In Vivo Activity of Metal Complexes Containing 1,10-Phenanthroline and 3,6,9-Trioxaundecanedioate Ligands against Pseudomonas aeruginosa Infection in Galleria mellonella Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pseudomonas aeruginosa Strains and Culture Conditions
2.2. Galleria mellonella Larvae Monitoring
2.3. Test Complexes
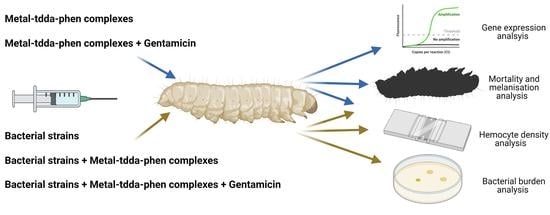
2.4. Galleria Mellonella Infection Studies with Pseudomonas aeruginosa Strains
2.4.1. Bacterial Infection of G. mellonella
2.4.2. Determination of Hemocyte Density
2.4.3. Determination of G. mellonella Hemolymph Burden of P. aeruginosa
2.5. Galleria mellonella Response to Metal-tdda-phen Complexes +/− Gentamicin
2.5.1. Toxicity Studies
2.5.2. Determination of Hemocyte Density
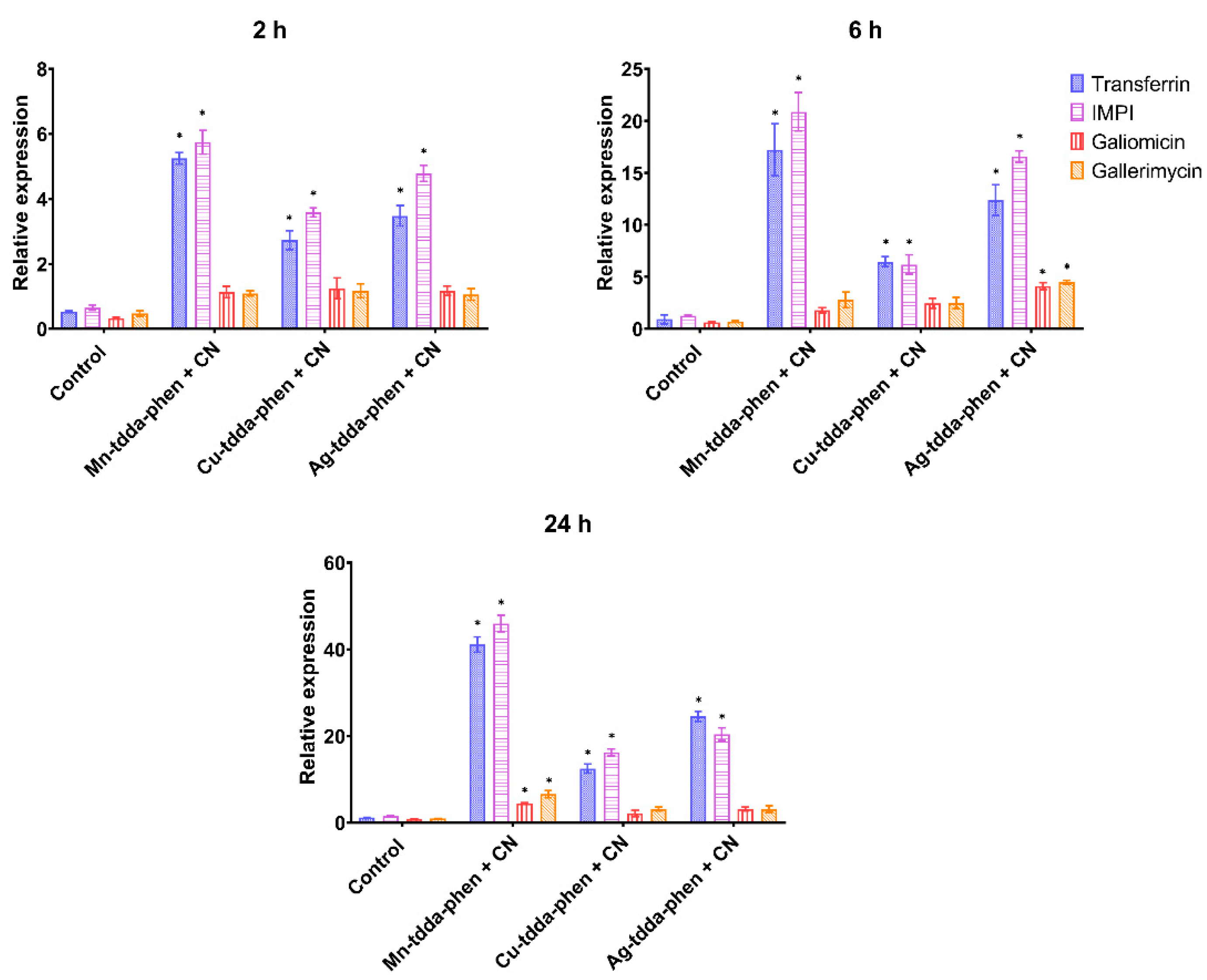
2.5.3. Gene Expression of Immune-Related Genes
2.6. Galleria mellonella Response to Pseudomonas aeruginosa Infection and Treatment with Metal-tdda-phen Complexes +/− Gentamicin
2.6.1. Treatment of Metal-tdda-phen Complexes in G. mellonella Infected with P. aeruginosa
2.6.2. Treatment of Metal-tdda-phen Complexes + Gentamicin in G. mellonella Infected with P. aeruginosa
2.7. Statistical Analysis
3. Results
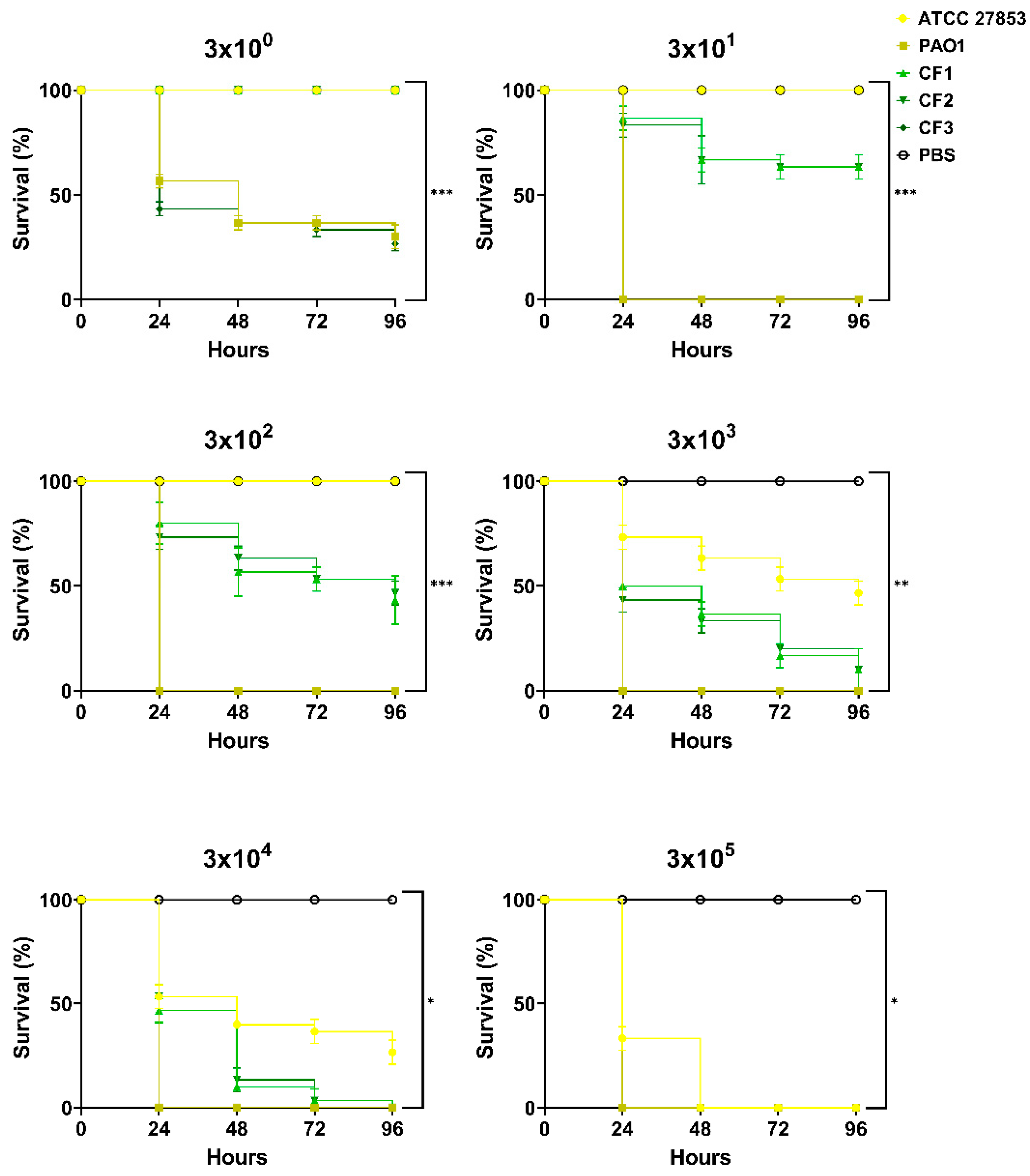
3.1. Response of Galleria mellonella to Pseudomonas aeruginosa Infection
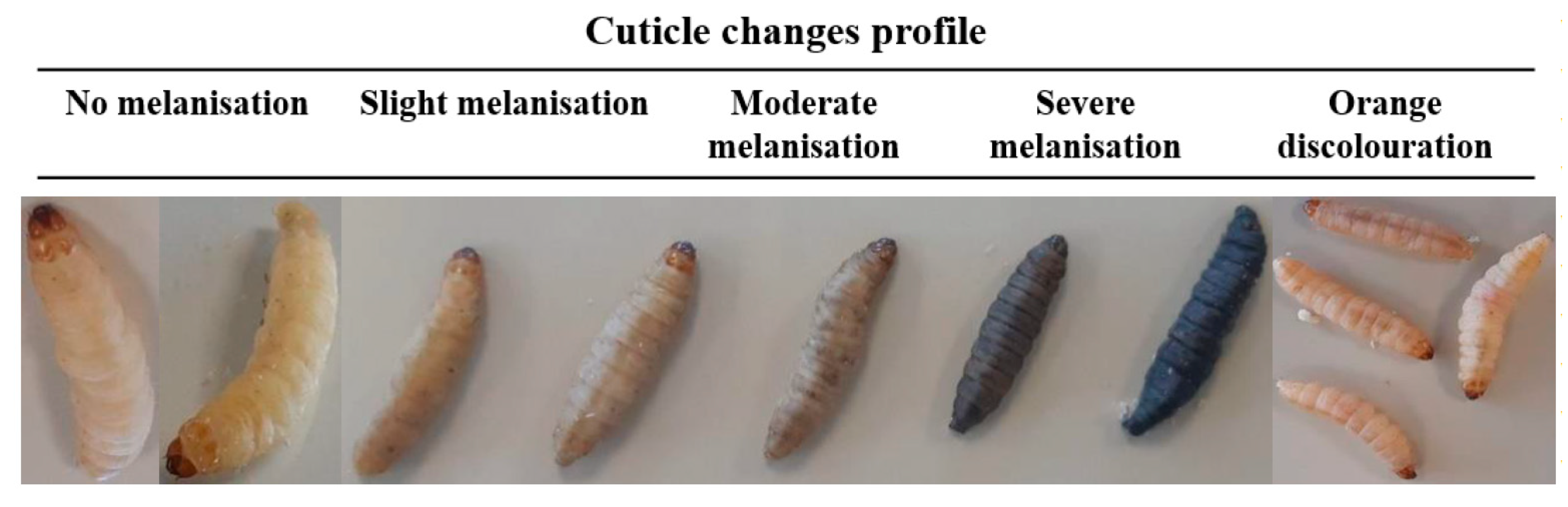
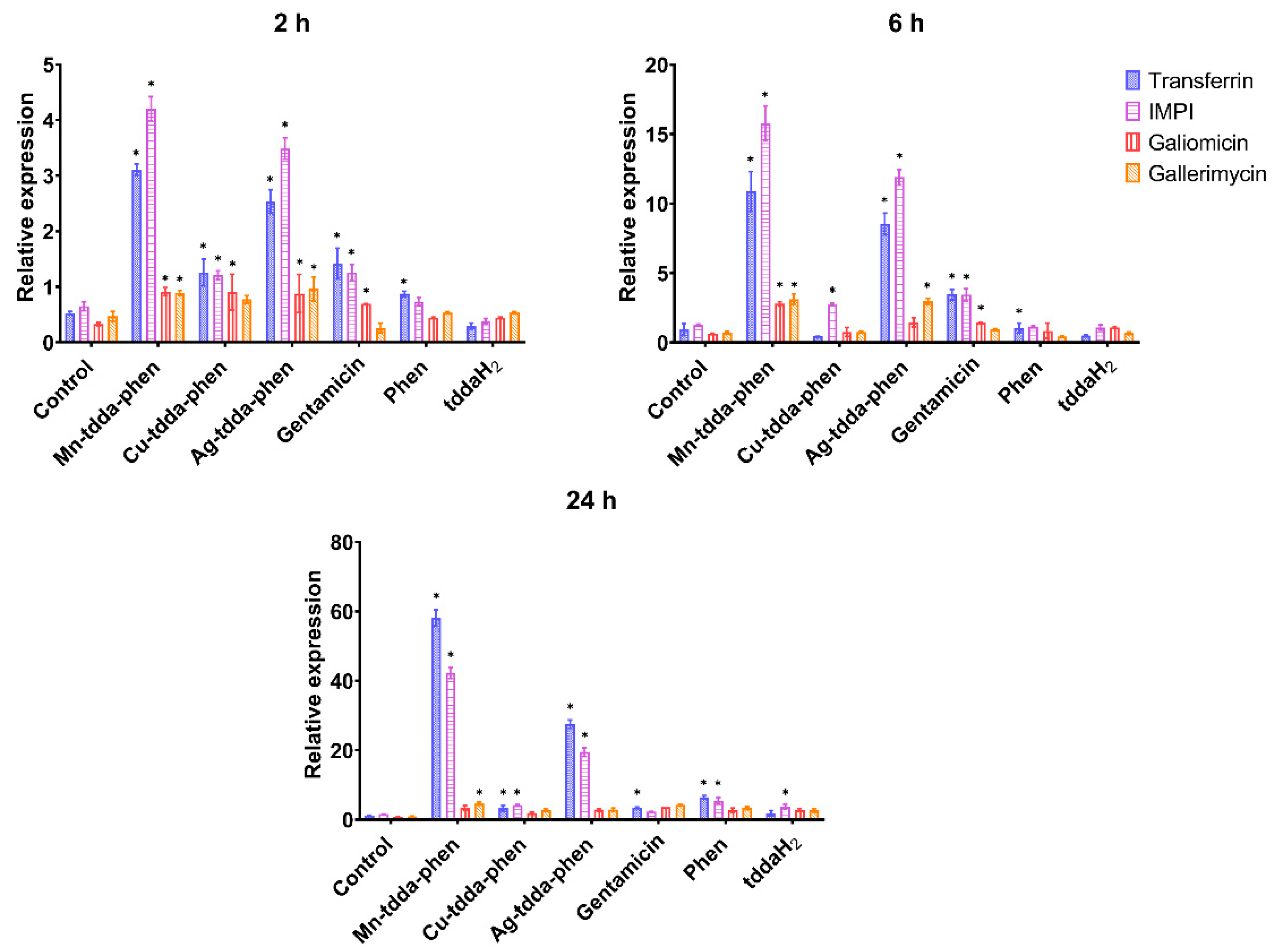
3.2. Immune Response of Galleria mellonella to Pseudomonas aeruginosa Infection
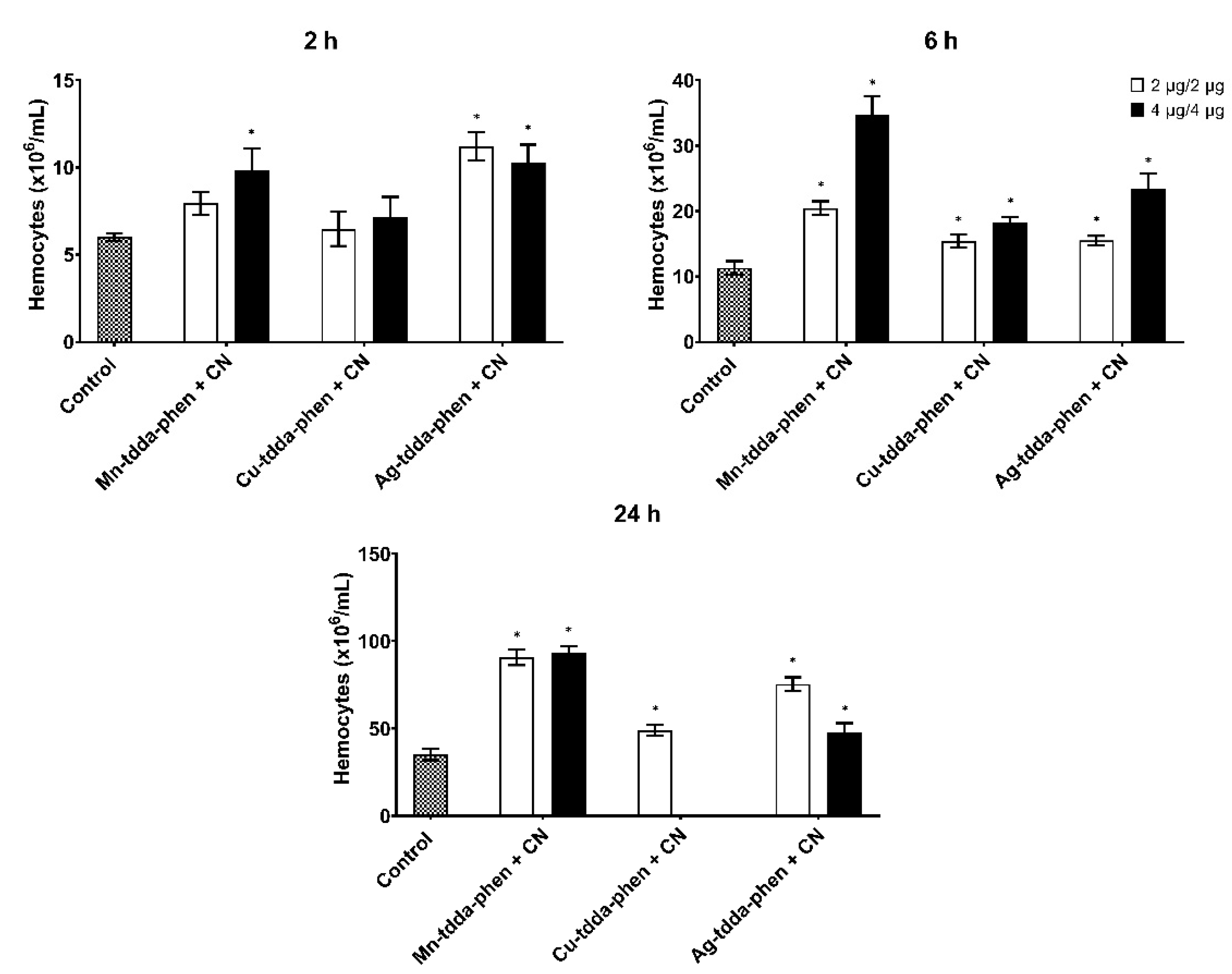
3.3. Galleria mellonella Response to Metal-tdda-phen Complexes
3.4. Galleria mellonella Response to Metal-tdda-phen Complexes and Gentamicin
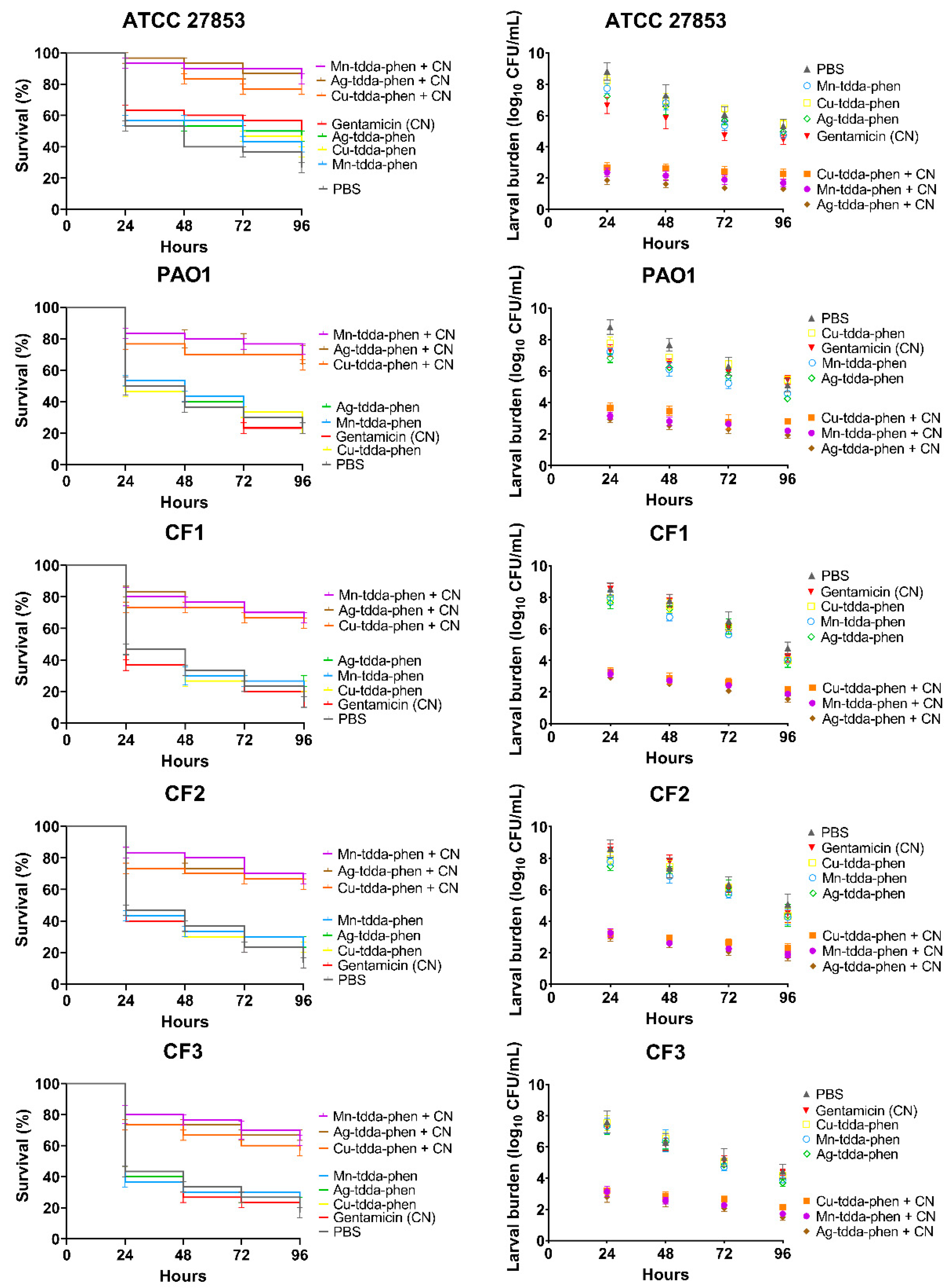
3.5. Effect of Metal-tdda-phen Complexes in Treating Pseudomonas aeruginosa Infection in Galleria mellonella +/− Gentamicin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2020 Antibacterial Agents in Clinical and Preclinical Development. Available online: https://www.who.int/publications/i/item/9789240021303 (accessed on 21 March 2021).
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Wunderink, R.G.; Waterer, G. Advances in the causes and management of community acquired pneumonia in adults. BMJ 2017, 358, j2471. [Google Scholar] [CrossRef]
- Garcia-Nuñez, M.; Marti, S.; Puig, C.; Perez-Brocal, V.; Millares, L.; Santos, S.; Ardanuy, C.; Moya, A.; Liñares, J.; Monsó, E. Bronchial microbiome, PA biofilm-forming capacity and exacerbation in severe COPD patients colonized by P. aeruginosa. Futur. Microbiol. 2017, 12, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.; O’Brien, S.; Brockhurst, M. Pseudomonas aeruginosa Evolutionary Adaptation and Diversification in Cystic Fibrosis Chronic Lung Infections. Trends Microbiol. 2016, 24, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theuretzbacher, U.; Gottwalt, S.; Beyer, P.; Butler, M.; Czaplewski, L.; Lienhardt, C.; Moja, L.; Paul, M.; Paulin, S.; Rex, J.; et al. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect. Dis. 2019, 19, e40–e50. [Google Scholar] [CrossRef]
- Amann, S.; Neef, K.; Kohl, S. Antimicrobial resistance (AMR). Eur. J. Hosp. Pharm. 2019, 26, 175–177. [Google Scholar] [CrossRef]
- Cafora, M.; Deflorian, G.; Forti, F.; Ferrari, L.; Binelli, G.; Briani, F.; Ghisotti, D.; Pistocchi, A. Phage therapy against Pseudomonas aeruginosa infections in a cystic fibrosis zebrafish model. Sci. Rep. 2019, 9, 1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, S.; Seixas, A.; Marques, J.; Leitão, J. Immunization and Immunotherapy Approaches against Pseudomonas aeruginosa and Burkholderia cepacia Complex Infections. Vaccines 2021, 9, 670. [Google Scholar] [CrossRef]
- Ruden, S.; Rieder, A.; Ster, I.C.; Schwartz, T.; Mikut, R.; Hilpert, K. Synergy Pattern of Short Cationic Antimicrobial Peptides Against Multidrug-Resistant Pseudomonas aeruginosa. Front. Microbiol. 2019, 10, 2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasir, M.; Dutta, D.; Willcox, M.D. Activity of Antimicrobial Peptides and Ciprofloxacin against Pseudomonas aeruginosa Biofilms. Molecules 2020, 25, 3843. [Google Scholar] [CrossRef]
- Rázquin-Olazarán, I.; Shahrour, H.; Martínez-De-Tejada, G. A synthetic peptide sensitizes multi-drug resistant Pseudomonas aeruginosa to antibiotics for more than two hours and permeabilizes its envelope for twenty hours. J. Biomed. Sci. 2020, 27, 1–19. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1,10- phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viganor, L.; Galdino, A.C.M.; Nunes, A.P.F.; Santos, K.R.N.; Branquinha, M.H.; Devereux, M.; Kellett, A.; McCann, M.; Santos, A.L.S. Anti- Pseudomonas aeruginosa activity of 1,10-phenanthroline-based drugs against both planktonic- and biofilm-growing cells. J. Antimicrob. Chemother. 2015, 71, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, R.F.; Galdino, A.C.M.; Viganor, L.; Schuenck, R.P.; Devereux, M.; McCann, M.; Santos, A.L.; Nunes, A.P.F. Antimicrobial action of 1,10-phenanthroline-based compounds on carbapenemase-producing Acinetobacter baumannii clinical strains: Efficacy against planktonic- and biofilm-growing cells. Braz. J. Microbiol. 2020, 51, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.Q.; Gonçalves, D.D.S.; Seabra, S.H.; McCann, M.; Devereux, M.; dos Santos, A.L.S.; Kneipp, L.F. 1,10-Phenanthroline-5,6-Dione–Based Compounds Are Effective in Disturbing Crucial Physiological Events of Phialophora verrucosa. Front. Microbiol. 2017, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.; Santos, A.L.S.; da Silva, B.A.; Romanos, M.T.V.; Pyrrho, A.S.; Devereux, M.; Kavanagh, K.; Fichtner, I.; Kellett, A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(ii) and silver(i) complexes. Toxicol. Res. 2012, 1, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Granato, M.Q.; Mello, T.P.; Nascimento, R.S.; Pereira, M.D.; Rosa, T.L.S.A.; Pessolani, M.C.V.; McCann, M.; Devereux, M.; Branquinha, M.H.; Santos, A.L.S.; et al. Silver(I) and Copper(II) Complexes of 1,10-Phenanthroline-5,6-Dione against Phialophora verrucosa: A Focus on the Interaction With Human Macrophages and Galleria mellonella Larvae. Front. Microbiol. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vargas Rigo, G.; Petro-Silveira, B.; Devereux, M.; McCann, M.; Souza Dos Santos, A.L.; Tasca, T. Anti-Trichomonas vaginalis activity of 1,10-phenanthroline-5,6-dione-based metallodrugs and synergistic effect with metronidazole. Parasitology 2019, 146, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Fricker, S.P.; Mosi, R.M.; Cameron, B.R.; Baird, I.; Zhu, Y.; Anastassov, V.; Cox, J.; Doyle, P.S.; Hansell, E.; Lau, G.; et al. Metal compounds for the treatment of parasitic diseases. J. Inorg. Biochem. 2008, 102, 1839–1845. [Google Scholar] [CrossRef]
- Lima, A.K.C.; Elias, C.G.R.; Oliveira, S.S.C.; Santos-Mallet, J.R.; McCann, M.; Devereux, M.; Branquinha, M.H.; Dutra, P.M.L.; Santos, A.L.S. Anti-Leishmania braziliensis activity of 1,10-phenanthroline-5,6-dione and its Cu(II) and Ag(I) complexes. Parasitol. Res. 2021, 120, 3273–3285. [Google Scholar] [CrossRef]
- Shulman, A.; White, D. Virostatic activity of 1,10-phenanthroline transition metal chelates: A structure-activity analysis. Chem. Interact. 1973, 6, 407–413. [Google Scholar] [CrossRef]
- Mazumder, A.; Gupta, M.; Perrin, D.M.; Sigman, D.S.; Rabinovitz, M.; Pommier, Y. Inhibition of Human Immunodeficiency Virus Type 1 Integrase by a Hydrophobic Cation: The Phenanthroline-Cuprous Complex. AIDS Res. Hum. Retrovir. 1995, 11, 115–125. [Google Scholar] [CrossRef]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt complexes as antiviral and antibacterial agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shaughnessy, M.; McCarron, P.; Viganor, L.; McCann, M.; Devereux, M.; Howe, O. The Antibacterial and Anti-biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas Aeruginosa from Irish Cystic Fibrosis Patients. Antibiotics 2020, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- McCarron, P.; McCann, M.; Devereux, M.; Kavanagh, K.; Skerry, C.; Karakousis, P.C.; Aor, A.C.; Mello, T.P.; Santos, A.L.S.; Campos, D.L.; et al. Unprecedented in Vitro Antitubercular Activitiy of Manganese(II) Complexes Containing 1,10-Phenanthroline and Dicarboxylate Ligands: Increased Activity, Superior Selectivity, and Lower Toxicity in Comparison to Their Copper(II) Analogs. Front. Microbiol. 2018, 9, 1432. [Google Scholar] [CrossRef] [PubMed]
- Gandra, R.M.; Mc Carron, P.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal Potential of Copper(II), Manganese(II) and Silver(I) 1,10-Phenanthroline Chelates against Multidrug-Resistant Fungal Species Forming the Candida haemulonii Complex: Impact on the Planktonic and Biofilm Lifestyles. Front. Microbiol. 2017, 8, 1257. [Google Scholar] [CrossRef]
- Browne, N.; Surlis, C.; Kavanagh, K. Thermal and physical stresses induce a short-term immune priming effect in Galleria mellonella larvae. J. Insect Physiol. 2014, 63, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, K.; Reeves, E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, G.; Kavanagh, K. Proteomic Analysis of the Responses of Candida albicans during Infection of Galleria mellonella Larvae. J. Fungi 2019, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, G.; Dixon, A.; Kavanagh, K. Utilization of Galleria mellonella larvae to characterize the development of Staphylococcus aureus infection. Microbiology 2019, 165, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Borghi, E.; Romagnoli, S.; Fuchs, B.B.; Cirasola, D.; Perdoni, F.; Tosi, D.; Braidotti, P.; Bulfamante, G.; Morace, G.; Mylonakis, E. Correlation between Candida albicans biofilm formation and invasion of the invertebrate host Galleria mellonella. Future Microbiol. 2014, 9, 163–173. [Google Scholar] [CrossRef]
- Rochford, G.; Molphy, Z.; Browne, N.; Surlis, C.; Devereux, M.; McCann, M.; Kellett, A.; Howe, O.; Kavanagh, K. In-vivo evaluation of the response of Galleria mellonella larvae to novel copper(II) phenanthroline-phenazine complexes. J. Inorg. Biochem. 2018, 186, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, L.; Dixit, V.; Assad, L.O.; Ribeiro, T.P.; Queiroz, D.; Kellett, A.; Casey, A.; Colleran, J.; Pereira, M.D.; Rochford, G.; et al. Water-soluble and photo-stable silver(I) dicarboxylate complexes containing 1,10-phenanthroline ligands: Antimicrobial and anticancer chemotherapeutic potential, DNA interactions and antioxidant activity. J. Inorg. Biochem. 2016, 159, 120–132. [Google Scholar] [CrossRef]
- Krezdorn, J.; Adams, S.; Coote, P.J. A Galleria mellonella infection model reveals double and triple antibiotic combination therapies with enhanced efficacy versus a multidrug-resistant strain of Pseudomonas aeruginosa. J. Med. Microbiol. 2014, 63, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignasiak, K.; Maxwell, A. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC Res. Notes 2017, 10, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandra, R.M.; McCarron, P.; Viganor, L.; Fernandes, M.F.; Kavanagh, K.; McCann, M.; Branquinha, M.H.; Santos, A.L.S.; Howe, O.; Devereux, M. In vivo Activity of Copper(II), Manganese(II), and Silver(I) 1,10-Phenanthroline Chelates against Candida haemulonii Using the Galleria mellonella Model. Front. Microbiol. 2020, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Jander, G.; Rahme, L.G.; Ausubel, F.M. Positive Correlation between Virulence of Pseudomonas aeruginosa Mutants in Mice and Insects. J. Bacteriol. 2000, 182, 3843–3845. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, G.; Garvey, A.; Croke, M.; Kavanagh, K. Innate humoral immune defences in mammals and insects: The same, with differences ? Virulence 2018, 9, 1625–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergin, D.; Reeves, E.P.; Renwick, J.; Wientjes, F.B.; Kavanagh, K. Superoxide Production in Galleria mellonella Hemocytes: Identification of Proteins Homologous to the NADPH Oxidase Complex of Human Neutrophils. Infect. Immun. 2005, 73, 4161–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.C.; De Barros, P.P.; de Oliveira Fugisaki, L.R.; Rossoni, R.D.; de Camargo Ribeiro, F.; De Menezes, R.T.; Junqueira, J.C.; Scorzoni, L. Recent Advances in the Use of Galleria mellonella Model to Study Immune Responses against Human Pathogens. J. Fungi 2018, 4, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A.P.; Coote, P.J. Utility of Greater Wax Moth Larva (Galleria mellonella) for Evaluating the Toxicity and Efficacy of New Antimicrobial Agents. Adv. Appl. Microbiol. 2012, 78, 25–53. [Google Scholar] [CrossRef]
- McCann, S.; McCann, M.; Casey, M.T.; Devereux, M.; McKee, V.; McMichael, P.; McCrea, J.G. Manganese(II) complexes of 3,6,9-trioxaundecanedioic acid (3,6,9-tddaH2): X-ray crystal structures of [Mn(3,6,9-tdda) (H2O)2]·2H2O and {[Mn(3,6,9-tdda)(phen)2·3H2O]·EtOH}n. Polyhedron 1997, 16, 4247–4252. [Google Scholar] [CrossRef]
- Kelly, J.; Kavanagh, K. Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J. Med. Microbiol. 2011, 60, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, N. An Analysis of the Cellular and Humoral Immune Responses of Galleria mellonella Larvae. Ph.D. Thesis, The National University of Ireland, Maynooth, Ireland, 2014; pp. 1–275. [Google Scholar]
- Andrejko, M.; Zdybicka-Barabas, A.; Cytryńska, M. Diverse effects of Galleria mellonella infection with entomopathogenic and clinical strains of Pseudomonas aeruginosa. J. Invertebr. Pathol. 2014, 115, 14–25. [Google Scholar] [CrossRef]
- Hill, L.; Veli, N.; Coote, P.J. Evaluation of Galleria mellonella larvae for measuring the efficacy and pharmacokinetics of antibiotic therapies against Pseudomonas aeruginosa infection. Int. J. Antimicrob. Agents 2014, 43, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Beeton, M.; Alves, D.; Enright, M.; Jenkins, A. Assessing phage therapy against Pseudomonas aeruginosa using a Galleria mellonella infection model. Int. J. Antimicrob. Agents 2015, 46, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Bergin, D.; Brennan, M.; Kavanagh, K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect. 2003, 5, 1389–1395. [Google Scholar] [CrossRef] [Green Version]
- Kellett, A.; O’Connor, M.; McCann, M.; Howe, O.; Casey, A.; McCarron, P.; Kavanagh, K.; McNamara, M.; Kennedy, S.; May, D.D.; et al. Water-soluble bis(1,10-phenanthroline) octanedioate Cu2+ and Mn2+ complexes with unprecedented nano and picomolar in vitro cytotoxicity: Promising leads for chemotherapeutic drug development. MedChemComm 2011, 2, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018, 7, 212527. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Liimatta, K.; Wong Fok Lung, T.; Fields, B.; Ahn, D.; Chen, D.; Lozano, C.; Sáenz, Y.; Uhlemann, A.-C.; Kahl, B.C.; et al. Pseudomonas aeruginosa Utilizes Host-Derived Itaconate to Redirect Its Metabolism to Promote Biofilm Formation. Cell Metab. 2020, 31, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosabiofilms to antimicrobial agents—How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Kovach, K.; Davis-Fields, M.; Irie, Y.; Jain, K.; Doorwar, S.; Vuong, K.; Dhamani, N.; Mohanty, K.; Touhami, A.; Gordon, V.D. Evolutionary adaptations of biofilms infecting cystic fibrosis lungs promote mechanical toughness by adjusting polysaccharide production. NPJ Biofilms Microbiomes 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, J.S. Some aspects of immune responses in insects. In Vitro 1967, 3, 120–128. [Google Scholar] [CrossRef]
- Pérez-Gallego, M.; Torrens, G.; Castillo-Vera, J.; Moya, B.; Zamorano, L.; Cabot, G.; Hultenby, K.; Alberti, S.; Mellroth, P.; Henriques-Normark, B.; et al. Impact of AmpC Derepression on Fitness and Virulence: The Mechanism or the Pathway? mBio 2016, 7, e01783-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomaz, L.; De Almeida, L.G.; Silva, F.R.O.; Cortez, M.; Taborda, C.P.; Spira, B. In vivo Activity of Silver Nanoparticles against Pseudomonas aeruginosa Infection in Galleria mellonella. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Maguire, R.; Duggan, O.; Kavanagh, K. Evaluation of Galleria mellonella larvae as an in vivo model for assessing the relative toxicity of food preservative agents. Cell Biol. Toxicol. 2016, 32, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [Green Version]
- Cutuli, M.A.; Petronio, G.P.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Utilising Galleria mellonella larvae for studying in vivo activity of conventional and novel antimicrobial agents. Pathog. Dis. 2020, 78, 1–10. [Google Scholar] [CrossRef]
- Moreno, R.G.; García-Clemente, M.; Diab-Cáceres, L.; Martínez-Vergara, A.; Martínez-García, M.; Gómez-Punter, R. Treatment of Pulmonary Disease of Cystic Fibrosis: A Comprehensive Review. Antibiotics 2021, 10, 486. [Google Scholar] [CrossRef]
- Magnet, S.; Blanchard, J.S. Molecular Insights into Aminoglycoside Action and Resistance. Chem. Rev. 2005, 105, 477–498. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Wierzbowski, J.; Cottarel, G.; Collins, J.J. Mistranslation of Membrane Proteins and Two-Component System Activation Trigger Antibiotic-Mediated Cell Death. Cell 2008, 135, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, A.; Kavanagh, K.A. Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens. J. Med. Microbiol. 2021, 70, 001363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Xu, X.; Gao, P.; Xu, Z.; Zhang, Q.; Li, H.; Yan, A.; Kao, R.Y.-T.; Sun, H. Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance. Nat. Commun. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Palermo, G.; Magistrato, A.; Riedel, T.; Von Erlach, T.; Davey, C.A.; Dyson, P.; Rothlisberger, U. Fighting Cancer with Transition Metal Complexes: From Naked DNA to Protein and Chromatin Targeting Strategies. ChemMedChem 2016, 11, 1199–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Test Complex | Dose µg/Larvae (µM) | Mean Mortality (%) +/− SE over Time (h) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| Mn-tdda-phen | 2 µg (2.71 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (5.42 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 µg (13.59 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 15 µg (20.39 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 30 µg (40.78 µM) | 0 ± 0 | 6.66 ± 5.77 | 6.66 ± 5.77 | |
| Cu-tdda-phen | 2 µg (2.68 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (5.36 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 µg (13.41 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 15 µg (20.15 µM) | 23.33 ± 5.77 | 46.66 ± 5.77 | 53.33 ± 5.77 | |
| 30 µg (40.3 µM) | 76.66 ± 5.77 | 83.33 ± 5.77 | 100 ± 0 | |
| Ag-tdda-phen | 2 µg (1.6 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (3.3 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 µg (8.3 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 15 µg (12.5 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 30 µg (24.9 µM) | 3.33 ± 5.77 | 23.33 ± 5.77 | 23.33 ± 5.77 | |
| Gentamicin | 2 µg (3.5 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (6.9 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 µg (17.4 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 15 µg (26.1 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 30 µg (52.1 µM) | 0 ± 0 | 13.33 ± 5.77 | 13.33 ± 5.77 | |
| Phen | 2 µg (11.1µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (22.2 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 µg (55.5 µM) | 53.3 ± 5.8 | 53.3 ± 5.8 | 53.3 ± 5.8 | |
| 15 µg (83.2 µM) | 76.7 ± 5.8 | 76.7 ± 5.8 | 76.7 ± 5.8 | |
| 30 µg (166.5 µM) | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| tddaH2 | 2 µg (9 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (18 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 10 µg (45 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 15 µg (67.5 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 30 µg (135 µM) | 46.7 ± 5.8 | 46.7 ± 5.8 | 46.7 ± 5.8 | |
| Test Agents | Dose µg/Larvae (µM) | Mean Mortality (%) +/− SE over Time (h) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| Mn-tdda-phen + Gentamicin | 2 µg (2.71 µM) + 2µg (3.5 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (5.42 µM) + 4 µg (6.9 µM) | 20 ± 5.8 | 20 ± 5.8 | 20 ± 5.8 | |
| 10 µg (13.59 µM) + 10 µg (17.4 µM) | 83.3 ± 3.3 | 86.7 ± 3.3 | 86.7 ± 3.3 | |
| 2 µg (2.71 µM) + 10 µg (17.4 µM) | 43.3 ± 3.3 | 46.7 ± 3.3 | 46.7 ± 3.3 | |
| 10 µg (13.59 µM) + 2 µg (3.5 µM) | 46.7 ± 3.3 | 50.0 ± 5.8 | 53.3 ± 3.3 | |
| Cu-tdda-phen + Gentamicin | 2 µg (2.68 µM) + 2µg (3.5 µM) | 26.7 ± 3.3 | 26.7 ± 3.3 | 26.7 ± 3.3 |
| 4 µg (5.36 µM) + 4 µg (6.9 µM) | 70 ± 5.8 | 73.3 ± 3.3 | 73.3 ± 3.3 | |
| 10 µg (13.41 µM) + 10 µg (17.4 µM) | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| 2 µg (2.68 µM) + 10 µg (17.4 µM) | 63.3 ± 3.3 | 66.7 ± 3.3 | 73.3 ± 3.3 | |
| 10 µg (13.41 µM) + 2 µg (3.5 µM) | 83.3 ± 3.3 | 83.3 ± 3.3 | 83.3 ± 3.3 | |
| Ag-tdda-phen + Gentamicin | 2 µg (1.6 µM) + 2µg (2.5 µM) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 4 µg (3.3 µM) + 4 µg (6.9 µM) | 26.7 ± 3.3 | 33.3 ± 3.3 | 33.3 ± 5.8 | |
| 10 µg (8.3 µM) + 10 µg (17.4 µM) | 83.3 ± 3.3 | 93.3 ± 6.7 | 93.3 ± 6.7 | |
| 2 µg (1.6 µM) + 10 µg (17.4 µM) | 43.3 ± 3.3 | 53.3 ± 3.3 | 53.3 ± 3.3 | |
| 10 µg (8.3 µM) + 2 µg (2.5 µM) | 46.7 ± 6.7 | 50 ± 5.8 | 53.3 ± 3.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Shaughnessy, M.; Piatek, M.; McCarron, P.; McCann, M.; Devereux, M.; Kavanagh, K.; Howe, O. In Vivo Activity of Metal Complexes Containing 1,10-Phenanthroline and 3,6,9-Trioxaundecanedioate Ligands against Pseudomonas aeruginosa Infection in Galleria mellonella Larvae. Biomedicines 2022, 10, 222. https://doi.org/10.3390/biomedicines10020222
O’Shaughnessy M, Piatek M, McCarron P, McCann M, Devereux M, Kavanagh K, Howe O. In Vivo Activity of Metal Complexes Containing 1,10-Phenanthroline and 3,6,9-Trioxaundecanedioate Ligands against Pseudomonas aeruginosa Infection in Galleria mellonella Larvae. Biomedicines. 2022; 10(2):222. https://doi.org/10.3390/biomedicines10020222
Chicago/Turabian StyleO’Shaughnessy, Megan, Magdalena Piatek, Pauraic McCarron, Malachy McCann, Michael Devereux, Kevin Kavanagh, and Orla Howe. 2022. "In Vivo Activity of Metal Complexes Containing 1,10-Phenanthroline and 3,6,9-Trioxaundecanedioate Ligands against Pseudomonas aeruginosa Infection in Galleria mellonella Larvae" Biomedicines 10, no. 2: 222. https://doi.org/10.3390/biomedicines10020222
APA StyleO’Shaughnessy, M., Piatek, M., McCarron, P., McCann, M., Devereux, M., Kavanagh, K., & Howe, O. (2022). In Vivo Activity of Metal Complexes Containing 1,10-Phenanthroline and 3,6,9-Trioxaundecanedioate Ligands against Pseudomonas aeruginosa Infection in Galleria mellonella Larvae. Biomedicines, 10(2), 222. https://doi.org/10.3390/biomedicines10020222