Abstract
The safety profile of hydroxyurea (HU) in patients with sickle-cell disease (SCD) is relatively well known. However, despite the suspected association of HU with myeloid neoplasms in myeloproliferative neoplasms (MPN), and the publication of sporadic reports of myeloid malignancies in SCD patients treated with HU, the possible excess risk imparted by HU in this population having an increasing life expectancy has failed to be demonstrated. Herein, we report one case of myelodysplastic syndrome emanating from the results on safety and effectiveness of HU on the largest European cohort of 1903 HU-treated adults and children who were followed-up prospectively in an observational setting over 10 years, accounting for a total exposure of 7309.5 patient-years. A comparison of this single case with previously published similar cases did not allow us to draw any significant conclusions due to the paucity of these events.
1. Introduction
Sickle-cell disease (SCD) refers to a group of inherited hemoglobin disorders characterized by sickling of erythrocytes when they are deoxygenated, leading to a significant burden of acute and chronic complications. Patients with SCD have acute and chronic pain due to vaso-occlusive pain crisis (VOCs) and have a reduced quality of life. The presence of hemoglobin A (HbA) and/or hemoglobin F (HbF) decreases the sickling and decelerates the polymerization process. Since the 1990s, hydroxyurea (HU) has been a cornerstone for the prevention of vaso-occlusive crises, the most frequent complication in SCD patients. HU is a chemotherapeutic agent that has been used for decades for the treatment of myeloproliferative neoplasms (MPNs). As a ribonucleotide reductase inhibitor, it interferes with DNA synthesis and repair mechanisms, causing cell cycle arrest and allowing γ globin genes to be more actively expressed. HU alters the kinetics of erythroid proliferation by enhancing the production of more F cells from primitive progenitors and subsequently the production of HbF. An increased risk of developing malignancies in SCD patients, especially myeloid neoplasms, emerged from epidemiological studies with incidence rates between 0.09 and 0.18% [1]. Despite the suspected association of HU with myeloid neoplasms, and the publication of sporadic reports of myeloid malignancies in SCD patients treated with HU, the possible excess risk imparted by HU in this population having an increasing life expectancy has failed to be demonstrated. Suspected mechanisms are chronic inflammation and disease-related immunomodulation, with potential additional risk of genomic alterations.
In the framework of the prospective observational study ESCORT-HU (NCT02516579), which evaluated the long-term safety and effectiveness of HU tablets in SCD patients across several European centers, one incident hematological malignancy was reported and described there [2]. Similarly, a combined analysis of all published MDS cases found in the scientific literature is also provided.
2. Case Report
The patient was a 40-year-old male with homozygous HbSS SCD enrolled in the ESCORT-HU study. Because of recurrent VOC episodes, he started HU treatment at the age of 23. At the time of inclusion, at the age of 30, he had experienced seven episodes of painful crises over the previous year (lasting for more than 48 h) and was switched to HU tablets at a dose of 25 mg/kg/day. He had no other complications related to SCD aside from priapism. Further improvement of the hematological and clinical response was shown by an increase in fetal hemoglobin rising from 20 to 26% and the absence of painful episodes during the first three-year follow-up in the study. The subsequent clinical course was notable for one episode of acute chest syndrome and painful crisis requiring hospitalization and the initiation of exchange transfusion at the age of 37. Because of iron overload, deferasirox was prescribed at the age of 38 and was maintained for only 20 days due to the subsequent clinical course. Mild heart failure (left ventricular ejection fraction of 55%) was detected at the age of 38 and was related to the underlying SCD. Severe pulmonary arterial hypertension (grade 4–5) was confirmed by right heart catheterization at the age of 41 during a further hospitalization due to an infection. A few months later, HU was withdrawn (after 17 years of exposure) because of a decreased hemoglobin level ranging from 8.7 to 7.0 g/dL and a decrease in the platelet count ranging from 160 to 116 × 109/L. Three months later, the patient was hospitalized for fever and the peripheral blood count at that time revealed these information: red blood cells: 2.55 × 1012; reticulocyte count: 8.59%; hemoglobin: 7.4 g/dL; leucocyte count: 24.11 × 109/L; and platelet count: 45 × 109/L. Examination of the bone marrow revealed hyperplasia and dyserythropoiesis of the red blood cell lineage, left-shift granulopoiesis, and also dysmegakaryopoiesis with an elevated number of megakaryocytes. There were 6% CD34+ blasts of the granulocyte lineage and also intermediate bone marrow infiltration (12–15%) by CD3+, CD2+, CD8+, CD5+, CD56+ (partially), CD7-, CD57-, TdT-, TIA-1-T lymphocytes. Thus, the diagnosis of refractory anemia with an excess of blasts-1 (RAEB-1) was confirmed. Cytogenetic analysis demonstrated a complex clonal abnormality including deletions of 5 q, 3 p, and 7 p, and monosomies of chromosomes 16, 17, and 18. There was no transformation into acute myeloid leukemia (AML). While the patient was screened for hematopoietic stem-cell transplantation (HSCT), the severe and persistent cytopenia led to his death three months after the established diagnosis.
3. Discussion
Conventionally, myeloid neoplasia occurring after exposure to a cytotoxic drug is classified as therapy-related. The WHO classification considers a single entity for t-AML (therapy-induced acute myeloid leukemia) and t-MDS (therapy-related myelodysplastic syndrome), in contrast to their de novo counterparts. The t-MDS/t-AML morphological classification has presumably limited relevance in predicting outcome, whereas cytogenetic stratification appears to provide more relevant information in terms of latency and prognosis [3].
A more frequent pattern of genetic rearrangements relative to their de novo counterparts has been identified for certain cytotoxic compounds such as alkylating agents/radiation therapy involving chromosomes 5 [del(5 q)] and/or 7 [-7/del(7 q)] and a complex karyotype, with a poor prognosis (median survival time of eight months). This pattern was also associated with a long latency (5–7 years) and with MDS, with a rapid progression into AML. A strong correlation has also been found between cytogenetic rearrangements leading to 17 p deletion and p53 mutations [4], which is the most encountered mutation in t-MDS/t-AML that occurs at a 2- to 4-fold higher frequency when compared to de novo myeloid neoplasms [5,6]. The complex chromosomal rearrangement exhibited by our patient had probably a detrimental impact on the MDS outcome. However, the cytogenetic pattern that was observed did not clearly superimpose with the abnormalities induced by alkylating agents.
Although the pathogenic mechanism is still unclear, mounting evidence indicates that instead of directly inducing mutations, chemotherapy (when combined with high dose radiations in some patients) may favor clonal expansion of selected progenitor or stem cells harboring mutations in specific genes (such as p53) by suppressing competing hematopoiesis—leading then to transformation into MDS/AML after subsequent acquisition of driver mutations [7].
The common observation of specific karyotypic abnormalities following the use of HU in MPN raised suspicion about the potential leukemogenicity of HU in this population [8], in addition to the intrinsic propensity of MPN to transform into MDS/AML even without prior cytotoxic therapy. However, case control and prospective studies with extended follow-up were unable to detect a clear excess risk that could be distinguished from concomitant cytotoxic exposure and the natural evolution of more progressive MPN.
In SCD patients, one British study suggested an increased risk of hematological malignancies, especially AML. A chronic inflammatory state, free radicals induced by free hemoglobin or heme, chronic hematopoietic stress, and premature “aging” of hematopoiesis with accrued genetic, epigenetic, and functional alteration of hematopoietic cells were suggested as possible explanations for this finding. HU may carry itself an intrinsic risk of oncogenic potential due to the increased frequency of acquired DNA mutations in SCD patients [9]. Nevertheless, no significant increase in myeloid malignancies occurred in a large population-based cohort following introduction of HU therapy in SCD patients. The largest cohort study in SCD patients treated with HU with a follow-up of up to 17 years did not provide evidence for a specific risk for patients treated with HU at conventional doses [10].
We retrieved further reports of MDS from the Medline database via Pubmed until May 2020, using keywords such as “sickle-cell disease”, “sickle-cell anemia”, “myelodysplastic” and “leukemia”. These cases are summarized in Table 1 and cumulative analysis of these reports is provided below. We chose to restrict the search to MDS to obtain further insights into the pathogenesis of the preleukemic process without the involvement of additional abnormalities acquired during transformation into AML. In total, five additional cases have been reported [11,12,13,14,15]. Importantly, even with a low estimation of 10% of patients treated with HU (from among 22,000 SCD patients in Europe and approximately 90,000 in the US), this reporting rate appears to be low.

Table 1.
Pathological features of MDS (past and present case)–only MDS according to the WHO 2016 classification were included (i.e., not including cases of MDS/MPN) &.
All patients were male and relatively young as the median age at diagnosis was 42 years, with two notable outliers: one 55-year-old patient without prior exposure to HU (case 2), and one 34-year-old (case 4) with a limited exposure of two years having received non-myeloablative chemotherapy and radiotherapy for HSCT with subsequent graft loss. All other patients were treated with HU for a median duration of 14 years (range: 10–20).
The long latencies of onset after starting HU (median: 15 years) that were observed for the five patients treated with HU at conventional doses were longer than those observed in t-MDS/AML patients treated with cytotoxic agents or immunosuppressants in non-malignant conditions (median 10.8 years). Abnormalities in chromosomes 5, 7, or both, in 4 of the 5 cases (case 1, 3–5) involving HU exposure were also found, consistent with the known cytogenetic profile of therapy-related myelogenous neoplasm observed in MDS, as well as in the case without prior exposure to HU (case 2). This latter patient, who was also older than the other patients of this series, showed a deletion in the short arm of chromosome 17, thus suggesting a common pathogenic pathway with the leukemogenicity of alkylating agents. In contrast, in the younger patient in case 4, who had a limited exposure to HU (only 2 years), the disease displayed shorter latency with partial loss of the long arm of chromosome 7 (but no reported alteration of chromosome 17). This patient had also received a non-myeloablative regimen and radiation, and the seven-year latency was more consistent with the commonly reported timelines associated with t-MDS.
Our patient developed MDS at an age comparable to the ones reported in other cases following a relatively similar duration of exposure to HU. Karyotype analysis of blast cells demonstrated features more commonly reported for patients with t-MDS, but no clear-cut cytogenetic profile has emerged when compared to the cases without prior exposure to HU.
Despite the abovementioned concerns, HU has a beneficial effect in many chronic complications of SCD and this is demonstrated in Figure 1. The death rate in patients treated with HU was significantly lower than that of SCD patients treated conventionally and without HU (13 deaths in HU group vs. 49 deaths in non-HU group). Figure 2 demonstrates that the probability of 10-year survival was 86% for the patients treated with HU (HU patients) vs. only 65% for the non-HU patients.
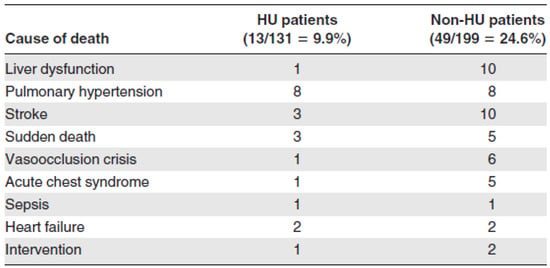
Figure 1.
Causes of death in HU and non-HU patients (adapted from [10]).
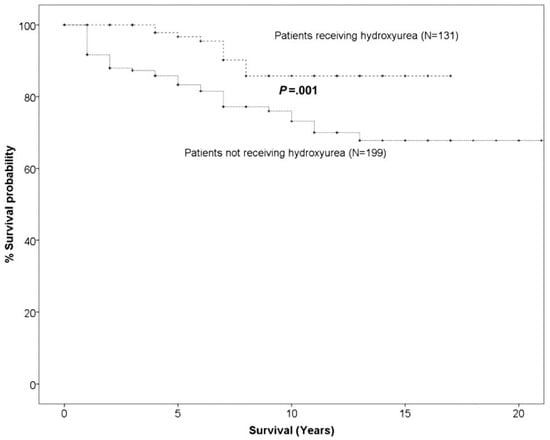
Figure 2.
Probability of 10-year overall survival in HU SCD patients versus non-HU SCD patients (adapted from [10]).
Additionally, HU is no longer the only available drug to relieve SCD patients from VOCs, improving their quality of life and increasing their life span. Crizanlizumab and Voxelotor are new emerging agents that are added to the management of SCD with HU, which still remains the gold standard.
4. Conclusions
One case of MDS in a 40-year-old male with homozygous HbSS SCD was collected in the prospective ESCORT-HU cohort study among the 1906 participants, of whom 55% were adults (total exposure of 7309.5 patient years). No other hematological malignancy was collected. Thus, the incidence of hematological malignancy appears to be low. Aside from the long duration of treatment with HU for SCD, no specific risk factor was identified for our patient, but additional surveillance with a longer follow-up is required.
Author Contributions
Conceptualization, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; methodology, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; validation, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; formal analysis, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; investigation, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; data curation, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; writing—original draft preparation, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; writing—review and editing, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; visualization, P.F., E.V., F.G., G.C., G.L., L.J., P.B., J.G.-D., E.B., C.C. and A.H.; supervision, P.F., E.V. and A.H.; project administration, P.F., E.V. and A.H.; funding acquisition, P.F., E.V. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
Addmedica Company provided funding for this prospective observational study ESCORT-HU (NCT02516579).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from patients involved in this study. Similarly, written or oral informed consent has been obtained from patients to publish this manuscript.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to all patients who participated in the ESCORT-HU study, and their families. The authors also thank the investigators who collaborated in this study.
Conflicts of Interest
Ersi Voskaridou reported receiving fees from CELGENE (Consultancy, Research funding), Bristol Myers Squibb (BMS) (Consultancy, Research funding), PROTAGONIST (Research funding), GENESIS (Consultancy, Research funding), NOVARTIS (Research funding), ADDMEDICA (Consultancy, Research funding), and IMARA (Research funding). Frédéric Galactéros reported receiving grants for consultancy from NOVARTIS, SANOFI, BLUEBIRDBIO, PFIZER, AGIOS, GBT, BMS and ADDMEDICA (Consultancy, Research funding). Giovanna Cannas reported receiving fees from ADDMEDICA (Research funding). Laure Joseph reported receiving fees from BLUEBIRDBIO (Consultancy), and ADDMEDICA (Research funding). Pablo Bartolucci reported receiving fees from NOVARTIS Company (Consultancy, Lecture, Research funding), BLUEBIRDBIO (Consultancy, Research funding), EMMAUS (Consultancy), HEMANEXT (Consultancy), AGIOS (Consultancy), JAZZPHARMA (Lecture), ADDMEDICA (Consultancy, Research funding), FOUNDATION FABRE (Research funding), Cofounder of INNOVHEM, ROCHE (Consultancy), and GBT (Consultancy). Justine Gellen-Dautremer reported receiving fees from ADDMEDICA (Research funding). Emmanuelle Bernit reported receiving fees from ADDMEDICA (Research funding). Anoosha Habibi reported receiving fees from NOVARTIS Company (Consultancy), BLUEBIRD BIO Company (Consultancy), and ADDMEDICA (Research funding). Addmedica Company was involved in the design and conduct of the study (including development of the study protocol and statistical analysis plan); collection, monitoring, analysis, and interpretation of the data; and preparation, approval and submission of this manuscript.
References
- Brunson, A.; Keegan, T.H.M.; Bang, H.; Mahajan, A.; Paulukonis, S.; Wun, T. Increased risk of leukemia among sickle cell disease patients in California. Blood 2017, 130, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- De Montalembert, M.; Voskaridou, E.; Oevermann, L.; Cannas, G.; Habibi, A.; Loko, G.; Joseph, L.; Colombatti, R.; Bartolucci, P.; Brousse, V.; et al. Real-Life experience with hydroxyurea in patients with sickle cell disease: Results from the prospective ESCORT-HU cohort study. Am. J. Hematol. 2021, 96, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Singh, Z.N.; Huo, D.; Anastasi, J.; Smith, S.M.; Karrison, T.; Le Beau, M.M.; Larson, R.A.; Vardiman, J.W. Therapy-related myelodysplastic syndrome: Morphologic subclassification may not be clinically relevant. Am. J. Clin. Pathol. 2007, 127, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Soenen, V.; Preudhomme, C.; Roumier, C.; Daudignon, A.; Lai, J.L.; Fenaux, P. 17 p Deletion in acute myeloid leukemia and myelodysplastic syndrome. Analysis of breakpoints and deleted segments by fluorescence in situ. Blood 1998, 91, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.Y.; Patel, K.P.; Garcia-Manero, G.; Routbort, M.J.; Fu, B.; Tang, G.; Goswami, M.; Singh, R.; Kanagal-Shamanna, R.; Pierce, S.A.; et al. Mutational profiling of therapy-related myelodysplastic syndromes and acute myeloid leukemia by next generation sequencing, a comparison with de novo diseases. Leuk. Res. 2015, 39, 348–354. [Google Scholar] [CrossRef] [PubMed]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-related myeloid neoplasms: When genetics and environment collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.N.; Ramsingh, G.; Young, A.L.; Miller, C.A.; Touma, W.; Welch, J.S.; Lamprecht, T.L.; Shen, D.; Hundal, J.; Fulton, R.S.; et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015, 518, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Sterkers, Y.; Preudhomme, C.; Lai, J.L.; Demory, J.L.; Caulier, M.T.; Wattel, E.; Bordessoule, D.; Bauters, F.; Fenaux, P. Acute myeloid leukemia and myelodysplastic syndromes following essential thrombocythemia treated with hydroxyurea: High proportion of cases with 17 p deletion. Blood 1998, 91, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Maia Filho, P.A.; Pereira, J.F.; Almeida Filho, T.P.; Cavalcanti, B.C.; Sousa, J.C.; Lemes, R.P.G. Is chronic use of hydroxyurea safe for patients with sickle cell anemia? An account of genotoxicity and mutagenicity. Environ. Mol. Mutagen 2019, 60, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Voskaridou, E.; Christoulas, D.; Bilalis, A.; Plata, E.; Varvagiannis, K.; Stamatopoulos, G.; Sinopoulou, K.; Balassopoulou, A.; Loukopoulos, D.; Terpos, E. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: Results of a 17-year, single-center trial (LaSHS). Blood 2010, 115, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Baz, W.; Najfeld, V.; Yotsuya, M.; Talwar, J.; Terjanian, T.; Forte, F. Development of myelodysplastic syndrome and acute myeloid leukemia 15 years after hydroxyurea use in a patient with sickle cell anemia. Clin. Med. Insights Oncol. 2012, 6, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Zemenides, S.; Erblich, T.; Luqmani, A.; Bain, B.J. Peripheral blood features of acute myeloid leukemia with myelodysplasia-related changes developing in a patient with sickle cell anemia. Am. J. Hematol. 2014, 89, 1010. [Google Scholar] [CrossRef] [PubMed]
- Aumont, C.; Driss, F.; Lazure, T.; Picard, V.; Creidy, R.; De Botton, S.; Saada, V.; Lambotte, O.; Bilhou-Nabera, C.; Tertian, G.; et al. Myelodysplastic syndrome with clonal cytogenetic abnormalities followed by fatal erythroid leukemia after 14 years of exposure to hydroxyurea for sickle cell anemia. Am. J. Hematol. 2015, 90, E131–E132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maule, J.; Neff, J.L.; McCall, C.M.; Rapisardo, S.; Lagoo, A.S.; Yang, L.H.; Crawford, R.D.; Zhao, Y.; Wang, E. Myeloid neoplasms in the setting of sickle cell disease: An intrinsic association with the underlying condition rather than a coincidence; report of 4 cases and review of the literature. Mod. Pathol. 2019, 32, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.M.; Bonner, M.; Pierciey, F.J.; Uchida, N.; Rottman, J.; Demopoulos, L.; Schmidt, M.; Kanter, J.; Walters, M.C.; Thompson, A.A.; et al. Myelodysplastic syndrome unrelated to lentiviral vector in a patient treated with gene therapy for sickle cell disease. Blood Adv. 2020, 4, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).