts (b), A. actinomycetemcomitans (c), P. gingivalis (d), and T. denticola (e), or a mixture of all four bacterial species (f). White arrows target remaining fibroblasts (b,f). The box and whisker graphs indicate the expression of G-CSF (g), CXCL-1 (h), and MCP-1 (i) in uninfected fibroblasts (CT) or after co-cultivation with S. oralis (So), A. actinomycetemcomitans (Aa), P. gingivalis (Pg), and T. denticola (Td), or a mixture of all species (So-Aa-Pg-Td). Significant changes according to the uninfected control were adjusted by Bonferroni correction for multiple tests. Symbols ***, **, and * indicate p < 0.001, p < 0.01, and p < 0.05, respectively.
3.2. Systemic Analysis of Serum Interleukin
Multiplex assay could determine an array of 19 inflammatory cytokines in mice serum including interleukin, chemokines, and growth-stimulating factors. The status of interleukin expressions from the infected implants or infected tissues without implants varied from the sterile implants (Figure 3 and Figure S3). Peri-implant infections had variable consequence in the interleukin expression than tissue infections without implants (Figure 3a and Figure S3). Expression of pro-inflammatory IL-6 was in a low detection range, but its secretion pattern was specific to the bacterial species and increased significantly (p = 0.0076 and 0.0058, respectively) in S. oralis- and T. denticola-infected implants compared to infected tissue without implants (Figure S3E, single species). Although in a low detection range, expression of pro-inflammatory IL-1α was specific to the bacterial species and increased in the presence of implants with A. actinomycetemcomitans or P. gingivalis compared to sterile implants (Figure S3A, single species). Dual species implant infections either with S. oralis–P. gingivalis or A. actinomycetemcomitans–T. denticola caused a significant decrease in the interleukin expression compared to single species infections or sterile implants (Figure 3 and Figure S3). Dual species infections with S. oralis–P. gingivalis caused significant (p = 0.002 and 0.004, respectively) decreases in the expression of IL-1α and IL-6 compared to sterile implants (Figure S3A,D, dual species). Serum levels of IL-12 (p40) significantly (p = 0.0059 and 0.0058, respectively) decreased with dual species infections compared to sterile implants (Figure 3b). IL-12 (p70) expression remained species-specific in single species infections and decreased in dual species infections (Figure 3c). The interspecies and single versus dual species differences regarding the expression of IL-17 were mild (Figure 3). Levels of IL-1β, IL-4, IL-10, and GMCSF were low or even below detection limits in either infected or sterile implants (Table S1). This analysis confirmed that implant material and dual species infections were playing an important role in the interleukin expression.
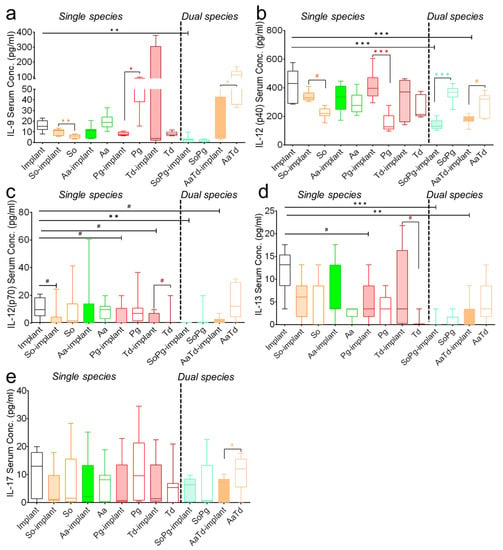
Figure 3.
Modulations in the systemic expression of interleukins in mice. The box and whiskers graphs indicate serum interleukin expression for IL-9 (a), IL-12p40 (b), IL-12p70 (c), IL-13 (d), and IL-17 (e) in animals with sterile implants (empty bars), infected implants (filled colored bars), and infected tissues (empty bars with colored borders). Data were analyzed animal-wise (biological replicates (n = 3), technical replicates (3 each)). Significant values have been adjusted by the Bonferroni correction for multiple tests. Symbols ***, **, *, and # indicate p < 0.001, p < 0.01, p < 0.05, and significant p-value prior to Bonferroni correction, respectively, between sterile and infected implants (black symbols). Significant differences between infections in the presence and absence of implants are indicated by colored symbols in each case. Abbreviations: So (S. oralis), Aa (A. actinomycetemcomitans), Pg (P. gingivalis), Td (T. denticola).
3.3. Systemic Analysis of Serum Chemokines
Since chemokines are an important family of cytokines and critical mediators of host immunity, the systemic status of chemokines was monitored in the multiplex assay as well []. The chemokine secretions in infected tissues with or without implants changed significantly compared to sterile implants (Figure 4). Chemokine expression was bacterium specific and showed different profiles in the presence of implants. For each bacterial species, the expression of chemokines was modified by the presence of implant (Figure 4). Dual species implant infections decreased chemokine concentrations compared to corresponding single species infections (Figure 4). CXCL1/KC expression seemed dependent on S. oralis and A. actinomycetemcomitans and significantly increased (p = 0.0058 and 0.038, respectively) in infected implants compared to sterile implants (Figure 4a). The level of pro-inflammatory chemokine CCL3/MIP1α in all infected groups was similar to the sterile implants (Figure S2F). The expression of CCL5/RANTES decreased in all infections in the presence or absence of implants compared to sterile implants (Figure 4c). Systemic expressions of the macrophage inflammatory protein CCL4/MIP-1β was consistently low in all infected implants or tissues compared to sterile implants (Figure 4b,d). In conclusion, there seemed in majority of the cases species-specific induction of chemokines.
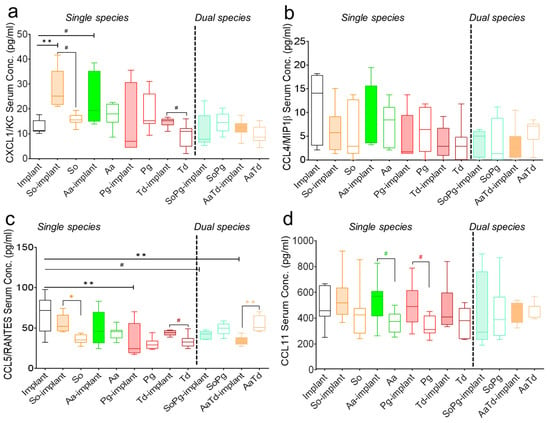
Figure 4.
Systemic expression of chemokines in mice. Black bars represent sterile implants, filled bars indicate infected implants, and empty colored bars indicate infected tissues for CXCL1/KC (a), CCL4/MIP1β (b), CCL5/RANTES (c), and CCL11 (d). Data were analyzed animal-wise (biological replicates (n = 3), technical replicates (3 each)). Significant values were adjusted by the Bonferroni correction for multiple tests. Symbols **, *, and # indicate p < 0.01, p < 0.05, and significant p-value prior to Bonferroni correction, respectively. Black symbols represent significant differences between sterile and infected implants. Significant differences between infected implants and infected sham-operated tissues are indicated by colored symbols. Abbreviations: So (S. oralis), Aa (A. actinomycetemcomitans), Pg (P. gingivalis), Td (T. denticola).
3.4. Systemic Analysis of Growth Factors and Cellular Regulators
Multiplex assay was further extended to the systemic investigation of host inflammatory responses, including interferon-gamma (IFN-γ), granulocyte colony-stimulating factor (G-CSF), tumor necrosis factor alpha (TNF-α), and monocyte chemotactic protein 1 (MCP-1) (Figure 5). Infections of implants or tissues showed the same effect in the expression of IFN-γ, G-CSF, and MCP-1 compared to sterile implants (Figure 5a). The effect of infection was clearly visible for the expression of G-CSF (Figure 5b). Serum TNF-α levels remained similar for sterile implants or after infections with implants or for infected tissues (Figure 5c). The systemic expression of MCP-1 decreased for all infections compared to sterile implants (Figure 5d). There was mild decrease in the serum levels of IFN-γ, G-CSF, CCL2/MCP-1, and IFN-γ after dual species infections compared to sterile implants (Figure 5a,b,d). To conclude, systemic expression of growth-stimulating factors was highly species-specific and decreased mostly for dual species infections.
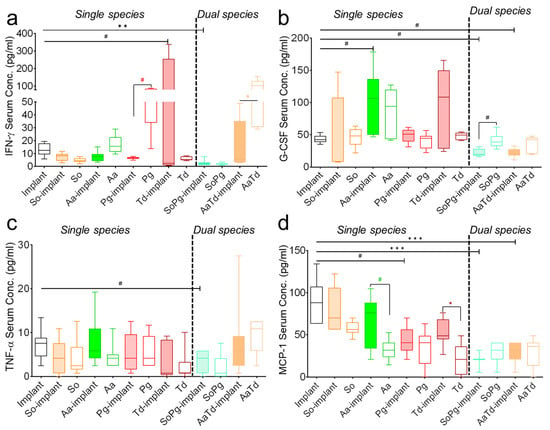
Figure 5.
Systemic levels of inflammatory cytokines. Graphs show serum levels of IFN-γ (a), G-CSF (b), TNF-α (c), and CCL2/MCP-1 (d) 21 days after implantations and infections. Empty bars represent sterile implants, colored bars indicate infected implants, and empty bars with colored borders indicate infected tissues. Data from three independent experiments are shown. Data were analyzed animal-wise (biological replicates (n = 3), technical replicates (3 each)). Significant values were adjusted by the Bonferroni correction for multiple tests. Symbols ***, **, *, and # indicate p < 0.001, p < 0.01, p < 0.05, and significant p-value prior to Bonferroni correction, respectively. Black symbols represent significant differences between sterile and infected implants. Colored symbols indicate significant differences between infected implants and infected tissues in the absence of implants. Abbreviation: So (S. oralis), Aa (A. actinomycetemcomitans), Pg (P. gingivalis), Td (T. denticola).
Significant changes between two different treatments (according to the presence or absence of implants, sterile vs. infected implants with a certain bacterium or combination of bacteria) are shown in tables to present further peculiarities (Table 1 and Table 2). Infected implants caused an increase in the cytokine induction for A. actinomycetemcomitans compared to sterile implants while causing a decrease for P. gingivalis infections compared to sterile implants (Table 1). Throughout dual species implant infections with A. actinomycetemcomitans–T. denticola or S. oralis–P. gingivalis consistently decreased more cytokines compared to any single species implant infections (Table 1). Statistical comparison between dual species and single species infections indicated significant downregulation of cytokine after dual species infections of implants when compared to single species infections (Table S2). Statistical analysis between single species-infected implants and infected tissues without implants indicated an upregulation in the expression of cytokines. However, in P. gingivalis, implant infections decreased the systemic expression of four cytokines compared to tissue infections without implants (Table 2). In dual species infection, a downregulation in the cytokine expression was observed in the presence of implants.

Table 1.
Effect of the single species or dual species bacterial infections around implants and status of inflammatory cytokine secretion. Symbols ***, **, *, and # indicate p < 0.001, p < 0.01, p < 0.05, and significant p-value prior to Bonferroni correction, respectively. (↑) indicate increase in the expression of cytokines, (↓) indicate decrease in the expression of cytokines.

Table 2.
Presence of implant material that play an important role in the systemic induction of inflammatory cytokines upon single or dual species infections. Symbols ***, **, *, and # indicate p < 0.001, p < 0.01, p < 0.05, and significant p-value prior to Bonferroni correction, respectively. (↑) indicate increase in the expression of cytokines, (↓) indicate decrease in the expression of cytokines.
4. Discussion
Infectious biofilms around dental implants cause uncontrolled cytokine secretions and are the first stage of peri-implant mucositis and peri-implantitis development []. In the latest finding, type I interferon-β expression around infected implants was dependent on the type of pathogen, implant material, and the mixture of two species. Notably, in-vivo expression of interferon-β decreased around implants infected with dual species compared to implant infections with the same bacteria as a single species [,]. While the interferon expression had entirely disappeared after three weeks, histological analysis of the infected implant–tissue interface indicated high recruitment of inflammatory cells. This recruitment of inflammatory cells at implant–tissue interfaces infected with dual species was more elevated than peri-implant tissues infected with single species. Such histological findings confirmed that a chronic inflammatory situation had developed around infected implants. The chronic inflammatory condition was not measurable by non-invasive in vivo imaging of type I interferon-β.
Fibroblasts are known to make immediate contact with implants or invading pathogens and express inflammatory cytokines [,]. Therefore, fibroblasts were exposed to S. oralis and A. actinomycetemcomitans, reported as initial colonizers, and P. gingivalis and T. denticola, reported as late colonizers. Four pathogens were mixed as multispecies to investigate the effect of polymicrobial biofilms, and fibroblasts were exposed. Murine fibroblasts seemed highly sensitive to direct exposure of S. oralis and could not proliferate. In another study that investigated the expression of IL-6, CCL-2, and CXCL-8 in human gingival epithelial cells (HGEps) and fibroblasts (HGFs) exposed indirectly to S. oralis, there was a variable expression of IL-6 and CCL-2 in both cell lines []. Notably, the expression of IL-6 significantly decreased in human gingival fibroblasts exposed to S. oralis while the CCL-2 showed a non-significant increase. Compared to this study, fibroblasts in the present study showed a non-significant increase in IL-6. The difference in IL-6 expression seemed mainly from a difference in the type of cells and the co-culture process. In the present study, direct exposure of murine fibroblasts to S. oralis damaged the cells and led to a proinflammatory situation, causing an increase in IL-6. Fibroblasts co-cultured with A. actinomycetemcomitans, P. gingivalis, and T. denticola managed to proliferate since these bacteria were anaerobic and could not exhibit optimum growth under standard cell culture conditions. In vitro cytokine analysis was restricted to detecting five cytokines, namely, IL-6, G-CSF, CXCL-1/KC, MCP-1 (MCAF), and IL-12 (p40). Among these detectable cytokines, T. denticola exposure led to a non-significant decrease in cytokine induction compared to uninfected fibroblasts. Such decrease in the cytokine production upon T. denticola could be related to previous observation for this bacteria to degrade cytokine for modulation in the immune responses [,]. Fibroblasts exposed to P. gingivalis showed a non-significant increase in IL-6 secretion compared to uninfected cells, and this type of trend was observed in epithelial cells, which showed high secretion of IL-1β, TNF-α, IL-6, and CXCL8/IL-8 after exposure to P. gingivalis [,]. Importantly, fibroblasts exposed to multi-species infections could not survive, and a decrease in the cytokine induction was observed. Overall, fibroblast seemed extremely sensitive to bacterial cultivations, and cytokine detection remained limited.
For in vivo evaluation, we selected a mouse model with subcutaneous implantations to investigate the detailed effect of biomaterial-associated infections by various bacterial species on the host. This sub-cutaneous mouse model allowed for easy implantation of large-sized titanium cylinders and the possibility of facilitating direct interaction of immune cells with infected implants. This subcutaneous mouse model quickly interpreted the effects of biomaterial-related infections and was less stressful for animals. The multiplex analysis in mice could measure the expression of 19 inflammatory cytokines, including interleukins, chemokines, and growth-stimulating factors. This systemic expression for most inflammatory cytokines downregulated after P. gingivalis or T. denticola, chronic inflammatory pathogens, in comparison with A. actinomycetemcomitans, responsible for aggressive inflammation [,]. The explanation could be the ability of P. gingivalis to secrete proteolytic enzymes that degrade cytokines and chemokines [,]. The majority of results indicated a decrease in the systemic cytokine induction after T. denticola infections with implants compared to sterile implants. The reduction in the cytokine after T. denticola infections could possibly be related to the ability of T. denticola to secrete proteinases that are known to degrade cytokines. The other reason could be associated with the limited capacity of T. denticola to interact with appropriate receptors for the release of cytokines [,]. Infections in the presence of implants showed different systemic effects in the secretions of cytokines compared to infected tissues without implants. From this observation, it can be assumed that tissues around implant materials allowed prolonged bacterial survival even in the presence of the host immune system. As shown in Table 2, A. actinomycetemcomitans infection of implants caused a significant increase in the systemic expression of IL-2, IL-3, IL-5, CCL-3/MIP1α, and CCL2/MCP-1 compared to tissue infections without implants. T. denticola single species implant infections systemically increased IL-6, IL-13, CXCL1/KC, CCL-3/MIP1α, and CCL2/MCP compared to tissue infection without the implant. Staphylococcus epidermidis infections of implants in tissues increased localized secretion of interleukin-1 [,]. Moreover, IL-1 and TNF-α production in human monocytes increased by the addition of LPS in the presence of biomaterials []. Pro-inflammatory IL-1β, IL-6, and TNF-α cytokines increased in patients bearing hip prosthetics after infection with coagulase-negative staphylococci []. The level of inflammatory cytokines IL-10 and TNF-α increased when biomaterials were infected with Staphylococcus epidermidis or Pseudomonas aeruginosa []. The current study observed a decrease in the systemic expression of IL-2 with single or dual species infections with or without implants. Other clinical and animal studies reported enhanced serum levels for IFN-γ, TNF-α, and IL-10, as well as decreased IL-2 levels in patients with A. actinomycetemcomitans- and P. gingivalis-associated periodontitis []. The present study systemically observed an increase in the expression of neutrophil chemoattractant CXCL1/Gro- upon single species implant infections, which was observed to increase in clinical studies and animal models during active bacterial infections [,,,].
Since cultural, immunologic, or DNA probe techniques have explained that several species of the oral microbiome interact with each other for the development of complex microbial biofilms []. Mixed species infections could trigger aggressive inflammation and rapid tissue loss in mice; therefore, complicated dual species bacterial infections were performed by mixing initial biofilm colonizers S. oralis and A. actinomycetemcomitans with late biofilm colonizers P. gingivalis and T. denticola []. Interestingly, and in agreement with observations documented from in vivo imaging of interferon-β in living mice, systemic levels of interleukins, chemokines, and growth-stimulating factors decreased upon dual species infections compared to single species infections. As indicated in Table 1, infection with A. actinomycetemcomitans as a single species caused a significant increase in IL-2, CXCL1, CCL-3/MIP1α, and G-CSF levels compared to sterile implants. T. denticola infection as single species on implants caused significant decrease in IL-2 expression compared to sterile implant. However, dual species infections with the mixture of A. actinomycetemcomitans and T. denticola significantly decreased IL-2, CCL-3/MIP1α, and G-CSF expression levels compared to sterile implants. Dual species infection with the combination of A. actinomycetemcomitans and T. denticola in the presence of implant material significantly decreased the expression of cytokines compared to tissue infection without implants. S. oralis infections as single species caused a significant increase in the expression of IL-1α, IL-6, IL-12 (p40), CXCL1/KC, CCL5/RANTES, and CCL11 compared to infection without implants. Interestingly, dual species infections with P. gingivalis and S. oralis decreased the expression of IL-6, IL-12 (p40), and CCL5/RANTES compared to tissue without implants. This observation can be explained since P. gingivalis antagonizes inflammatory response to other periodontal pathogens particularly because of its lipopolysaccharide (LPS) []. This has been already observed in human monocytes where co-stimulation with LPS from P. gingivalis and Campylobacter rectus sufficiently antagonized secretion of IL-6 and IL-8 [,]. In addition, P. gingivalis LPS prevented the apoptosis of neutrophils and countered the interaction of LPS from Escherichia coli for the regulation of inflammatory reactions in cells [,]. Decrease in cytokine induction mainly seemed to be arising from P. gingivalis or T. denticola since it was found that dual species P. gingivalis–T. denticola infections decreased IL-6, IL-1β, and CCL5/RANTES compared to single species infections with each of the bacterium in macrophage/epithelial cell co-culture model []. It can be speculated that inter-bacterial interactions that frequently occur in pathological oral biofilms have the capacity to undermine critical components of host immunity, including receptors, subsequent intracellular signaling pathways, and cytokine inductions. Therefore, it is proposed that dual species implant infections decrease the expression of inflammatory cytokines to promote prolonged persistence of pathogenic biofilm communities and to cause chronic inflammatory responses responsible for tissue destruction []. The overview of cytokine modification with dual species infections shows an exciting pattern and underlines the validity for preclinical studies regarding multispecies infection. Overall, implant-related infections by periodontal pathogens modulate the systemic expression of inflammatory cytokines. Such a modulation in inflammatory cytokines is suggestive of a challenge for the treatment of biomaterial-related bacterial infections that resist host immunity and conventional antibiotics therapy.
5. Conclusions
This study described the status of inflammatory cytokines in fibroblasts and then systemically studied them in mice with chronic peri-implant inflammation after infections with four different periodontal pathogens (alone or in combination). Fibroblasts were sensitive to bacterial co-cultivation and could only express five cytokines, suggesting that this in vitro experiment was insufficient to investigate complex bacteria–tissue interactions. The systemic modulation in the expression of inflammatory cytokines was specific to the type of pathogen, dependent on the presence of implants, and decreased with dual species infections. This suggests that bacterial infections as dual species decrease systemic cytokine expression to hide from the host inflammatory events. Such systemic modulation in the inflammatory cytokines reflects additional challenges associated with the treatment of biomaterial-associated infections. Moreover, these findings suggest the inclusion of multispecies to investigate biomaterial-related diseases for future animal models. Results from this sub-cutaneous model encourage extending this investigation to analyze inflammatory cytokines in future mouse models involving the implantation of infected implants inside bones in the oral cavity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines10020286/s1, Figure S1: Classification of mice into respective groups for the measurement of cytokines. Figure S2: Expression of interleukins IL-6 and IL-12 in murine fibroblast. Figure S3: Systemic profiles of inflammatory cytokines 21 days post-implantation. Systemic cytokine expressed in the concentration of 0–10 pg/mL for IL-1α (A), IL-2 (B), IL-3 (C), IL-5 (D), IL-6 (E), and CCL3/MIP1α (F) were determined in the blood serum of mice carrying infected implants. Table S1: List of cytokines in pictograms per microliter (pg/mL) expressed near or below detection limits. (biological replicates (n = 3), technical replicates (3 each)). Table S2: Dual vs. single species infections: cytokine expression decreased with dual species infections compared to single species infections.
Author Contributions
Conceptualization: M.I.R. and M.E.; methodology: M.I.R., S.L., A.W. and A.I.-T.; software: M.I.R.; validation: M.I.R., M.E., M.S. and M.S.; formal analysis: M.I.R. and A.W.; investigation: M.I.R.; resources: M.E. and M.S.; data curation: M.I.R.; writing—original draft preparation: M.I.R.; writing—review and editing: M.I.R., M.E., M.S., and A.W.; visualization: M.E., M.S. and A.W.; supervision: M.E. and M.S.; project administration: M.I.R.; funding acquisition: M.E. and M.S., C.S.F., methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BIOFABRICATION FOR NIFE Initiative. NIFE is the Lower Saxony Center for Biomedical Engineering, Implant Research and Development, a joint translational research center of the Hannover Medical School, the Leibniz University Hannover, the University of Veterinary Medicine Hannover, and the Laser Zentrum Hannover e. V. The BIOFABRICATION FOR NIFE Initiative is financially supported by the Ministry of Lower Saxony and the Volkswagen Foundation (both BIOFABRICATION FOR NIFE: VWZN2860). The APC was funded by the German Research Foundation (DFG) and the Open Access Fund of Hannover Medical School (MHH).
Institutional Review Board Statement
All animal experiments were performed at Institute for Laboratory Animal Science and Central Animal facility, Hannover Medical School, according to the permission from the Lower Saxony State Office for Consumer Protection and Food Safety, Germany, with number 33.12-42502-04-17/2580.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are thankful to Szymon P. Szafrański for performing the statistical analysis. We are grateful to Marly Dalton and Rainer Schreeb for their support in the completion of experiments. We acknowledge Kerstin Beushausen and Jana Keil for excellent technical assistance. MIR was supported by the Alexander von Humboldt Foundation. This research project was supported by BIOFABRICATION FOR NIFE Initiative. NIFE is the Lower Saxony Center for Biomedical Engineering, Implant Research and Development, a joint translational research center of the Hannover Medical School, the Leibniz University Hannover, the University of Veterinary Medicine Hannover, and the Laser Zentrum Hannover e. V. The BIOFABRICATION FOR NIFE Initiative is financially supported by the Ministry of Lower Saxony and the Volkswagen Foundation (both BIOFABRICATION FOR NIFE: VWZN2860). The graphical abstract was generated by BioRender.com (https://biorender.com/, accessed on 4 November 2021).
Conflicts of Interest
The authors declare that they have no conflict of interest (such as a financial relationship with one or more companies whose products are featured in the manuscript).
References
- Giannobile, W.; Lang, N. Are dental implants a panacea or should we better strive to save teeth. J. Dent. Res. 2016, 95, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. Npj Biofilms Microbiomes 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Kabir, L.; Stiesch, M.; Grischke, J. The effect of keratinized mucosa on the severity of peri-implant mucositis differs between periodontally healthy subjects and the general population: A cross-sectional study. Clin. Oral Investig. 2021, 25, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Bremer, F.; Grade, S.; Kohorst, P.; Stiesch, M. In vivo biofilm formation on different dental ceramics. Quintessence Int. 2011, 42, 565–574. [Google Scholar]
- Jakobi, M.L.; Stumpp, S.N.; Stiesch, M.; Eberhard, J.; Heuer, W. The Peri-Implant and Periodontal Microbiota in Patients with and without Clinical Signs of Inflammation. Dent. J. 2015, 3, 24–42. [Google Scholar] [CrossRef]
- Ingendoh-Tsakmakidis, A.; Mikolai, C.; Winkel, A.; Szafrański, S.P.; Falk, C.S.; Rossi, A.; Walles, H.; Stiesch, M. Commensal and pathogenic biofilms differently modulate peri-implant oral mucosa in an organotypic model. Cell. Microbiol. 2019, 21, e13078. [Google Scholar] [CrossRef]
- Vantucci, C.E.; Ahn, H.; Schenker, M.L.; Pradhan, P.; Wood, L.B.; Guldberg, R.E.; Roy, K.; Willett, N.J. Development of Systemic Immune Dysregulation in a Rat Trauma Model with Biomaterial-Associated Infection. Biomaterials 2021, 264, 120405. [Google Scholar] [CrossRef]
- Torrado, E.; Cooper, A.M. Cytokines in the balance of protection and pathology during mycobacterial infections. Adv. Exp. Med. Biol. 2013, 783, 121–140. [Google Scholar]
- Rahim, M.I.; Babbar, A.; Lienenklaus, S.; Pils, M.C.; Rohde, M. Degradable magnesium implant-associated infections by bacterial biofilms induce robust localized and systemic inflammatory reactions in a mouse model. Biomed. Mater. 2017, 12, 055006. [Google Scholar] [CrossRef]
- Rochford, E.T.J.; Sabaté Brescó, M.; Zeiter, S.; Kluge, K.; Poulsson, A.; Ziegler, M.; Richards, R.G.; O’Mahony, L.; Moriarty, T.F. Monitoring immune responses in a mouse model of fracture fixation with and without Staphylococcus aureus osteomyelitis. Bone 2016, 83, 82–92. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H.F. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J., Jr.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef]
- Alves, L.A.; de Carli, T.R.; Harth-Chu, E.N.; Mariano, F.S.; Höfling, J.F.; Stipp, R.N.; Mattos-Graner, R.O. Oral streptococci show diversity in resistance to complement immunity. J. Med. Microbiol. 2019, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Bullon, P.; Fioroni, M.; Goteri, G.; Rubini, C.; Battino, M. Immunohistochemical analysis of soft tissues in implants with healthy and peri-implantitis condition, and aggressive periodontitis. Clin. Oral Implant. Res. 2004, 15, 553–559. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S278–S285. [Google Scholar] [CrossRef]
- Radaelli, K.; Alberti, A.; Corbella, S.; Francetti, L. The Impact of Peri-Implantitis on Systemic Diseases and Conditions: A Review of the Literature. Int. J. Dent. 2021, 2021, 5536566. [Google Scholar] [CrossRef]
- Rahim, M.I.; Winkel, A.; Lienenklaus, S.; Stumpp, N.S.; Szafrański, S.P.; Kommerein, N.; Willbold, E.; Reifenrath, J.; Mueller, P.P.; Eisenburger, M.; et al. Non-Invasive Luciferase Imaging of Type I Interferon Induction in a Transgenic Mouse Model of Biomaterial Associated Bacterial Infections: Microbial Specificity and Inter-Bacterial Species Interactions. Microorganisms 2020, 8, 1624. [Google Scholar] [CrossRef]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The Unknown Sentinels Eliciting Immune Responses Against Microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef]
- Kommerein, N.; Stumpp, S.N.; Müsken, M.; Ehlert, N.; Winkel, A.; Häussler, S.; Behrens, P.; Buettner, F.F.R.; Stiesch, M. An oral multispecies biofilm model for high content screening applications. PLoS ONE 2017, 12, e0173973. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.S.; Siboo, R.; Keng, T.; Psarra, N.; Hurley, R.; Cheng, S.-L.; Iugovaz, I. Treponema denticola (ex Brumpt 1925) sp. nov., nom. rev., and Identification of New Spirochete Isolates from Periodontal Pockets. Int. J. Syst. Evol. Microbiol. 1993, 43, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Tavares, A.; Evertz, F.; Kieke, M.; Seitz, J.-M.; Eifler, R.; Weizbauer, A.; Willbold, E.; Maier, H.J.; Glasmacher, B.; et al. Phosphate conversion coating reduces the degradation rate and suppresses side effects of metallic magnesium implants in an animal model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Kommerein, N.; Doll, K.; Stumpp, N.S.; Stiesch, M. Development and characterization of an oral multispecies biofilm implant flow chamber model. PLoS ONE 2018, 13, e0196967. [Google Scholar] [CrossRef]
- Moser, B.; Willimann, K. Chemokines: Role in inflammation and immune surveillance. Ann. Rheum. Dis. 2004, 63, ii84–ii89. [Google Scholar] [CrossRef]
- Laine, M.L.; Leonhardt, Å.; Roos-Jansåker, A.-M.; Peña, A.S.; Van Winkelhoff, A.J.; Winkel, E.G.; Renvert, S. IL-1RN gene polymorphism is associated with peri-implantitis. Clin. Oral Implant. Res. 2006, 17, 380–385. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Jinbu, Y.; Itoh, H.; Kusama, M. Increased IL-6 Levels in Peri-Implant Crevicular Fluid Correlate with Peri-Implantitis. Oral Med. Pathol. 2005, 10, 95–99. [Google Scholar] [CrossRef]
- Fonseca, F.J.P.O.; Junior, M.M.; Lourenço, E.J.V.; de Moraes Teles, D.; Figueredo, C.M. Cytokines expression in saliva and peri-implant crevicular fluid of patients with peri-implant disease. Clin. Oral Implant. Res. 2014, 25, e68–e72. [Google Scholar] [CrossRef]
- Liskmann, S.; Vihalemm, T.; Salum, O.; Zilmer, K.; Fischer, K.; Zilmer, M. Correlations Between Clinical Parameters and lnterleukin-6 and lnterleukin-10 Levels in Saliva from Totally Edentulous Patients with Peri-implant Disease. Int. J. Oral Maxillofac. Implant. 2006, 21, 543–550. [Google Scholar]
- Faot, F.; Nascimento, G.G.; Bielemann, A.M.; Campão, T.D.; Leite, F.R.M.; Quirynen, M. Can Peri-Implant Crevicular Fluid Assist in the Diagnosis of Peri-Implantitis? A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 631–645. [Google Scholar] [CrossRef]
- Zani, S.R.; Moss, K.; Shibli, J.A.; Teixeira, E.R.; de Oliveira Mairink, R.; Onuma, T.; Feres, M.; Teles, R.P. Peri-implant crevicular fluid biomarkers as discriminants of peri-implant health and disease. J. Clin. Periodontol. 2016, 43, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Kobayashi, Y.; Yamasaki, S.; Kawakami, A.; Eguchi, K.; Sasaki, H.; Sakai, H. Protein Expression and Functional Difference of Membrane-Bound and Soluble Receptor Activator of NF-κB Ligand: Modulation of the Expression by Osteotropic Factors and Cytokines. Biochem. Biophys. Res. Commun. 2000, 275, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Severino, V.O.; Napimoga, M.H.; de Lima Pereira, S.A. Expression of IL-6, IL-10, IL-17 and IL-8 in the peri-implant crevicular fluid of patients with peri-implantitis. Arch. Oral Biol. 2011, 56, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Rath-Deschner, B.; Memmert, S.; Damanaki, A.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Götz, W.; Deschner, J.; Jäger, A.; Nogueira, A.V.B. CXCL1, CCL2, and CCL5 modulation by microbial and biomechanical signals in periodontal cells and tissues—In vitro and in vivo studies. Clin. Oral Investig. 2020, 24, 3661–3670. [Google Scholar] [CrossRef]
- Gemmell, E.; Carter, C.L.; Seymour, G.J. Chemokines in human periodontal disease tissues. Clin. Exp. Immunol. 2001, 125, 134–141. [Google Scholar] [CrossRef]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 alpha)/CCL3: As a Biomarker. Gen. Methods Biomark. Res. Appl. 2015, 27, 223–249. [Google Scholar] [CrossRef]
- De Wilde, E.A.W.J.; Jimbo, R.; Wennerberg, A.; Naito, Y.; Coucke, P.; Bryington, M.S.; Vandeweghe, S.; De Bruyn, H. The Soft Tissue Immunologic Response to Hydroxyapatite-Coated Transmucosal Implant Surfaces: A Study in Humans. Clin. Implant. Dent. Relat. Res. 2015, 17, e65–e74. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Vissink, A.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Liefers, S.C.; Kroese, F.G.M.; Raghoebar, G.M. Biomarker levels in peri-implant crevicular fluid of healthy implants, untreated and non-surgically treated implants with peri-implantitis. J. Clin. Periodontol. 2021, 48, 590–601. [Google Scholar] [CrossRef]
- Petković, A.B.; Matić, S.M.; Stamatović, N.V.; Vojvodić, D.V.; Todorović, T.M.; Lazić, Z.R.; Kozomara, R.J. Proinflammatory cytokines (IL-1β and TNF-α) and chemokines (IL-8 and MIP-1α) as markers of peri-implant tissue condition. Int. J. Oral Maxillofac. Surg. 2010, 39, 478–485. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.-M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lienenklaus, S.; Cornitescu, M.; Zietara, N.; Łyszkiewicz, M.; Gekara, N.; Jabłónska, J.; Edenhofer, F.; Rajewsky, K.; Bruder, D.; Hafner, M.; et al. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J. Immunol. 2009, 183, 3229–3236. [Google Scholar] [CrossRef] [PubMed]
- Ingendoh-Tsakmakidis, A.; Eberhard, J.; Falk, C.S.; Stiesch, M.; Winkel, A. In Vitro Effects of Streptococcus oralis Biofilm on Peri-Implant Soft Tissue Cells. Cells 2020, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Belton, C.M.; Reife, R.A.; Lamont, R.J. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 1998, 66, 1660–1665. [Google Scholar] [CrossRef]
- Mikolajczyk-Pawlinska, J.; Travis, J.; Potempa, J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: Implications for pathogenicity of periodontal disease. FEBS Lett. 1998, 440, 282–286. [Google Scholar] [CrossRef]
- Sandros, J.; Karlsson, C.; Lappin, D.F.; Madianos, P.N.; Kinane, D.F.; Papapanou, P.N. Cytokine Responses of Oral Epithelial Cells to Porphyromonas gingivalis Infection. J. Dent. Res. 2000, 79, 1808–1814. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kita, M.; Oseko, F.; Nakamura, T.; Imanishi, J.; Kanamura, N. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J. Periodontal Res. 2006, 41, 554–559. [Google Scholar] [CrossRef]
- Tan, K.H.; Seers, C.A.; Dashper, S.G.; Mitchell, H.L.; Pyke, J.S.; Meuric, V.; Slakeski, N.; Cleal, S.M.; Chambers, J.L.; McConville, M.J.; et al. Porphyromonas gingivalis and Treponema denticola Exhibit Metabolic Symbioses. PLoS Pathog. 2014, 10, e1003955. [Google Scholar] [CrossRef]
- Åberg, C.H.; Kelk, P.; Johansson, A. Aggregatibacter actinomycetemcomitans: Virulence of its leukotoxin and association with aggressive periodontitis. Virulence 2015, 6, 188–195. [Google Scholar] [CrossRef]
- Palm, E.; Khalaf, H.; Bengtsson, T. Porphyromonas gingivalis downregulates the immune response of fibroblasts. BMC Microbiol. 2013, 13, 155. [Google Scholar] [CrossRef]
- Abdi, K.; Chen, T.; Klein, B.A.; Tai, A.K.; Coursen, J.; Liu, X.; Skinner, J.; Periasamy, S.; Choi, Y.; Kessler, B.M.; et al. Mechanisms by which Porphyromonas gingivalis evades innate immunity. PLoS ONE 2017, 12, e0182164. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.S.; Steffen, M.J.; Ebersole, J.L. Cytokine responses to treponema pectinovorum and treponema denticola in human gingival fibroblasts. Infect. Immun. 2000, 68, 5284–5292. [Google Scholar] [CrossRef][Green Version]
- Asai, Y.; Jinno, T.; Ogawa, T. Oral treponemes and their outer membrane extracts activate human gingival epithelial cells through toll-like receptor. Infect. Immun. 2003, 71, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Boelens, J.J.; Zaat, S.A.J.; Murk, J.L.; Weening, J.J.; van der Poll, T.; Dankert, J. Enhanced Susceptibility to Subcutaneous Abscess Formation and Persistent Infection around Catheters Is Associated with Sustained Interleukin-1β Levels. Infect. Immun. 2000, 68, 1692–1695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boelens, J.J.; van der Poll, T.; Zaat, S.A.J.; Murk, J.L.A.N.; Weening, J.J.; Dankert, J. Interleukin-1 Receptor Type I Gene-Deficient Mice Are Less Susceptible to Staphylococcus epidermidis Biomaterial-Associated Infection than Are Wild-Type Mice. Infect. Immun. 2000, 68, 6924–6931. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M.A.; Simmons, R.L.; Kaplan, S.S. TNF and IL-1 generation by human monocytes in response to biomaterials. J. Biomed. Mater. Res. 1992, 26, 851–859. [Google Scholar] [CrossRef]
- Nilsdotter-Augustinsson, Å.; Briheim, G.; Herder, A.; Ljunghusen, O.; Wahlström, O.; öhman, L. Inflammatory response in 85 patients with loosened hip prostheses: A prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007, 78, 629–639. [Google Scholar] [CrossRef]
- Skadiņš, I.; Kroiča, J.; Salma, I.; Reinis, A.; Sokolova, M.; Rostoka, D. The Level of Inflammatory Cytokines and Antimicrobial Peptides after Composite Material Implantation and Contamination with Bacterial Culture. Key Eng. Mater. 2017, 721, 245–250. [Google Scholar] [CrossRef]
- Andrukhov, O.; Ulm, C.; Reischl, H.; Nguyen, P.Q.; Matejka, M.; Rausch-Fan, X. Serum Cytokine Levels in Periodontitis Patients in Relation to the Bacterial Load. J. Periodontol. 2011, 82, 885–892. [Google Scholar] [CrossRef]
- Sawant, K.V.; Poluri, K.M.; Dutta, A.K.; Sepuru, K.M.; Troshkina, A.; Garofalo, R.P.; Rajarathnam, K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016, 6, 33123. [Google Scholar] [CrossRef]
- Ritzman, A.M.; Hughes-Hanks, J.M.; Blaho, V.A.; Wax, L.E.; Mitchell, W.J.; Brown, C.R. The chemokine receptor CXCR2 ligand KC (CXCL1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect. Immun. 2010, 78, 4593–4600. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.J.; Martin, T.R.; Frevert, C.W.; Quan, J.M.; Wong, V.A.; Mongovin, S.M.; Hagen, T.R.; Steinberg, K.P.; Goodman, R.B. Expression and Function of the Chemokine Receptors CXCR1 and CXCR2 in Sepsis. J. Immunol. 1999, 162, 2341. [Google Scholar] [PubMed]
- Jin, L.; Batra, S.; Douda, D.N.; Palaniyar, N.; Jeyaseelan, S. CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J. Immunol. 2014, 193, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Darveau, R.P.; Pham, T.T.; Lemley, K.; Reife, R.A.; Bainbridge, B.W.; Coats, S.R.; Howald, W.N.; Way, S.S.; Hajjar, A.M. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun. 2004, 72, 5041–5051. [Google Scholar] [CrossRef]
- Bostanci, N.; Allaker, R.P.; Belibasakis, G.N.; Rangarajan, M.; Curtis, M.A.; Hughes, F.J.; McKay, I.J. Porphyromonas gingivalis antagonises Campylobacter rectus induced cytokine production by human monocytes. Cytokine 2007, 39, 147–156. [Google Scholar] [CrossRef]
- Bostanci, N.; Allaker, R.; Johansson, U.; Rangarajan, M.; Curtis, M.A.; Hughes, F.J.; McKay, I.J. Interleukin-1α stimulation in monocytes by periodontal bacteria: Antagonistic effects of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2007, 22, 52–60. [Google Scholar] [CrossRef]
- Murray, D.A.; Wilton, J.M.A. Lipopolysaccharide from the periodontal pathogen Porphyromonas gingivalis prevents apoptosis of HL60-derived neutrophils in vitro. Infect. Immun. 2003, 71, 7232–7235. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Martin, M.; Schifferle, R.E.; Genco, R.J. Counteracting interactions between lipopolysaccharide molecules with differential activation of toll-like receptors. Infect. Immun. 2002, 70, 6658–6664. [Google Scholar] [CrossRef]
- Bodet, C.; Chandad, F.; Grenier, D. Inflammatory responses of a macrophage/epithelial cell co-culture model to mono and mixed infections with Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. Microbes Infect. 2006, 8, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Krauss, J.L.; Liang, S.; McIntosh, M.L.; Lambris, J.D. Pathogenic microbes and community service through manipulation of innate immunity. Adv. Exp. Med. Biol. 2012, 946, 69–85. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).