Vitisin A, a Resveratrol Tetramer, Improves Scopolamine-Induced Impaired Learning and Memory Functions in Amnesiac ICR Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical and Reagents
2.2. Preparations of Crude Extracts and Partitioned Fractions of VT and VTT
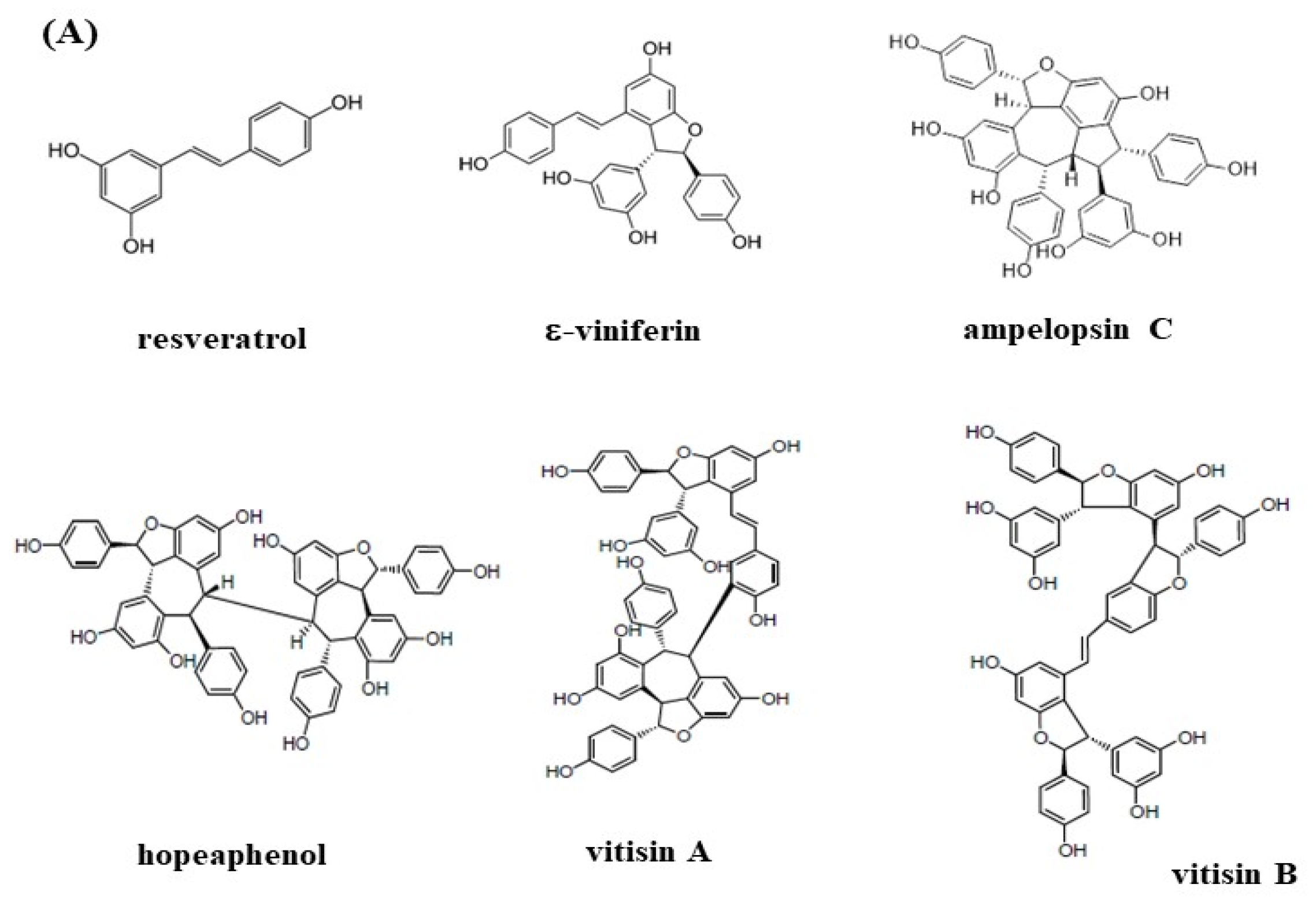
2.3. Isolation of Resveratrol Tetramers and Fingerprinting Analysis of Extracts and Fractions
2.4. AChE Inhibitory Activities In Vitro
2.5. MAO-B Inhibitory Activities In Vitro
2.6. Effects of Resveratrol and Resveratrol Tetramers on Neuroprotective Activities against MGO-Treated SH-SY5Y Cells
2.7. Treatments of VT-95EE or VT-95EE-EA or Purified Compounds of (+)-Vitisin A or (−)-Vitisin B in Scopolamine-Induced Amnesiac ICR Mice
2.8. Learning and Memory Evalustions by Passive Avoidance Tests of Amnesiac ICR Mice
2.9. AChE Activities, MDA Levels, and Protein Expressions of Brain-Derived Neurotrophic Factor (BDNF) and Tropomyosin Receptor Kinase B (TrkB) in Mouse Brain Tissue Extracts
2.10. Statistical Analyses
3. Results
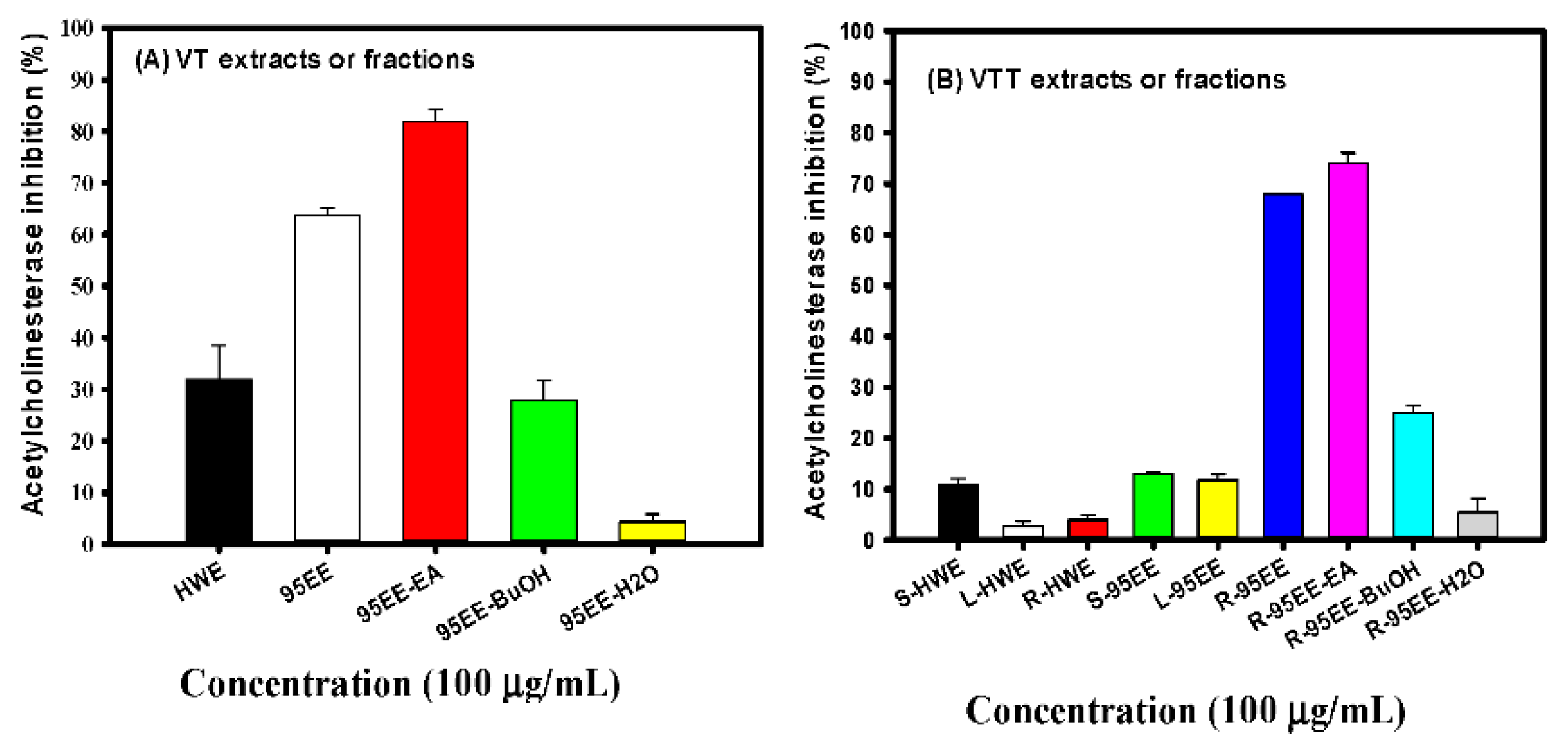
3.1. Effects of VT Extracts, Different Parts of VTT Extracts, VT- or VTT-Partitioned Fractions on AChE Inhibitory Activities
3.2. The HPLC Fingerprinting Analyses
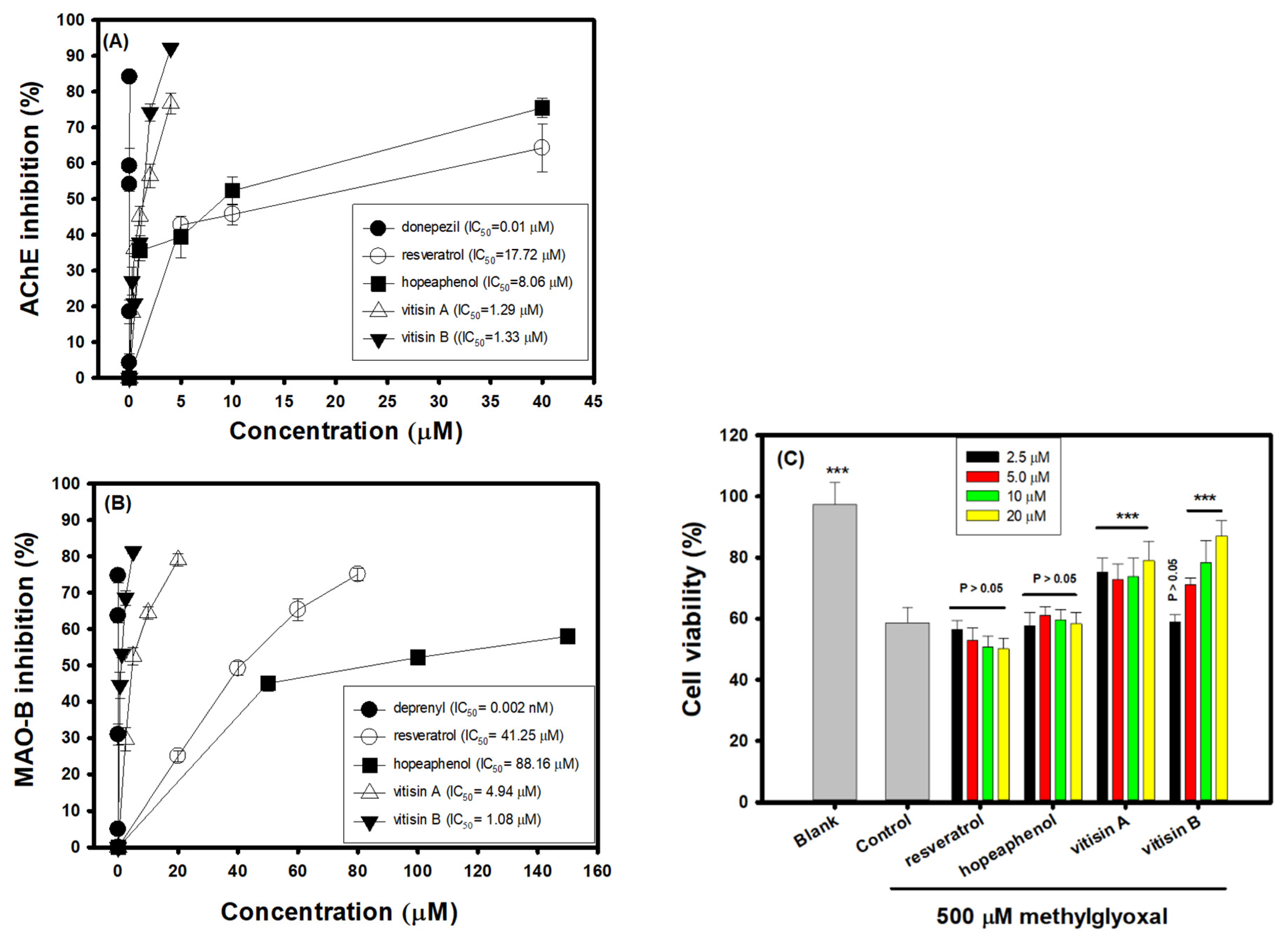
3.3. Effects of Resveratrol Tetramers on Inhibitory Activities of AChE and MAO-B and Neuroprotective Activities on MGO-Induced SH-SY5Y Cell Deaths
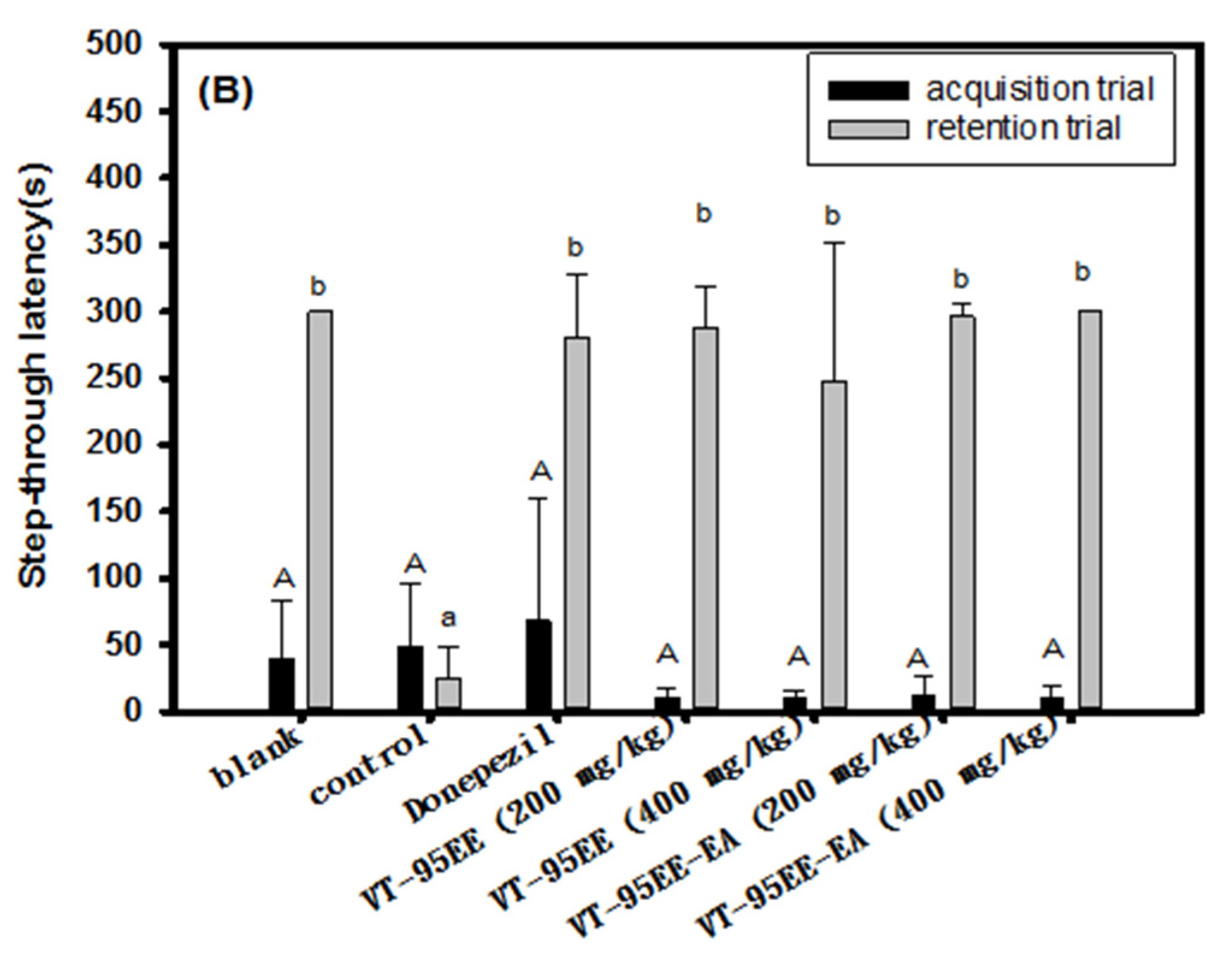
3.4. Effects of VT-95EE and VT-95EE-EA Pretreatments on the Improvements of Impaired Learning and Memory Functions in Scopolamine-Treated Amnesiac ICR Mice
3.5. Effects of of Resveratrol Tetramers with the Same Molecular Mass of Vitisin A and Vitisin B Pretreatments on Impaired Learning and Memory Functions in Scopolamine-Treated Amnesiac ICR Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Dementia. 2019. Available online: https://www.who.int/en/news-room/fact-sheets/detail/dementia (accessed on 26 December 2021).
- Plassman, B.L.; Lang, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 8, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Čolović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Micheau, J.; Marighetto, A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behav. Brain Res. 2011, 221, 424–429. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [Green Version]
- Herholz, K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. 1), S25–S29. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J.F. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Nachum, Z.; Shupak, A.; Gordon, C.R. Transdermal scopolamine for prevention of motion sickness. Clinical pharmacokinetics and therapeutic applications. Clin. Pharmacokinet. 2006, 45, 543–566. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.A.; Baker, J.D.; Locke, C.; Rueter, L.E.; Mohler, E.G.; Wesnes, K.; Abi-Saab, W.; Saltarelli, M.D. The scopolamine model as a pharmacodynamic marker in early drug development. Psychopharmacology 2012, 220, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. The neuropsychology of human memory. Annu. Rev. Neurosci. 1982, 5, 241–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Mobley, W.C. Exploring the pathogenesis of Alzheimer disease in basal forebrain cholinergic neurons: Onverging insights from alternative hypotheses. Front. Neurosci. 2019, 3, 446. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.D.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Chen, L.G.; Lin, S.Y.; Lee, Y.S.; Wang, C.C.; Hou, W.C. Hydrolysable tannins exhibit acetylcholinesterase inhibitory and anti-glycation activities in vitro and learning and memory function improvements in scopolamine-induced amnesiac mice. Biomedicines 2021, 9, 1066. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lee, C.J.; Chen, L.C.; Lee, T.L.; Hsieh, Y.Y.; Han, C.H.; Yang, C.H.; Huang, W.J.; Hou, W.C. Acetylcholinesterase inhibitory activity and neuroprotection in vitro, molecular docking, and improved learning and memory functions of demethylcurcumin in scopolamine-induced amnesia ICR mice. Food Funct. 2020, 11, 2328–2338. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Baik, S.H.; Kang, S.; Cho, S.W.; Bae, J.; Cha, M.Y.; Sailor, M.J.; Inhee, M.J.; Ahn, K.H. Close correlation of monoamine oxidase activity with progress of Alzheimer’s disease in mice, observed by in vivo two-photon imaging. ACS Cent. Sci. 2016, 2, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Calcutt, N.A.; Cooper, M.E.; Kern, T.S.; Schmidt, A.M. Therapies for hyperglycaemia-induced diabetic complications: From animal models to clinical trials. Nature Rev. Drug Discov. 2009, 8, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Newurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adh. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.M.; Lin, Y.Z.; Lee, T.L.; Imtiyaz, Z.; Hou, W.C.; Lee, M.H. In vitro and in vivo evaluation of the neuroprotective activity of Uncaria hirsuta Haviland. J. Food Drug Anal. 2020, 28, 147–158. [Google Scholar] [CrossRef]
- Han, C.H.; Liu, J.C.; Fang, S.U.; Hou, W.C. Antioxidant activities of the synthesized thiol-contained peptides derived from computer-aided pepsin hydrolysis of yam tuber storage protein, dioscorin. Food Chem. 2013, 138, 923–930. [Google Scholar] [CrossRef]
- Huang, Y.L.; Tsai, W.J.; Shen, C.C.; Chen, C.C. Resveratrol derivatives from the roots of Vitis thunbergii. J. Nat. Prod. 2005, 68, 217–220. [Google Scholar] [CrossRef]
- Huang, Y.L.; Liu, Y.W.; Huang, Y.J.; Chiou, W.F. A special ingredient (VtR) containing oligostilbenes isolated from Vitis thunbergii prevents bone loss in ovariectomized mice: In vitro and in vivo srudy. Evid. Complement. Altern. Med. 2013, 2013, 409421. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.S.; Lu, Y.L.; Wang, G.J.; Chen, L.G.; Wen, C.L.; Hou, W.C. Ethanolic extracts and isolated compounds from small-leaf grape (Vitis thunbergii var. taiwaniana) with antihypertensive activities. J. Agric. Food Chem. 2012, 60, 7435–7441. [Google Scholar] [CrossRef]
- Lin, S.Y.; Huang, G.C.; Hsieh, Y.Y.; Lin, Y.S.; Han, C.H.; Wen, C.L.; Chang, C.I.; Hou, W.C. Vitis thunbergii var. taiwaniana extracts and purified compounds ameliorate obesity in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 9286–9294. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Chen, C.R.; Wu, W.H.; Wen, C.L.; Chang, C.I.; Hou, W.C. Anti-α-glucosidase and anti-dipeptidyl peptidase-IV activities of extracts and purified compounds from Vitis thunbergii var. taiwaniana. J. Agric. Food Chem. 2015, 63, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Wang, C.C. Preparative separation of oligostilbenes from Vitis thunbergii var. taiwaniana by centrifugal partition chromatography followed by Sephadex LH-20 column chromatography. Sep. Purif. Technol. 2009, 66, 65–70. [Google Scholar] [CrossRef]
- Morinan, A.; Garratt, H.M. An improved fluorimetric assay for brain monoamine oxidase. J. Pharmacol. Methods 1985, 13, 213–223. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Ferreira, G.C.; Schuck, P.F.; Bosco, S.M.D. Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem. Biol. Interact. 2015, 242, 396–406. [Google Scholar] [CrossRef]
- Gacar, N.; Mutlu, O.; Utkan, T.; Celikyurt, I.K.; Gocmez, S.S.; Ulak, G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 2011, 99, 316–323. [Google Scholar] [CrossRef]
- Yamada, K.; Toshitaka Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.S.; Li, W.P.; Yao, Z.B.; Zhou, X.F. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999, 835, 259–265. [Google Scholar] [CrossRef]
- Schaaf, M.J.; De Kloet, E.R.; Vreugdenhil, E. Corticosterone effects on BDNF expression in the hippocampus implications for memory formation. Stress 2000, 3, 201–208. [Google Scholar] [CrossRef]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death. Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.Y.; Jang, M.J.; Choi, Y.H.; Hwang, H.; Rhim, H.; Lee, B.; Choi, C.W.; Min Soo Kim, M.S. Central administration of afzelin extracted from Ribes fasciculatum improves cognitive and memory function in a mouse model of dementia. Sci. Rep. 2021, 11, 9182. [Google Scholar] [CrossRef] [PubMed]
- Moghbelinejad, S.; Nassiri-Asl, M.; Farivar, T.N.; Abbasi, E.; Sheikhi, M.; Taghiloo, M.; Farsad, F.; Samimi, A.; Hajiali, F. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol. Lett. 2014, 224, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Minichiello, L.; Korte, M.; Wolfer, D.; Kühn, R.; Unsicker, K.; Cestari, V.; Rossi-Arnaud, C.; Lipp, H.P.; Bonhoeffer, T.; Klein, R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 1999, 24, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; You, Y.; Gupta, V.B.; Klistorner, A.; Graham, S.L. TrkB receptor signaling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef]
- Kingsbury, T.J.; Murray, P.D.; Bambrick, L.L.; Krueger, B.K. Ca2+-dependent regulation of TrkB expression in neurons. J. Biol. Chem. 2003, 278, 40744–40748. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
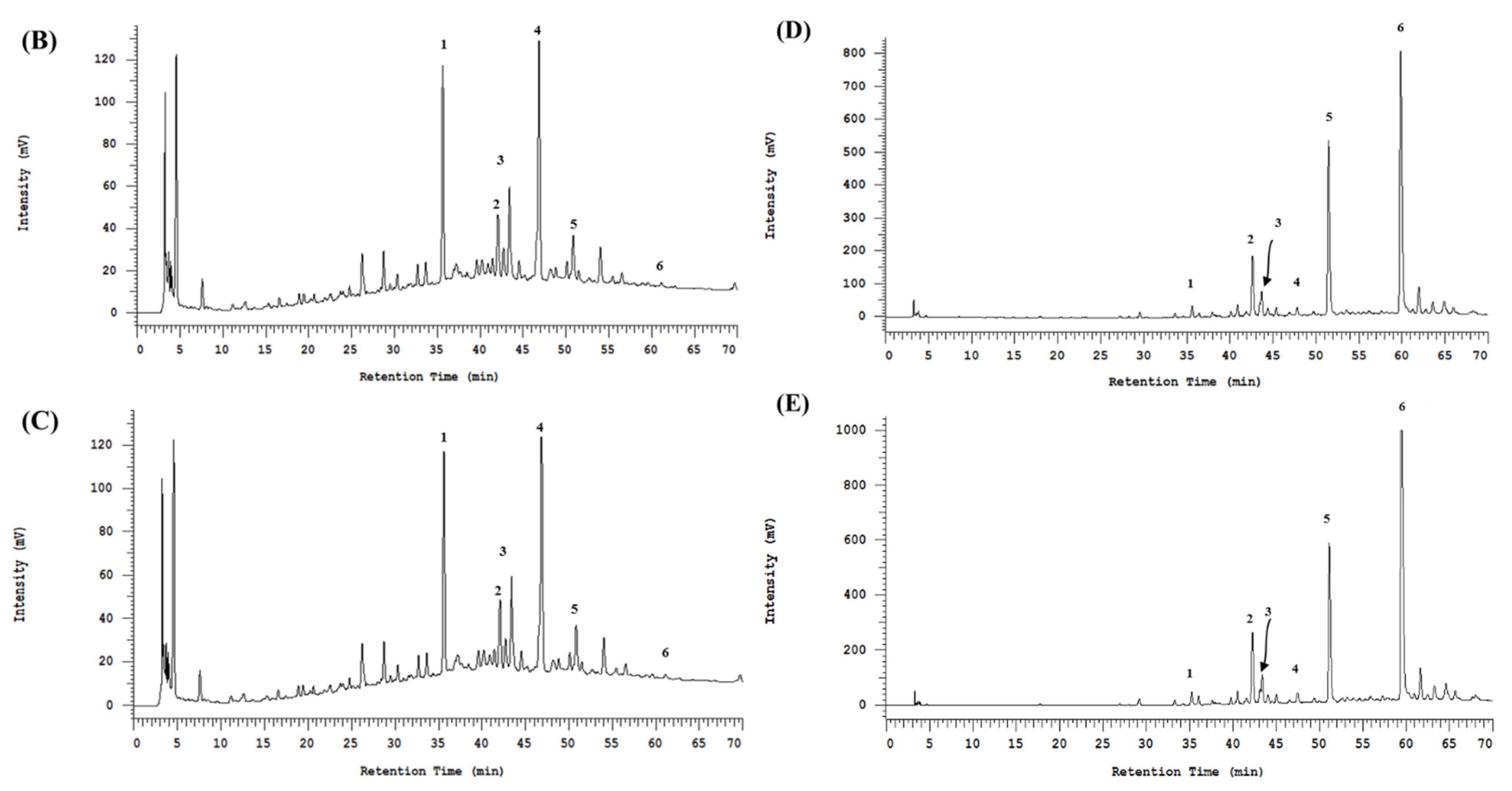



| Extracts | VT-95EE | VT-95EE-EA | VTT-R-95EE | VTT-R-95EE-EA | ||
|---|---|---|---|---|---|---|
| Area (%) | ||||||
| Compound | ||||||
| Monomer resveratrol (peak 1) | 33.97 # | 33.90 | 0.41 | 0.76 | ||
| Dimer (+)-ε-viniferin (peak 4) | 47.89 | 44.43 | 0.86 | 0.96 | ||
| Trimer (+)-ampelopsin C (peak 3) | 12.64 | 10.51 | 1.10 | 1.12 | ||
| Tetramer (+)- hopeaphenol (peak 2) | 2.37 | 2.24 | 8.30 | 8.09 | ||
| Tetramer (+)-vitisin A (peak 5) | 2.52 | 2.30 | 24.37 | 19.33 | ||
| Tetramer (-)-vitisin B (peak 6) | 0.60 | 0.62 | 41.20 | 45.14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-G.; Wang, C.-C.; Lee, Y.-S.; Sie, Y.-Y.; Chang, C.-I.; Hou, W.-C. Vitisin A, a Resveratrol Tetramer, Improves Scopolamine-Induced Impaired Learning and Memory Functions in Amnesiac ICR Mice. Biomedicines 2022, 10, 273. https://doi.org/10.3390/biomedicines10020273
Chen L-G, Wang C-C, Lee Y-S, Sie Y-Y, Chang C-I, Hou W-C. Vitisin A, a Resveratrol Tetramer, Improves Scopolamine-Induced Impaired Learning and Memory Functions in Amnesiac ICR Mice. Biomedicines. 2022; 10(2):273. https://doi.org/10.3390/biomedicines10020273
Chicago/Turabian StyleChen, Lih-Geeng, Ching-Chiung Wang, Yi-Shan Lee, Yi-Yan Sie, Chi-I Chang, and Wen-Chi Hou. 2022. "Vitisin A, a Resveratrol Tetramer, Improves Scopolamine-Induced Impaired Learning and Memory Functions in Amnesiac ICR Mice" Biomedicines 10, no. 2: 273. https://doi.org/10.3390/biomedicines10020273
APA StyleChen, L.-G., Wang, C.-C., Lee, Y.-S., Sie, Y.-Y., Chang, C.-I., & Hou, W.-C. (2022). Vitisin A, a Resveratrol Tetramer, Improves Scopolamine-Induced Impaired Learning and Memory Functions in Amnesiac ICR Mice. Biomedicines, 10(2), 273. https://doi.org/10.3390/biomedicines10020273







