L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Human Samples
2.2. Experimental Spinal Cord Injury and Recombinant Arginase-I Treatment
2.3. Assessment of Locomotor Recovery after Spinal Cord Injury
2.4. Plasma Preparation and L-Arginine ELISA
2.5. Immunohistochemistry
2.6. Quantitative Image Analysis
2.7. Splenocyte and Peripheral Blood Mononuclear Cell Isolation
2.8. T-Cell Characterization after Spinal Cord Injury
2.9. T-Cell Proliferation Assay
2.10. Quantitative PCR
2.11. Western Blotting
2.12. Microglial Cell Isolation
2.13. Isolation and Culture of Bone Marrow-Derived Macrophages
2.14. Griess Assay
2.15. MTT Viability Assay
2.16. Experimental Design and Statistical Analysis
3. Results
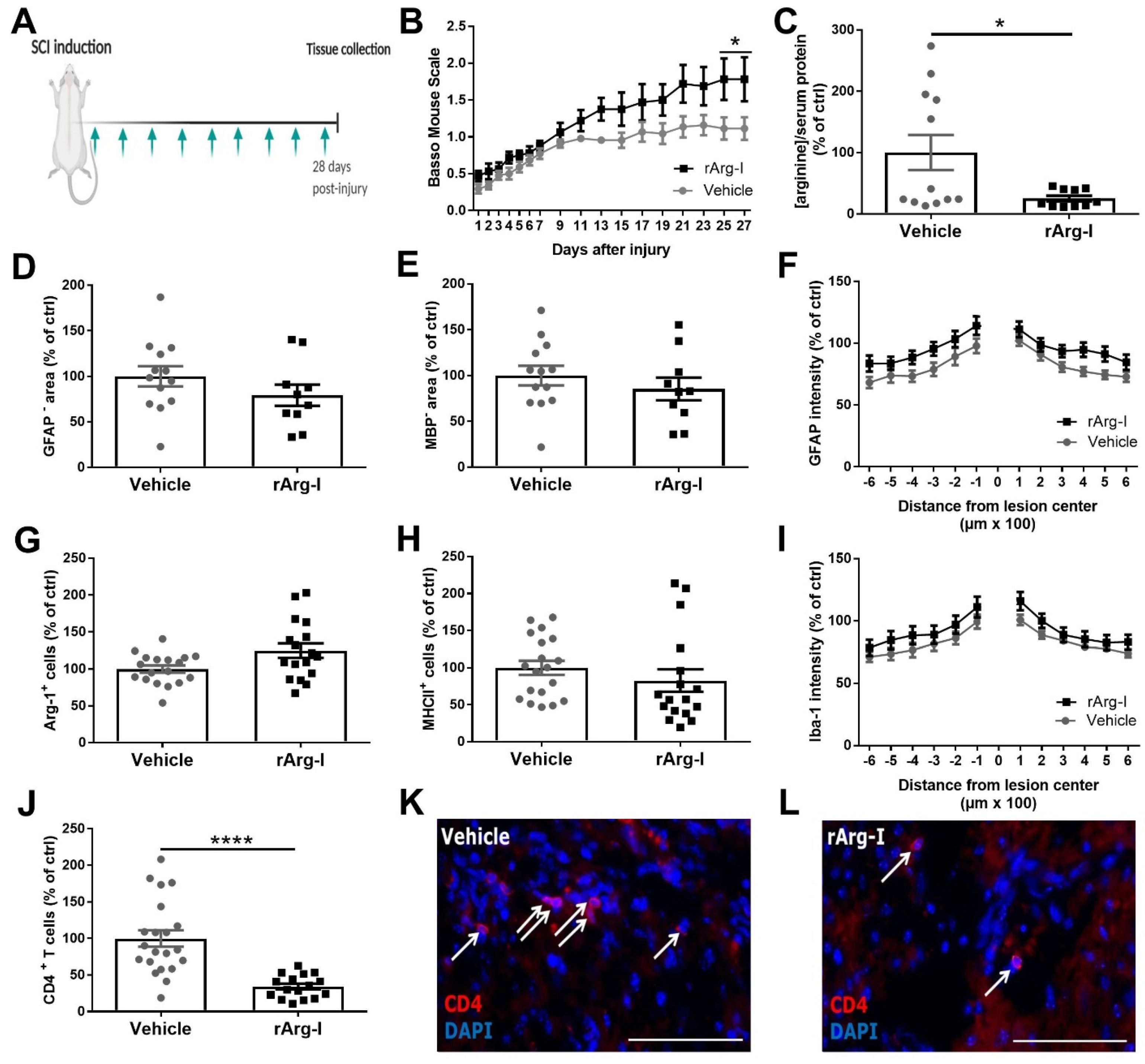
3.1. Systemic L-Arginine Depletion via rArg-I Administration Results in Improved Functional Recovery and Strongly Reduced CD4+ T-Cell Numbers after Spinal Cord Injury
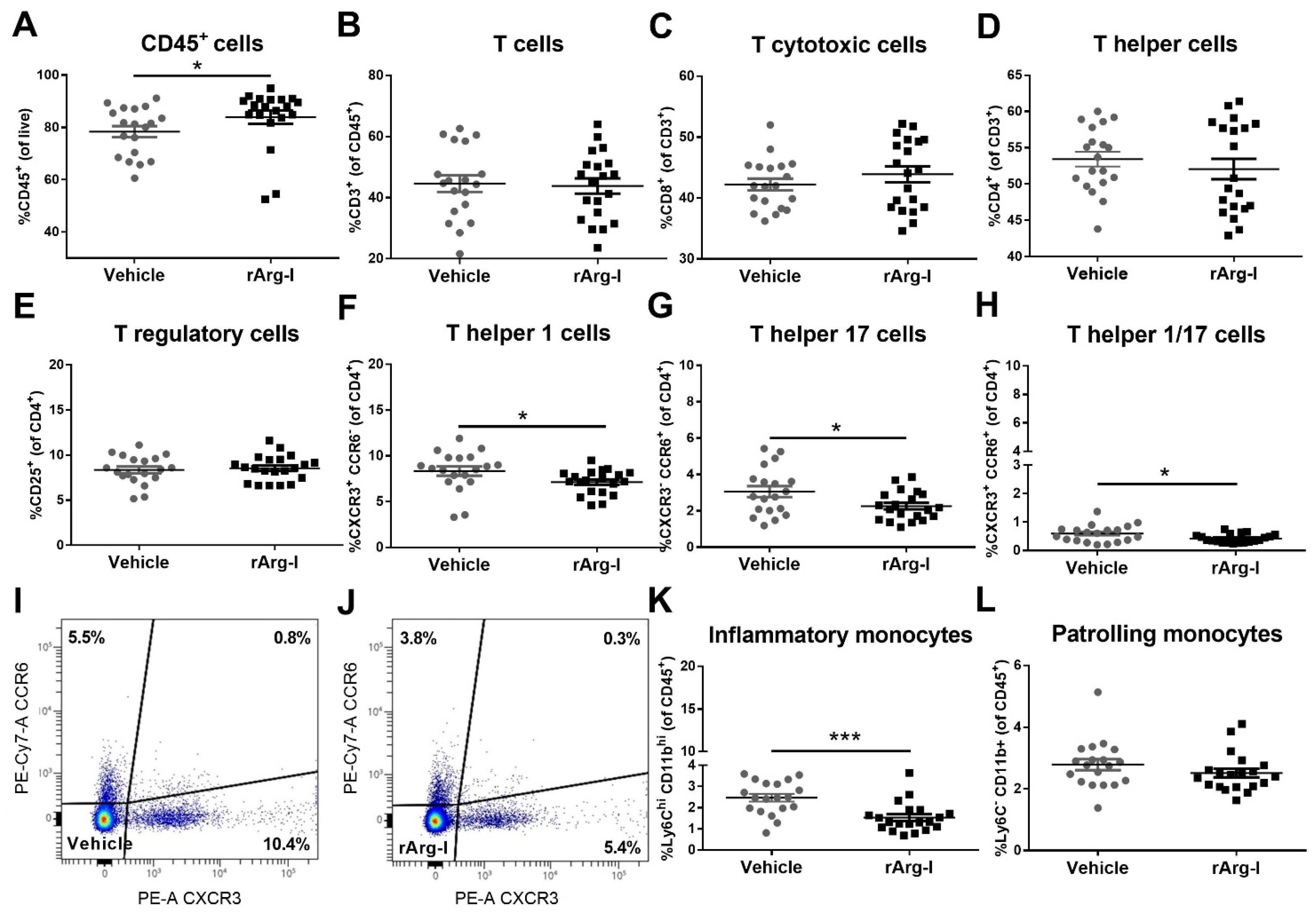
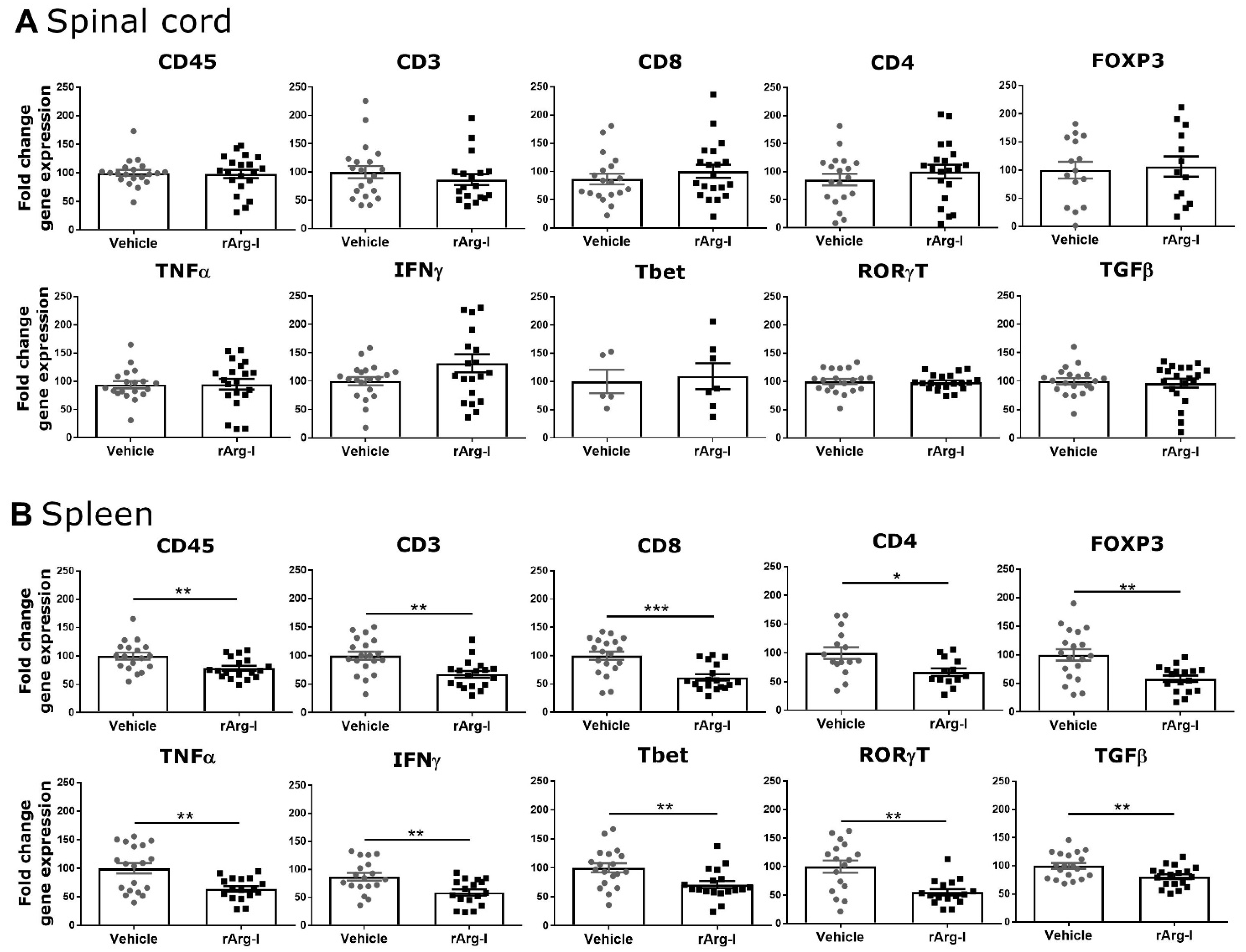
3.2. rArg-I Treatment Alters the Systemic Immune Response after Spinal Cord Injury
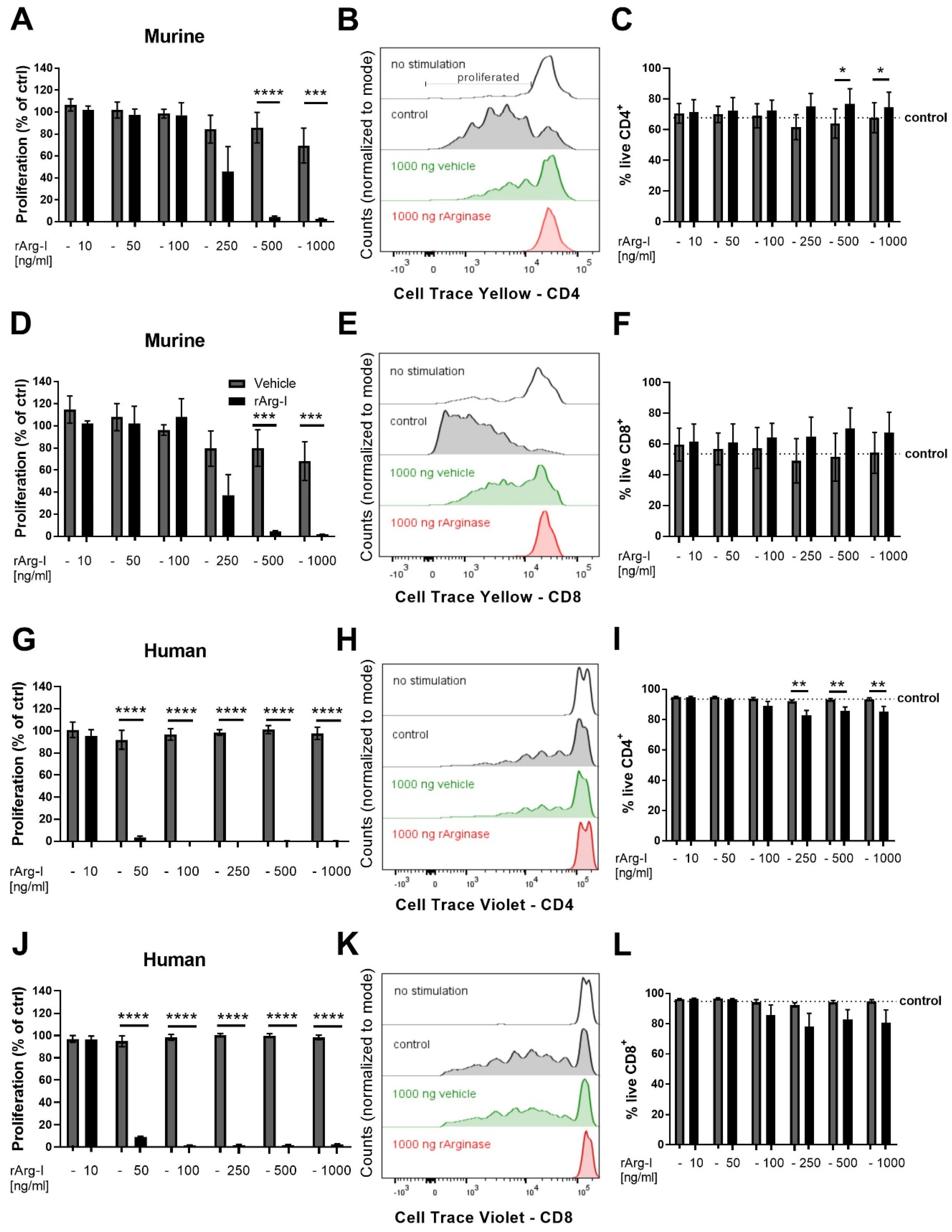
3.3. L-Arginine Is Essential for T-Cell Activation
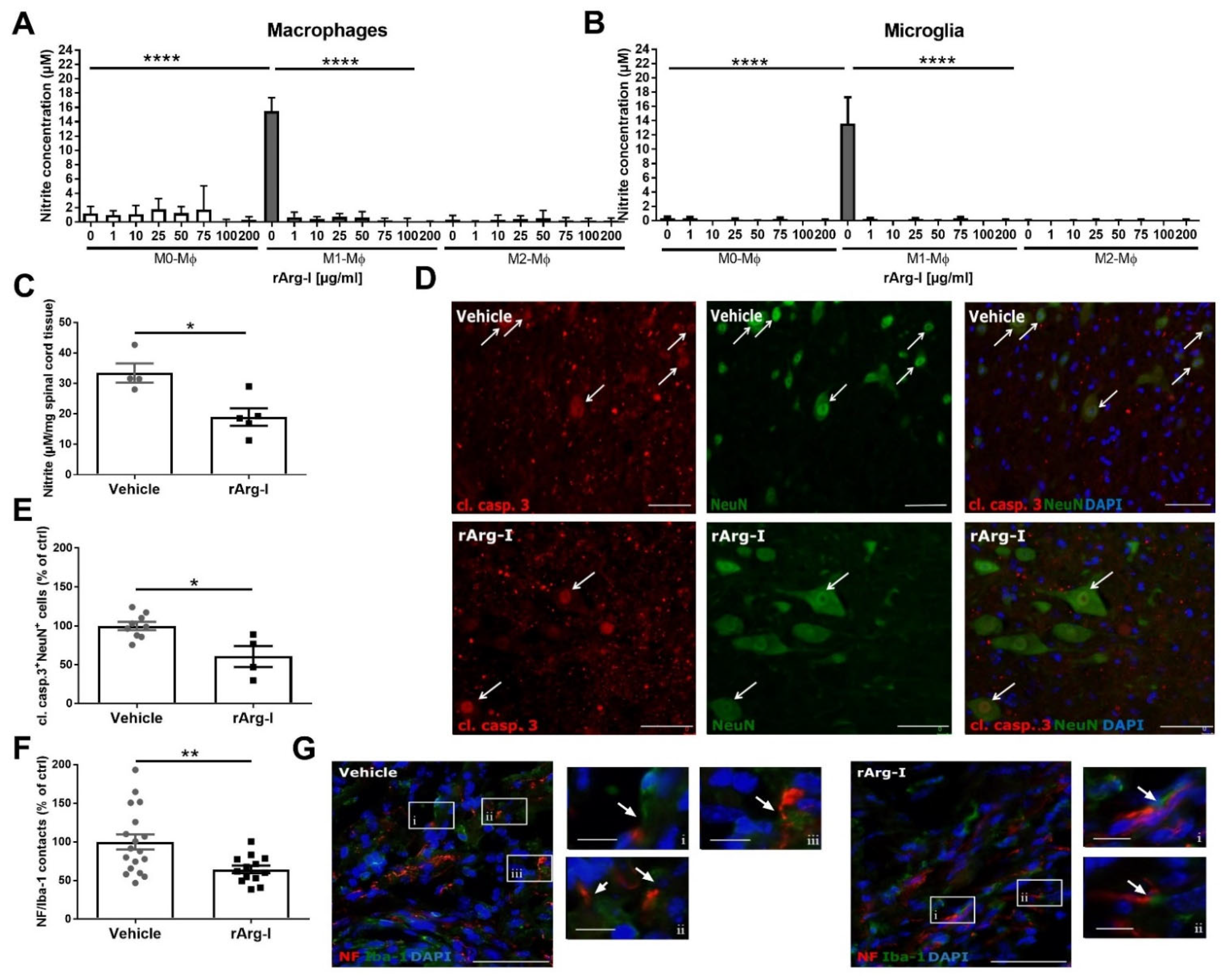
3.4. L-Arginine Depletion Reduces NO Production, Neurotoxicity, and the Number of Phagocyte/Axon Contacts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouanet, C.; Reges, D.; Rocha, E.; Gagliardi, V.; Silva, G.S. Traumatic spinal cord injury: Current concepts and treatment update. Arq. Neuropsiquiatr. 2017, 75, 387–393. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133 (Pt. 2), 433–447. [Google Scholar] [CrossRef]
- Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006, 494, 578–594. [Google Scholar] [CrossRef]
- Popovich, P.G.; Wei, P.; Stokes, B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997, 377, 443–464. [Google Scholar] [CrossRef]
- Sroga, J.M.; Jones, T.B.; Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J. Comp. Neurol. 2003, 462, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.L.; Parrish, M.E.; Springer, J.E.; Doty, K.; Dossett, L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998, 151, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Lymphocytes and autoimmunity after spinal cord injury. Exp. Neurol. 2014, 258, 78–90. [Google Scholar] [CrossRef]
- Hendrix, S.; Nitsch, R. The role of T helper cells in neuroprotection and regeneration. J. Neuroimmunol. 2007, 184, 100–112. [Google Scholar] [CrossRef]
- Hauben, E.; Nevo, U.; Yoles, E.; Moalem-Taylor, G.; Agranov, E.; Mor, F.; Akselrod, S.; Neeman, M.; Cohen, I.; Schwartz, M. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet 2000, 355, 286–287. [Google Scholar] [CrossRef]
- Schwartz, M.; Hauben, E. Chapter 29 T cell-based therapeutic vaccination for spinal cord injury. Prog. Brain Res. 2002, 137, 401–406. [Google Scholar] [CrossRef]
- Hu, J.-G.; Shi, L.-L.; Chen, Y.-J.; Xie, X.-M.; Zhang, N.; Zhu, A.-Y.; Jiang, Z.-S.; Feng, Y.-F.; Zhang, C.; Xi, J.; et al. Differential effects of myelin basic protein-activated Th1 and Th2 cells on the local immune microenvironment of injured spinal cord. Exp. Neurol. 2016, 277, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Ankeny, D.P.; Guan, Z.; McGaughy, V.; Fisher, L.C.; Basso, D.M.; Popovich, P.G. Passive or Active Immunization with Myelin Basic Protein Impairs Neurological Function and Exacerbates Neuropathology after Spinal Cord Injury in Rats. J. Neurosci. 2004, 24, 3752–3761. [Google Scholar] [CrossRef]
- Ishii, H.; Jin, X.; Ueno, M.; Tanabe, S.; Kubo, T.; Serada, S.; Naka, T.; Yamashita, T. Adoptive transfer of Th1-conditioned lymphocytes promotes axonal remodeling and functional recovery after spinal cord injury. Cell Death Dis. 2012, 3, e363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, J.; Wang, D.; Niu, X.; Hu, J.; Zhou, Y.; Li, Z. MicroRNA-155 Deficiency Suppresses Th17 Cell Differentiation and Improves Locomotor Recovery after Spinal Cord Injury. Scand. J. Immunol. 2015, 81, 284–290. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, R.; Glaser, J.; Liu, M.T.; Lane, T.E.; Keirstead, H.S. Reducing inflammation decreases secondary degeneration and func-tional deficit after spinal cord injury. Exp. Neurol. 2003, 184, 456–463. [Google Scholar] [CrossRef]
- Potas, J.R.; Zheng, Y.; Moussa, C.; Venn, M.; Gorrie, C.A.; Deng, C.; Waite, P.M.E. Augmented locomotor recovery after spinal cord injury in the athymic nude rat. J. Neurotrauma 2006, 23, 660–673. [Google Scholar] [CrossRef]
- Wu, B.; Matic, D.; Djogo, N.; Szpotowicz, E.; Schachner, M.; Jakovcevski, I. Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp. Neurol. 2012, 237, 274–285. [Google Scholar] [CrossRef]
- Luchetti, S.; Beck, K.D.; Galvan, M.D.; Silva, R.; Cummings, B.J.; Anderson, A.J. Comparison of Immunopathology and Locomotor Recovery in C57BL/6, BUB/BnJ, and NOD-SCID Mice after Contusion Spinal Cord Injury. J. Neurotrauma 2010, 27, 411–421. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Imagama, T.; Ogino, K.; Takemoto, K.; Kato, Y.; Kataoka, H.; Suzuki, H.; Ran, Z.; Setiawan, H.; Fujikura, Y.; Taguchi, T. Regulation of nitric oxide generation by up-regulated arginase I in rat spinal cord injury. J. Clin. Biochem. Nutr. 2012, 51, 68–75. [Google Scholar] [CrossRef]
- Nemoto, T.; Sekikawa, T.; Suzuki, T.; Moriya, H.; Nakaya, H. Inhibition of nitric oxide synthesis accelerates the recovery of polysynaptic reflex potentials after transient spinal cord ischemia in cats. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 355, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Chatzipanteli, K.; Marcillo, A.E.; Bunge, M.B.; Dietrich, W.D. Comparison of iNOS inhibition by antisense and phar-macological inhibitors after spinal cord injury. J. Neuropathol. Exp. Neurol. 2003, 62, 1096–1107. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Serafini, P.; Mazzoni, A.; Segal, D.M.; Zanovello, P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003, 24, 301–305. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef]
- Sahin, E.; Haubenwallner, S.; Kuttke, M.; Kollmann, I.; Halfmann, A.; Dohnal, A.M.; Chen, L.; Cheng, P.; Hoesel, B.; Einwallner, E.; et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J. Immunol. 2014, 193, 1717–1727. [Google Scholar] [CrossRef]
- Farooque, M.; Hillered, L.; Holtz, A.; Olsson, Y. Changes of extracellular levels of amino acids after graded compression trau-ma to the spinal cord: An experimental study in the rat using microdialysis. J. Neurotrauma 1996, 13, 537–548. [Google Scholar] [CrossRef]
- Liu, D.; McAdoo, D.J. Methylprednisolone reduces excitatory amino acid release following experimental spinal cord injury. Brain Res. 1993, 609, 293–297. [Google Scholar] [CrossRef]
- Esquivel-Aguilar, A.; Castañeda-Hernández, G.; Martínez-Cruz, A.; Franco-Bourland, R.E.; Madrazo, I.; Guizar-Sahagun, G. Early Administration of l-Arginine in Experimental Acute Spinal Cord Injury Impairs Long-Term Motor Function Recovery. J. Trauma Acute Care Surg. 2011, 70, 1198–1202. [Google Scholar] [CrossRef]
- Tuncer, M.C.; Hatipoglu, E.S.; Ozturk, H.; Kervancioglu, P.; Buyukbayram, H. The Effects of L-Arginine on Neurological Function, Histopathology, and Expression of Hypoxia-Inducible Factor-1 Alpha following Spinal Cord Ischemia in Rats. Eur. Surg. Res. 2005, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Savas, C.; Altuntas, I.; Adiloglu, A. The correlation between nitric oxide and vascular endothelial growth factor in spinal cord injury. Spinal Cord. 2008, 46, 113–117. [Google Scholar] [CrossRef]
- Yüceer, N.; Attar, A.; Sargon, M.F.; Egemen, N.; Türker, R.K.; Demirel-Yilmaz, E. The early protective effects of L-arginine and Ng-nitro-L-arginine methyl ester after experimental acute spinal cord injury. A light and electron microscopic study. J. Clin. Neurosci. 2000, 7, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-M.; Lam, T.-L.; Lam, W.-M.; Tsui, S.-M.; Cheng, A.W.-M.; Lo, W.-H.; Leung, Y.-C. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007, 67, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sommer, D.; Corstjens, I.; Sanchez, S.; Dooley, D.; Lemmens, S.; Van Broeckhoven, J.; Bogie, J.; Vanmierlo, T.; Vidal, P.M.; Rose-John, S.; et al. ADAM17-deficiency on microglia but not on macrophages promotes phagocytosis and functional recovery after spinal cord injury. Brain Behav. Immun. 2019, 80, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Vangansewinkel, T.; Geurts, N.; Quanten, K.; Nelissen, S.; Lemmens, S.; Geboes, L.; Dooley, D.; Vidal, P.M.; Pejler, G.; Hendrix, S. Mast cells promote scar remodeling and functional recovery after spinal cord injury via mouse mast cell protease. FASEB J. 2016, 30, 2040–2057. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Man Leung, Y.; Chung, L.O.; Lo, W. Bio-Cancer Treatment International Limited [CN]/[CN], assignee. Pharmaceutical preparation and method of treatment of human malignancies with arginine deprivation. U.S. Patent 20050244398A1, 20 June 2002. [Google Scholar]
- Ikemoto, M.; Tabata, M.; Murachi, T.; Totani, M. Purification and Properties of Human Erythrocyte Arginase. Ann. Clin. Biochem. Int. J. Lab. Med. 1989, 26, 547–553. [Google Scholar] [CrossRef]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; A Clark, M. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar] [PubMed]
- Morris, S.M., Jr. Arginine Metabolism Revisited. J. Nutr. 2016, 146, 2579s–2586s. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects dif-ferences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]
- Tamashiro, T.T.; Dalgard, C.L.; Byrnes, K.R. Primary Microglia Isolation from Mixed Glial Cell Cultures of Neonatal Rat Brain Tissue. J. Vis. Exp. 2012, 66. [Google Scholar] [CrossRef]
- Sanchez, S.; Lemmens, S.; Baeten, P.; Sommer, D.; Dooley, D.; Hendrix, S.; Gou-Fabregas, M. HDAC3 Inhibition Promotes Alternative Activation of Macrophages but Does Not Affect Functional Recovery after Spinal Cord Injury. Exp. Neurobiol. 2018, 27, 437–452. [Google Scholar] [CrossRef]
- Badurdeen, S.; Mulongo, M.; Berkley, J.A. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr. Res. 2014, 77, 290–297. [Google Scholar] [CrossRef]
- Xu, J.; Kim, G.-M.; Chen, S.; Yan, P.; Ahmed, S.H.; Ku, G.; Beckman, J.S.; Xu, X.M.; Hsu, C.Y. iNOS and Nitrotyrosine Expression After Spinal Cord Injury. J. Neurotrauma 2001, 18, 523–532. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expres-sion by L-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef]
- Zea, A.H.; Rodriguez, P.C.; Culotta, K.S.; Hernandez, C.P.; DeSalvo, J.; Ochoa, J.B.; Park, H.; Zabaleta, J.; Ochoa, A.C. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004, 232, 21–31. [Google Scholar] [CrossRef]
- Liu, D.; Ling, X.; Wen, J.; Liu, J. The role of reactive nitrogen species in secondary spinal cord injury: Formation of nitric oxide, peroxynitrite, and nitrated protein. J. Neurochem. 2000, 75, 2144–2154. [Google Scholar] [CrossRef]
- Ishkitiev, N.; Yaegaki, K.; Imai, T.; Tanaka, T.; Nakahara, T.; Ishikawa, H.; Mitev, V.; Haapasalo, M. High-purity Hepatic Lineage Differentiated from Dental Pulp Stem Cells in Serum-free Medium. J. Endod. 2012, 38, 475–480. [Google Scholar] [CrossRef]
- Vincent, V.A.M.; Tilders, F.J.H.; Van Dam, A.-M. Production, regulation and role of nitric oxide in glial cells. Mediat. Inflamm. 1998, 7, 239–255. [Google Scholar] [CrossRef]
- Khandoker, A.R.; Mahbub, H.; Ji-Eun, S. Severity of the autoimmune encephalomyelitis symptoms in mouse model by inhi-bition of LAT-1 transporters. J. Pharm. Investig. 2020, 50, 481–491. [Google Scholar]
- Boato, F.; Hendrix, S.; Huelsenbeck, S.C.; Hofmann, F.; Große, G.; Djalali, S.; Klimaschewski, L.; Auer, M.; Just, I.; Ahnert-Hilger, G.; et al. C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J. Cell Sci. 2010, 123, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Steward, O. Concepts and Methods for the Study of Axonal Regeneration in the CNS. Neuron 2012, 74, 777–791. [Google Scholar] [CrossRef]
- Seifter, E.; Rettura, G.; Barbul, A.; Levenson, S.M. Arginine: An essential amino acid for injured rats. Surgery 1978, 84, 224–230. [Google Scholar] [PubMed]
- Ahmadi, S.A.; Jafari, M.; Darabi, M.R.; Chehrei, A.; Rezaei, M.; Mirsalehi, M. The Effect of l-Arginine on Dural Healing After Experimentally Induced Dural Defect in a Rat Model. World Neurosurg. 2017, 97, 98–103. [Google Scholar] [CrossRef]
- Dusart, I.; Schwab, M.E. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur. J. Neurosci. 1994, 6, 712–724. [Google Scholar] [CrossRef]
- Ghirnikar, R.S.; Lee, Y.L.; Eng, L.F. Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat. J. Neurosci. Res. 2001, 64, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Hart, R.P.; Popovich, P.G. Molecular Control of Physiological and Pathological T-Cell Recruitment after Mouse Spinal Cord Injury. J. Neurosci. 2005, 25, 6576–6583. [Google Scholar] [CrossRef]
- Wang, L.; Yu, W.-B.; Tao, L.-Y.; Xu, Q. Myeloid-derived suppressor cells mediate immune suppression in spinal cord injury. J. Neuroimmunol. 2016, 290, 96–102. [Google Scholar] [CrossRef]
- Werner, A.; Amann, E.; Schnitzius, V.; Habermeier, A.; Luckner-Minden, C.; Leuchtner, N.; Rupp, J.; Closs, E.I.; Munder, M. Induced arginine transport via cationic amino acid transporter-1 is necessary for human T-cell proliferation. Eur. J. Immunol. 2015, 46, 92–103. [Google Scholar] [CrossRef]
- Hoeks, C.; Vanheusden, M.; Peeters, L.M.; Stinissen, P.; Broux, B.; Hellings, N. Treg-Resistant Cytotoxic CD4+ T Cells Dictate T Helper Cells in Their Vicinity: TH17 Skewing and Modulation of Proliferation. Int. J. Mol. Sci. 2021, 22, 5660. [Google Scholar] [CrossRef]
- Werner, A.; Koschke, M.; Leuchtner, N.; Luckner-Minden, C.; Habermeier, A.; Rupp, J.; Heinrich, C.; Conradi, R.; Closs, E.I.; Munder, M. Reconstitution of T Cell Prolifera-tion under Arginine Limitation: Activated Human T Cells Take Up Citrulline via L-Type Amino Acid Transporter 1 and Use It to Regenerate Arginine after Induction of Argininosuccinate Synthase Expression. Front Immunol. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Lanzavecchia, A.; Sallusto, F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 2003, 101, 4260–4266. [Google Scholar] [CrossRef]
- Munder, M.; Choi, B.-S.; Rogers, M.; Kropf, P. L -Arginine deprivation impairs Leishmania major -specific T-cell responses. Eur. J. Immunol. 2009, 39, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Blomster, L.V.; Brennan, F.H.; Lao, H.W.; Harle, D.W.; Harvey, A.R.; Ruitenberg, M.J. Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp. Neurol. 2013, 247, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Thawer, S.G.; Mawhinney, L.; Chadwick, K.; de Chickera, S.N.; Weaver, L.C.; Brown, A.; Dekaban, G.A. Temporal changes in monocyte and macrophage subsets and microglial macrophages following spinal cord injury in the lys-egfp-ki mouse model. J. Neuroimmunol. 2013, 261, 7–20. [Google Scholar] [CrossRef]
- Estévez, A.G.; Sahawneh, M.A.; Lange, P.S.; Bae, N.; Egea, M.; Ratan, R.R. Arginase 1 Regulation of Nitric Oxide Production Is Key to Survival of Trophic Factor-Deprived Motor Neurons. J. Neurosci. 2006, 26, 8512–8516. [Google Scholar] [CrossRef]
- Horn, K.P.; Busch, S.A.; Hawthorne, A.L.; van Rooijen, N.; Silver, J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 2008, 28, 9330–9341. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Lorenz, U. Engulfment of apoptotic cells: Signals for a good meal. Nat. Rev. Immunol. 2007, 7, 964–974. [Google Scholar] [CrossRef]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erens, C.; Van Broeckhoven, J.; Hoeks, C.; Schabbauer, G.; Cheng, P.N.; Chen, L.; Hellings, N.; Broux, B.; Lemmens, S.; Hendrix, S. L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction. Biomedicines 2022, 10, 205. https://doi.org/10.3390/biomedicines10020205
Erens C, Van Broeckhoven J, Hoeks C, Schabbauer G, Cheng PN, Chen L, Hellings N, Broux B, Lemmens S, Hendrix S. L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction. Biomedicines. 2022; 10(2):205. https://doi.org/10.3390/biomedicines10020205
Chicago/Turabian StyleErens, Céline, Jana Van Broeckhoven, Cindy Hoeks, Gernot Schabbauer, Paul N. Cheng, Li Chen, Niels Hellings, Bieke Broux, Stefanie Lemmens, and Sven Hendrix. 2022. "L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction" Biomedicines 10, no. 2: 205. https://doi.org/10.3390/biomedicines10020205
APA StyleErens, C., Van Broeckhoven, J., Hoeks, C., Schabbauer, G., Cheng, P. N., Chen, L., Hellings, N., Broux, B., Lemmens, S., & Hendrix, S. (2022). L-Arginine Depletion Improves Spinal Cord Injury via Immunomodulation and Nitric Oxide Reduction. Biomedicines, 10(2), 205. https://doi.org/10.3390/biomedicines10020205





