High Risk, High Dose?—Pharmacotherapeutic Prescription Patterns of Offender and Non-Offender Patients with Schizophrenia Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Null Hypothesis Significance Testing (NHST)
3.1.1. Hypothesis I: Offender Patients Receive Higher Doses of Antipsychotic Drugs
3.1.2. Hypothesis II: Offender Patients Are More Often Subjected to Antipsychotic Polypharmacy
3.1.3. Hypothesis III: Offender Patients Receive Benzodiazepines for Sedation More Frequently
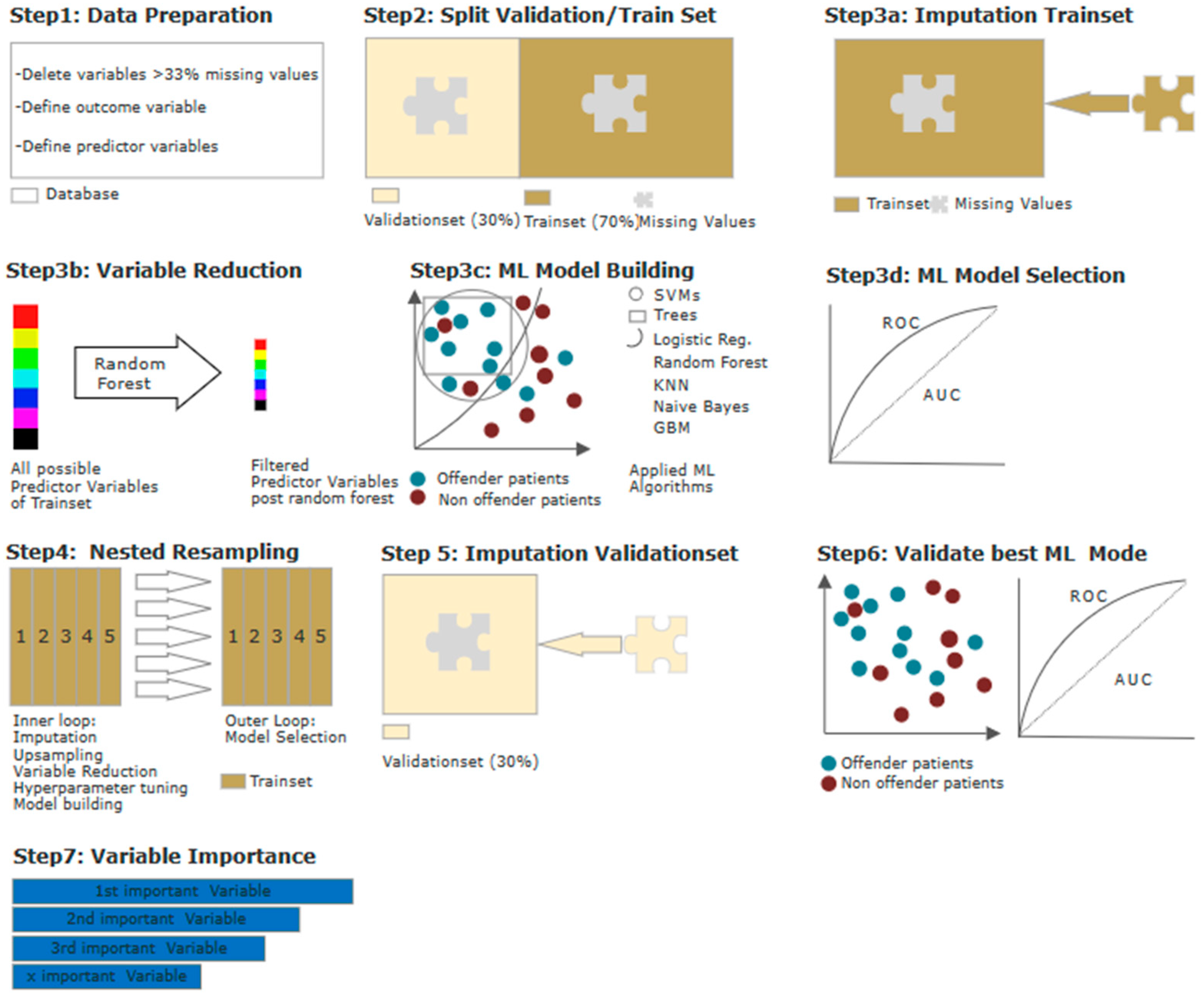
3.2. Model Calculation Using Machine Learning (ML)
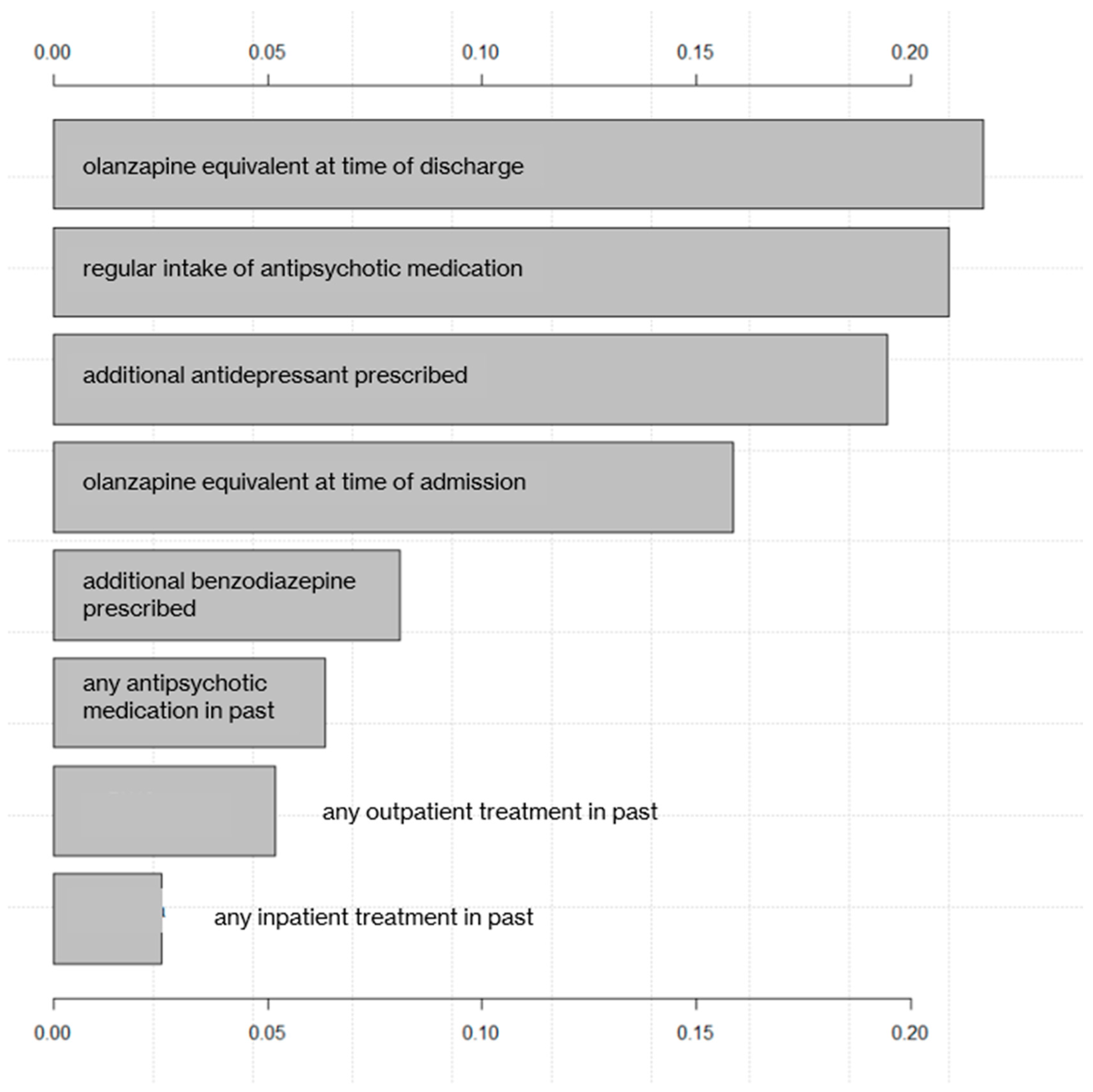
3.3. Ranking of Predictor Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edworthy, R.; Sampson, S.; Völlm, B. Inpatient forensic-psychiatric care: Legal frameworks and service provision in three Euro-pean countries. Int. J. Law Psychiatry 2016, 47, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, A.; Grounds, A. Forensic psychiatry and public protection. Br. J. Psychiatry 2011, 198, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Habermeyer, E.; Mokros, A.; Briken, P. "Die Relevanz eines kohärenten forensischen Beurteilungs- und Behandlungsprozesses": Großer Wurf oder alter Wein in undichtem Schlauch? Forens. Psychiatr. Psychol. Kriminol. 2020, 14, 212–219. [Google Scholar] [CrossRef]
- Howner, K.; Andiné, P.; Engberg, G.; Ekström, E.H.; Lindström, E.; Nilsson, M.; Radovic, S.; Hultcrantz, M. Pharmacological Treatment in Forensic Psychiatry—A Systematic Review. Front. Psychiatry 2019, 10, 963. [Google Scholar] [CrossRef]
- Hodgins, S. The major mental disorders and crime: Stop debating and start treating and preventing. Int. J. Law Psychiatry 2001, 24, 427–446. [Google Scholar] [CrossRef]
- Gunn, J.; Taylor, P.; Hutcheon, I.D. Forensic Psychiatry: Clinical, Legal and Ethical Issues; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Palijan, T.Ž.; Mužinić, L.; Radeljak, S. Psychiatric comorbidity in forensic psychiatry. Psychiatr. Danub. 2009, 21, 429–436. [Google Scholar]
- Goethals, K.R.; Vorstenbosch, E.C.; Van Marle, H.J. Diagnostic Comorbidity in Psychotic Offenders and Their Criminal History: A Review of the Literature. Int. J. Forensic Ment. Health 2008, 7, 147–156. [Google Scholar] [CrossRef]
- Fazel, S.; Långström, N.; Hjern, A.; Grann, M.; Lichtenstein, P. Schizophrenia, Substance Abuse, and Violent Crime. JAMA 2009, 301, 2016–2023. [Google Scholar] [CrossRef]
- Lelliott, P.; Paton, C.; Harrington, M.; Konsolaki, M.; Sensky, T.; Okocha, C. The influence of patient variables on polypharmacy and combined high dose of antipsychotic drugs prescribed for in-patients. Psychiatr. Bull. 2002, 26, 411–414. [Google Scholar] [CrossRef]
- Barnes, T.R.; Drake, R.; Paton, C.; Cooper, S.J.; Deakin, B.; Ferrier, I.N.; Gregory, C.J.; Haddad, P.M.; Howes, O.D.; Jones, I. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2020, 34, 3–78. [Google Scholar] [CrossRef]
- Connolly, A.; Taylor, D. Factors associated with non evidence-based prescribing of antipsychotics. Ther. Adv. Psychopharmacol. 2014, 4, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Völlm, B.A.; Chadwick, K.; Abdelrazek, T.; Smith, J. Prescribing of psychotropic medication for personality disordered patients in secure forensic settings. J. Forensic Psychiatry Psychol. 2012, 23, 200–216. [Google Scholar] [CrossRef]
- Acosta-Armas, A.; Cooper, M.; Jacob, C.; Churchward, S. High-dose antipsychotic prescriptions at a forensic psychiatric hospital: Is there a need for implementation of a monitoring form? Br. J. Forensic Pract. 2004, 6, 18–24. [Google Scholar] [CrossRef]
- Parker, J.; Villiers, J.D.; Churchward, S. High-dose antipsychotic drug use in a forensic setting. J. Forensic Psychiatry 2002, 13, 407–415. [Google Scholar] [CrossRef]
- Farrell, C.; Brink, J. The Prevalence and Factors Associated With Antipsychotic Polypharmacy in a Forensic Psychiatric Sample. Front. Psychiatry 2020, 11, 263. [Google Scholar] [CrossRef]
- Di Giacomo, E.; Stefana, A.; Candini, V.; Bianconi, G.; Canal, L.; Clerici, M.; Conte, G.; Ferla, M.T.; Iozzino, L.; Sbravati, G.; et al. Prescribing Patterns of Psychotropic Drugs and Risk of Violent Behavior: A Prospective, Multicenter Study in Italy. Int. J. Neuropsychopharmacol. 2020, 23, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Mandarelli, G.; Carabellese, F.; di Sciascio, G.; Catanesi, R. Antipsychotic Polypharmacy and High-Dose Antipsychotic Regimens in the Residential Italian Forensic Psychiatric Population (REMS). Front. Psychol. 2022, 13, 722985. [Google Scholar] [CrossRef]
- Hassan, L.; Senior, J.; Webb, R.T.; Frisher, M.; Tully, M.P.; While, D.; Shaw, J.J. Prevalence and appropriateness of psychotropic medication prescribing in a nationally representative cross-sectional survey of male and female prisoners in England. BMC Psychiatry 2016, 16, 346. [Google Scholar] [CrossRef]
- Pelizza, L.; Maestri, D.; Paulillo, G.; Pellegrini, P. Prevalence and Appropriateness of Antipsychotic Prescribing in an Italian Prison: Is Everything Always Really Overprescribed? J. Clin. Psychopharmacol. 2022, 42, 31–36. [Google Scholar] [CrossRef]
- Günther, M.P.; Kirchebner, J.; Kling, S.; Lau, S. Antipsychotic Overdosing and Polypharmacy in Schizophrenic Delinquents Explored. Int. J. Offender Ther. Comp. Criminol. 2020, 64, 938–952. [Google Scholar] [CrossRef]
- World Health Organization ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision 1992; World Health Organization: Geneva, Switzerland, 2004; Volume 1.
- Lau, S.; Günther, M.P.; Kling, S.; Kirchebner, J. Latent class analysis identified phenotypes in individuals with schizophrenia spectrum disorder who engage in aggressive behaviour towards others. Eur. Psychiatry 2019, 60, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-F.; Shannon, S.E. Three Approaches to Qualitative Content Analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Seifert, D. Die entwicklung des psychiatrischen massregelvollzzugs (§ 63StGB) in Nordrhein-Wesfalen. Psychiat. Prax. 1997, 24, 237–244. [Google Scholar]
- Leucht, S.; Samara, M.; Heres, S.; Patel, M.X.; Furukawa, T.; Cipriani, A.; Geddes, J.; Davis, J.M. Dose equivalents for sec-ond-generation antipsychotic drugs: The classical mean dose method. Schizophr. Bull. 2015, 41, 1397–1402. [Google Scholar] [CrossRef]
- Leucht, S.; Samara, M.; Heres, S.; Patel, M.X.; Woods, S.W.; Davis, J.M. Dose Equivalents for Second-Generation Antipsychotics: The Minimum Effective Dose Method. Schizophr. Bull. 2014, 40, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.M.; Murphy, A.L.; O’Donnell, H.; Centorrino, F.; Baldessarini, R.J. International Consensus Study of Antipsychotic Dosing. Am. J. Psychiatry 2010, 167, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Bower, K.M. When to Use Fisher’s Exact Test. In Six Sigma Forum Magazine; American Society for Quality: Milwaukee, MI, USA, 2003; Volume 2, pp. 35–37. [Google Scholar]
- McKnight, P.E.; Najab, J. Mann-Whitney U Test. In The Corsini Encyclopedia of Psychology; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Zwinderman, A.H. On the Benjamini–Hochberg method. Ann. Stat. 2006, 34, 1827–1849. [Google Scholar] [CrossRef]
- Kirchebner, J.; Lau, S.; Kling, S.; Sonnweber, M.; Günther, M.P. Individuals with schizophrenia who act violently towards others profit unequally from inpatient treatment—Identifying subgroups by latent class analysis. Int. J. Methods Psychiatr. Res. 2021, 30, e1856. [Google Scholar] [CrossRef]
- Yarkoni, T.; Westfall, J. Choosing Prediction Over Explanation in Psychology: Lessons from Machine Learning. Perspect. Psychol. Sci. 2017, 12, 1100–1122. [Google Scholar] [CrossRef]
- Browne, M.W. Cross-Validation Methods. J. Math. Psychol. 2000, 44, 108–132. [Google Scholar] [CrossRef] [PubMed]
- van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef]
- Hofmann, L.A.; Lau, S.; Kirchebner, J. Maintaining social capital in offenders with schizophrenia spectrum disorder—An explorative analysis of influential factors. Front. Psychiatry 2022, 13, 945732. [Google Scholar] [CrossRef]
- Świtaj, P.; Anczewska, M.; Chrostek, A.; Sabariego, C.; Cieza, A.; Bickenbach, J.; Chatterji, S. Disability and schizophrenia: A systematic review of experienced psychosocial difficulties. BMC Psychiatry 2012, 12, 193. [Google Scholar] [CrossRef]
- Fazel, S.; Yu, R. Psychotic Disorders and Repeat Offending: Systematic Review and Meta-analysis. Schizophr. Bull. 2011, 37, 800–810. [Google Scholar] [CrossRef]
- Witt, K.; van Dorn, R.; Fazel, S. Risk factors for violence in psychosis: Systematic review and meta-regression analysis of 110 studies. PLoS ONE 2013, 8, e55942. [Google Scholar] [CrossRef]
- Stompe, T.; Schanda, H. Psychopharmocotherapy of schizophrenia in forensic and general psychiatry. Neuropsychiatr. Klin. Diagn. Ther. Rehabilit. Org. Ges. Oster. Nerven. Psychiater 2011, 25, 75–84. [Google Scholar]
- Stone-Brown, K.; Naji, M.; Francioni, A.; Myers, K.; Samarendra, H.; Mushtaq-Chaudhry, H.; Heslop, S.; Sengupta, S.; Ross, C.C.; Larkin, F.; et al. Psychotropic prescribing in seriously violent men with schizophrenia or personality disorder in a UK high security hospital. CNS Spectrums 2016, 21, 60–69. [Google Scholar] [CrossRef]
- Mancuso, C.E.; Tanzi, M.G.; Gabay, M. Paradoxical Reactions to Benzodiazepines: Literature Review and Treatment Options. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 1177–1185. [Google Scholar] [CrossRef]
- Kramer, M.S.; Vogel, W.H.; DiJohnson, C.; Dewey, D.A.; Sheves, P.; Cavicchia, S.; Little, P.; Schmidt, R.; Kimes, I. Antide-pressants in ‘depressed’ schizophrenic inpatients: A controlled trial. Arch. Gen. Psychiatry 1989, 46, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-D.; Mao, Y.-M. Augmentation with antidepressants in schizophrenia treatment: Benefit or risk. Neuropsychiatr. Dis. Treat. 2015, 11, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Helfer, B.; Samara, M.T.; Huhn, M.; Klupp, E.; Leucht, C.; Zhu, Y.; Engel, R.R.; Leucht, S. Efficacy and Safety of Antide-pressants Added to Antipsychotics for Schizophrenia: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2016, 173, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Veerman, S.R.T.; Schulte, P.F.J.; De Haan, L. Treatment for Negative Symptoms in Schizophrenia: A Comprehensive Review. Drugs 2017, 77, 1423–1459. [Google Scholar] [CrossRef]
- Karabekiroğlu, A.; Pazvantoğlu, O.; Karabekiroğlu, K.; Böke, Ö.; Korkmaz, I.Z. Associations with violent and homicidal be-haviour among men with schizophrenia. Nord. J. Psychiatry 2016, 70, 303–308. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Zhu, X.-M.; Zhang, S.-M.; Zhou, J.-S.; Li, Q.-G.; Wang, Q.; Zhong, S.-L.; Ng, C.H.; Ungvari, G.S.; et al. Association between schizophrenia and violence among Chinese female offenders. Sci. Rep. 2017, 7, 818. [Google Scholar] [CrossRef]
- Saavedra, J.; López, M.; Trigo, M.E. Association between Violent Crime and Psychosis in Men Serving Prison Terms. Span. J. Psychol. 2017, 20, E30. [Google Scholar] [CrossRef]
- Gabrielsen, G.; Kramp, P. Forensic psychiatric patients among immigrants in Denmark–Diagnoses and criminality. Nord. J. Psychiatry 2009, 63, 140–147. [Google Scholar] [CrossRef]
- Huber, D.A.; Lau, S.; Sonnweber, M.; Günther, M.P.; Kirchebner, J. Exploring similarities and differences of non-European mi-grants among forensic patients with schizophrenia. Int. J. Environ. Res. Public Health 2020, 17, 7922. [Google Scholar] [CrossRef]
- Rezansoff, S.N.; Moniruzzaman, A.; Fazel, S.; McCandless, L.; Somers, J.M. Adherence to Antipsychotic Medication and Criminal Recidivism in a Canadian Provincial Offender Population. Schizophr. Bull. 2017, 43, 1002–1010. [Google Scholar] [CrossRef]
- Fazel, S.; Zetterqvist, J.; Larsson, H.; Långström, N.; Lichtenstein, P. Antipsychotics, mood stabilisers, and risk of violent crime. Lancet 2014, 384, 1206–1214. [Google Scholar] [CrossRef]
- Walsh, E.; Gilvarry, C.; Samele, C.; Harvey, K.; Manley, C.; Tattan, T.; Tyrer, P.; Creed, F.; Murray, R.; Fahy, T. Predicting violence in schizophrenia: A prospective study. Schizophr. Res. 2004, 67, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.A.; Lau, S.; Kirchebner, J. Advantages of Machine Learning in Forensic Psychiatric Research—Uncovering the Complexities of Aggressive Behavior in Schizophrenia. Appl. Sci. 2022, 12, 819. [Google Scholar] [CrossRef]
- Zhou, J.S.; Zhong, B.L.; Xiang, Y.T.; Chen, Q.; Cao, X.L.; Correll, C.U.; Ungvari, G.S.; Chiu, H.F.; Lai, K.Y.; Wang, X.P. Prevalence of aggression in hospitalized patients with schizophrenia in China: A meta-analysis. Asia. Pac. Psychiatry 2016, 8, 60–69. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Stewart, D.; Bowers, L. Self-harm and attempted suicide within inpatient psychiatric services: A review of the Literature. Int. J. Ment. Health Nurs. 2012, 21, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Eidgenossenschaft, D.B.S. Schweizerisches Strafgesetzbuch; Bundesamt f. Bauten + Logistik: Bern, Switzerland, 2022. [Google Scholar]
- Schultze-Lutter, F.; Meisenzahl, E.; Michel, C. Psychotische Störungen in der ICD-11: Die Revisionen. Z. Kinder. Jugendpsychiatr. Psychother. 2021, 49, 453–462. [Google Scholar] [CrossRef] [PubMed]
| Variable Description | Offender Patients n/N (%) | Non-Offender Patients n/N (%) | p-Value * |
|---|---|---|---|
| Sociodemographic Data | |||
| Age at admission (mean) | 34.8 (10.5) | 35.4 (11.2) | 0.702 |
| Gender: male | 161/176 (91.5) | 187/206 (90.8) | 0.703 |
| Country of birth, Switzerland | 98/206 (47.6) | 108/177 (61) | 0.017 * |
| No school graduation (at admission) | 128/189 (67.7) | 75/161 (46.6) | 0.000 * |
| Psychiatric Data | |||
| Any outpatient psychiatric treatment in the past | 115/196 (58.7) | 141/159 (88.7) | 0.000 * |
| Any inpatient psychiatric treatment in the past | 152/200 (76) | 174/177 (98.3) | 0.000 * |
| Any antipsychotic medication in the past | 134/206 (65) | 165/173 (95.4) | 0.000 * |
| Regular intake of antipsychotic medication | 14/122 (11.5) | 79/148 (54.4) | 0.000 * |
| Comorbid alcohol use disorder | 125/192 (65.1) | 76/168 (45.2) | 0.000 * |
| Comorbid substance use disorder | 156/206 (75.7) | 107/172 (62.2) | 0.009 * |
| Comorbid personality disorder | 31/206 (15) | 14/150 (9.3) | 0.183 |
| Any compulsory measure in the past | 100/184 (54.3) | 62/142 (43.7) | 0.089 |
| Any compulsory measure currently | 81/204 (39.7) | 26/176 (14.8) | 0.000 * |
| Length of stay (in weeks) | 134.4 (124.7) | 8.8 (7) | 0.000 * |
| PANSS at admission | 24.7 (12.8) | 22.1 (10) | 0.114 |
| PANSS at discharge | 12.2 (9.9) | 12.9 (10.7) | 0.834 |
| Data on current medication | |||
| Olanzapine equivalent at admission (mg) | 21.4 (14.3) | 14.6 (12.1) | 0.000 * |
| Olanzapine equivalent at discharge (mg) | 22.1 (12.3) | 19.3 (14.2) | 0.008 * |
| Polypharmacy 1 at admission | 34/145 (23.4) | 54/146 (37) | 0.024 * |
| Polypharmacy 1 at discharge | 72/206 (35) | 72/178 (40.4) | 0.333 |
| Typical antipsychotic prescribed | 37/204 (18.1) | 24/177 (13.6) | 0.188 |
| Clozapine prescribed | 77/204 (37.7) | 54/177 (30.5) | 0.192 |
| Additional benzodiazepine prescribed | 37/204 (18.1) | 68/178 (38.2) | 0.000 * |
| Additional antidepressant prescribed | 18/204 (8.8) | 63/178 (35.4) | 0.000 * |
| Scheme | Balanced Accuracy (%) | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Logistic Regression | 74.9 | 0.84 | 68.90 | 81.10 | 76.30 | 75 |
| Tree | 73.9 | 0.79 | 70.90 | 77.10 | 72.60 | 75.5 |
| Random Forest | 75.3 | 0.84 | 70.7 | 79.9 | 75.3 | 76.5 |
| GradientBoosting | 76.2 | 0.85 | 69.8 | 82.6 | 78.2 | 76 |
| KNN | 74.6 | 0.82 | 70.9 | 78.2 | 74.1 | 75.7 |
| SVM | 77.8 | 0.87 | 77.8 | 77.9 | 74.2 | 79.3 |
| Naïve Bayes | 77 | 0.85 | 76.5 | 77.6 | 74.5 | 79.5 |
| Performance Measures | % (95% CI) |
|---|---|
| Balanced Accuracy | 73.7 (65.6–81.1) |
| AUC | 0.83 (0.76–0.90) |
| Sensitivity | 66.7 (52.4–78.5) |
| Specificity | 82.3 (70.1–90.4) |
| PPV | 76.6 (61.6–87.2) |
| NPV | 73.9 (61.7–83.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machetanz, L.; Günther, M.P.; Lau, S.; Kirchebner, J. High Risk, High Dose?—Pharmacotherapeutic Prescription Patterns of Offender and Non-Offender Patients with Schizophrenia Spectrum Disorder. Biomedicines 2022, 10, 3243. https://doi.org/10.3390/biomedicines10123243
Machetanz L, Günther MP, Lau S, Kirchebner J. High Risk, High Dose?—Pharmacotherapeutic Prescription Patterns of Offender and Non-Offender Patients with Schizophrenia Spectrum Disorder. Biomedicines. 2022; 10(12):3243. https://doi.org/10.3390/biomedicines10123243
Chicago/Turabian StyleMachetanz, Lena, Moritz Philipp Günther, Steffen Lau, and Johannes Kirchebner. 2022. "High Risk, High Dose?—Pharmacotherapeutic Prescription Patterns of Offender and Non-Offender Patients with Schizophrenia Spectrum Disorder" Biomedicines 10, no. 12: 3243. https://doi.org/10.3390/biomedicines10123243
APA StyleMachetanz, L., Günther, M. P., Lau, S., & Kirchebner, J. (2022). High Risk, High Dose?—Pharmacotherapeutic Prescription Patterns of Offender and Non-Offender Patients with Schizophrenia Spectrum Disorder. Biomedicines, 10(12), 3243. https://doi.org/10.3390/biomedicines10123243






