Prospective Analysis of Functional and Structural Changes in Patients with Spinal Muscular Atrophy—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in Later-Onset Spinal Muscular Atrophy: Long-Term Results from the Phase 1/2 Studies. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Montes, J.; Dunaway Young, S.; McDermott, M.P.; Martens, W.; Pasternak, A.; Quigley, J.; Mirek, E.; Glanzman, A.M.; Civitello, M.; et al. Quantitative Evaluation of Lower Extremity Joint Contractures in Spinal Muscular Atrophy: Implications for Motor Function. Pediatr. Phys. Ther. 2018, 30, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.; Mazurkiewicz, Ł.; Maślanko, K.; Rekowski, W.; Jędrzejowska, M. Cervical Rotation, Chest Deformity and Pelvic Obliquity in Patients with Spinal Muscular Atrophy. BMC Musculoskelet Disord. 2020, 21, 726. [Google Scholar] [CrossRef]
- Van den Engel-Hoek, L.; Erasmus, C.E.; van Bruggen, H.W.; de Swart, B.J.M.; Sie, L.T.L.; Steenks, M.H.; de Groot, I.J.M. Dysphagia in Spinal Muscular Atrophy Type II: More than a Bulbar Problem? Neurology 2009, 73, 1787–1791. [Google Scholar] [CrossRef]
- Modrzejewska, S.; Kotulska, K.; Kopyta, I.; Grędowska, E.; Emich-Widera, E.; Tomaszek, K.; Paprocka, J.; Chmielewski, D.; Pilch, J.; Pietruszewski, J.; et al. Nusinersen Treatment of Spinal Muscular Atrophy Type 1—Results of Expanded Access Programme in Poland. Neurol. Neurochir. Pol. 2021, 55, 289–294. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Robertson, A.; Leary, R.; McMacken, G.; König, K.; Kirschner, J.; Jones, C.C.; Cook, S.F.; Lochmüller, H. A Multi-Source Approach to Determine SMA Incidence and Research Ready Population. J. Neurol. 2017, 264, 1465–1473. [Google Scholar] [CrossRef]
- SMA Foundation in Poland. Available online: https://www.fsma.pl/rdzeniowy-zanik-miesni/jak-czeste-jest-sma/ (accessed on 23 January 2022).
- Chong, L.C.; Gandhi, G.; Lee, J.M.; Yeo, W.W.Y.; Choi, S.-B. Drug Discovery of Spinal Muscular Atrophy (SMA) from the Computational Perspective: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 8962. [Google Scholar] [CrossRef]
- Chen, T.-H. New and Developing Therapies in Spinal Muscular Atrophy: From Genotype to Phenotype to Treatment and Where Do We Stand? Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Gajewska, E. Przegląd metod oceny funkcjonalnej analizujących motorykę małą i dużą u chorych z rdzeniowym zanikiem mięśni. Neurol. Dziecięca 2019, 28, 23–26. [Google Scholar] [CrossRef]
- Mercuri, E.; Baranello, G.; Boespflug-Tanguy, O.; De Waele, L.; Goemans, N.; Kirschner, J.; Masson, R.; Mazzone, E.S.; Pechmann, A.; Pera, M.C.; et al. Risdiplam in Types 2 and 3 Spinal Muscular Atrophy: A Randomised, Placebo-controlled, Dose-finding Trial Followed by 24 Months of Treatment. Euro. J. Neurol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Risdiplam: First Approval. Drugs 2020, 80, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Claborn, M.K.; Gildon, B.L.; Kessler, T.L.; Walker, C. Onasemnogene Abeparvovec-Xioi: Gene Therapy for Spinal Muscular Atrophy. Ann. Pharmacother. 2020, 54, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, M.; Moschou, M.; Papaliagkas, V.; Notas, K.; Chatzikyriakou, E.; Papagiannopoulos, S.; Arnaoutoglou, M.; Kimiskidis, V.K. Nusinersen in Adults with 5q Spinal Muscular Atrophy: A Systematic Review and Meta-Analysis. Neurotherapeutics 2022, 19, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Finkel, R.S.; Bertini, E.S.; Schroth, M.; Simonds, A.; Wong, B.; Aloysius, A. Consensus Statement for Standard of Care in Spinal Muscular Atrophy. J. Child Neurolog. 2007, 22, 1027–1049. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and Management of Spinal Muscular Atrophy: Part 2: Pulmonary and Acute Care; Medications, Supplements and Immunizations; Other Organ Systems; and Ethics. Neuromuscul. Disord. 2018, 28, 197–207. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and Management of Spinal Muscular Atrophy: Part 1: Recommendations for Diagnosis, Rehabilitation, Orthopedic and Nutritional Care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Stępień, A.; Jędrzejowska, M.; Guzek, K.; Rekowski, W.; Stępowska, J. Reliability of Four Tests to Assess Body Posture and the Range of Selected Movements in Individuals with Spinal Muscular Atrophy. BMC Musculoskelet. Disord. 2019, 20, 54. [Google Scholar] [CrossRef]
- Fujak, A.; Kopschina, C.; Gras, F.; Forst, R.; Forst, J. Contractures of the Lower Extremities in Spinal Muscular Atrophy Type II. Descriptive Clinical Study with Retrospective Data Collection. Ortop. Traumatol. Rehabil. 2011, 13, 27–36. [Google Scholar] [CrossRef]
- Glanzman, A.M.; Mazzone, E.; Main, M.; Pelliccioni, M.; Wood, J.; Swoboda, K.J.; Scott, C.; Pane, M.; Messina, S.; Bertini, E.; et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): Test Development and Reliability. Neuromuscul. Disord. 2010, 20, 155–161. [Google Scholar] [CrossRef]
- Main, M.; Kairon, H.; Mercuri, E.; Muntoni, F. The Hammersmith Functional Motor Scale for Children with Spinal Muscular Atrophy: A Scale to Test Ability and Monitor Progress in Children with Limited Ambulation. Eur. J. Paediatr. Neurol. 2003, 7, 155–159. [Google Scholar] [CrossRef]
- Haataja, L.; Mercuri, E.; Regev, R.; Cowan, F.; Rutherford, M.; Dubowitz, V.; Dubowitz, L. Optimality Score for the Neurologic Examination of the Infant at 12 and 18 Months of Age. J. Pediatrics 1999, 135, 153–161. [Google Scholar] [CrossRef]
- Bérard, C.; Payan, C.; Hodgkinson, I.; Fermanian, J.; The MFM Collaborative Study Group A Motor Function Measure Scale for Neuromuscular Diseases. Construction and Validation Study. Neuromuscul. Disord. 2005, 15, 463–470. [Google Scholar] [CrossRef]
- Mazzone, E.S.; Mayhew, A.; Montes, J.; Ramsey, D.; Fanelli, L.; Young, S.D.; Salazar, R.; De Sanctis, R.; Pasternak, A.; Glanzman, A.; et al. Revised Upper Limb Module for Spinal Muscular Atrophy: Development of a New Module. Muscle Nerve 2017, 55, 869–874. [Google Scholar] [CrossRef]
- Dunaway Young, S.; Montes, J.; Kramer, S.S.; Marra, J.; Salazar, R.; Cruz, R.; Chiriboga, C.A.; Garber, C.E.; De Vivo, D.C. Six-Minute Walk Test Is Reliable and Valid in Spinal Muscular Atrophy: 6MWT in SMA. Muscle Nerve 2016, 54, 836–842. [Google Scholar] [CrossRef]
- O’Hagen, J.M.; Glanzman, A.M.; McDermott, M.P.; Ryan, P.A.; Flickinger, J.; Quigley, J.; Riley, S.; Sanborn, E.; Irvine, C.; Martens, W.B.; et al. An Expanded Version of the Hammersmith Functional Motor Scale for SMA II and III Patients. Neuromuscul. Disord. 2007, 17, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.; Gajewska, E.; Rekowski, W. Motor Function of Children with SMA1 and SMA2 Depends on the Neck and Trunk Muscle Strength, Deformation of the Spine, and the Range of Motion in the Limb Joints. Int. J. Environ. Res. Public Health 2021, 18, 9134. [Google Scholar] [CrossRef]
- Pechmann, A.; Langer, T.; Schorling, D.; Stein, S.; Vogt, S.; Schara, U.; Kölbel, H.; Schwartz, O.; Hahn, A.; Giese, K.; et al. Evaluation of Children with SMA Type 1 Under Treatment with Nusinersen within the Expanded Access Program in Germany. J. Neuromuscul. Dis. 2018, 5, 135–143. [Google Scholar] [CrossRef]
- Gavriilaki, M.; Moschou, M.; Papaliagkas, V.; Notas, K.; Chatzikyriakou, E.C.; Zafeiridou, G.; Papagiannopoulos, S.; Arnaoutoglou, M.; Kimiskidis, V.K.K. Biomarkers of Disease Progression in Adolescents and Adults with 5q Spinal Muscular Atrophy: A Systematic Review and Meta-Analysis. Neuromuscul. Disord. 2022, 32, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Schorling, D.C.; Pechmann, A.; Kirschner, J. Advances in Treatment of Spinal Muscular Atrophy—New Phenotypes, New Challenges, New Implications for Care. J. Neuromuscul. Dis. 2020, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ojala, K.S.; Reedich, E.J.; DiDonato, C.J.; Meriney, S.D. In Search of a Cure: The Development of Therapeutics to Alter the Progression of Spinal Muscular Atrophy. Brain Sci. 2021, 11, 194. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.; Montes, J.; Mazzone, E.S.; Sormani, M.P.; Main, M.; Ramsey, D.; Mayhew, A.; Glanzman, A.M.; Dunaway, S.; et al. Patterns of Disease Progression in Type 2 and 3 SMA: Implications for Clinical Trials. Neuromuscul. Disord. 2016, 26, 126–131. [Google Scholar] [CrossRef]
- Pera, M.C.; Coratti, G.; Bovis, F.; Pane, M.; Pasternak, A.; Montes, J.; Sansone, V.A.; Dunaway Young, S.; Duong, T.; Messina, S.; et al. Nusinersen in Pediatric and Adult Patients with Type III Spinal Muscular Atrophy. Ann. Clin. Transl. Neurol. 2021, 8, 1622–1634. [Google Scholar] [CrossRef]
- Tscherter, A.; Rüsch, C.T.; Baumann, D.; Enzmann, C.; Hasselmann, O.; Jacquier, D.; Jung, H.H.; Kruijshaar, M.E.; Kuehni, C.E.; Neuwirth, C.; et al. Evaluation of Real-Life Outcome Data of Patients with Spinal Muscular Atrophy Treated with Nusinersen in Switzerland. Neuromuscul. Disord. 2022, 32, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Pane, M.; Coratti, G.; Sansone, V.A.; Messina, S.; Bruno, C.; Catteruccia, M.; Sframeli, M.; Albamonte, E.; Pedemonte, M.; D’Amico, A.; et al. Nusinersen in Type 1 Spinal Muscular Atrophy: Twelve-month Real-world Data. Ann. Neurol. 2019, 86, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.; Sikora-Chojak, J.; Maślanko, K.; Kiebzak, W. Hip Abduction and Supported Standing Affect the Ranges of Hips Extension in Spinal Muscular Atrophy Patients. Pol. Ann. Med. 2020, 28, 50–56. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ju, Y.H.; Chen, S.M.; Lo, S.K.; Jong, Y.J. Joint Range of Motion Limitations in Children and Young Adults with Spinal Muscular Atrophy. Arch. Phys. Med. Rehabil. 2004, 85, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Fujak, A.; Raab, W.; Schuh, A.; Richter, S.; Forst, R.; Forst, J. Natural Course of Scoliosis in Proximal Spinal Muscular Atrophy Type II and IIIa: Descriptive Clinical Study with Retrospective Data Collection of 126 Patients. BMC Musculoskelet. Disord. 2013, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.; Owens, H.; Hynan, L.S.; Iannaccone, S.T. AmSMART Group the Gross Motor Function MeasureTM Is a Valid and Sensitive Outcome Measure for Spinal Muscular Atrophy. Neuromuscul. Disord. 2006, 16, 374–380. [Google Scholar] [CrossRef]
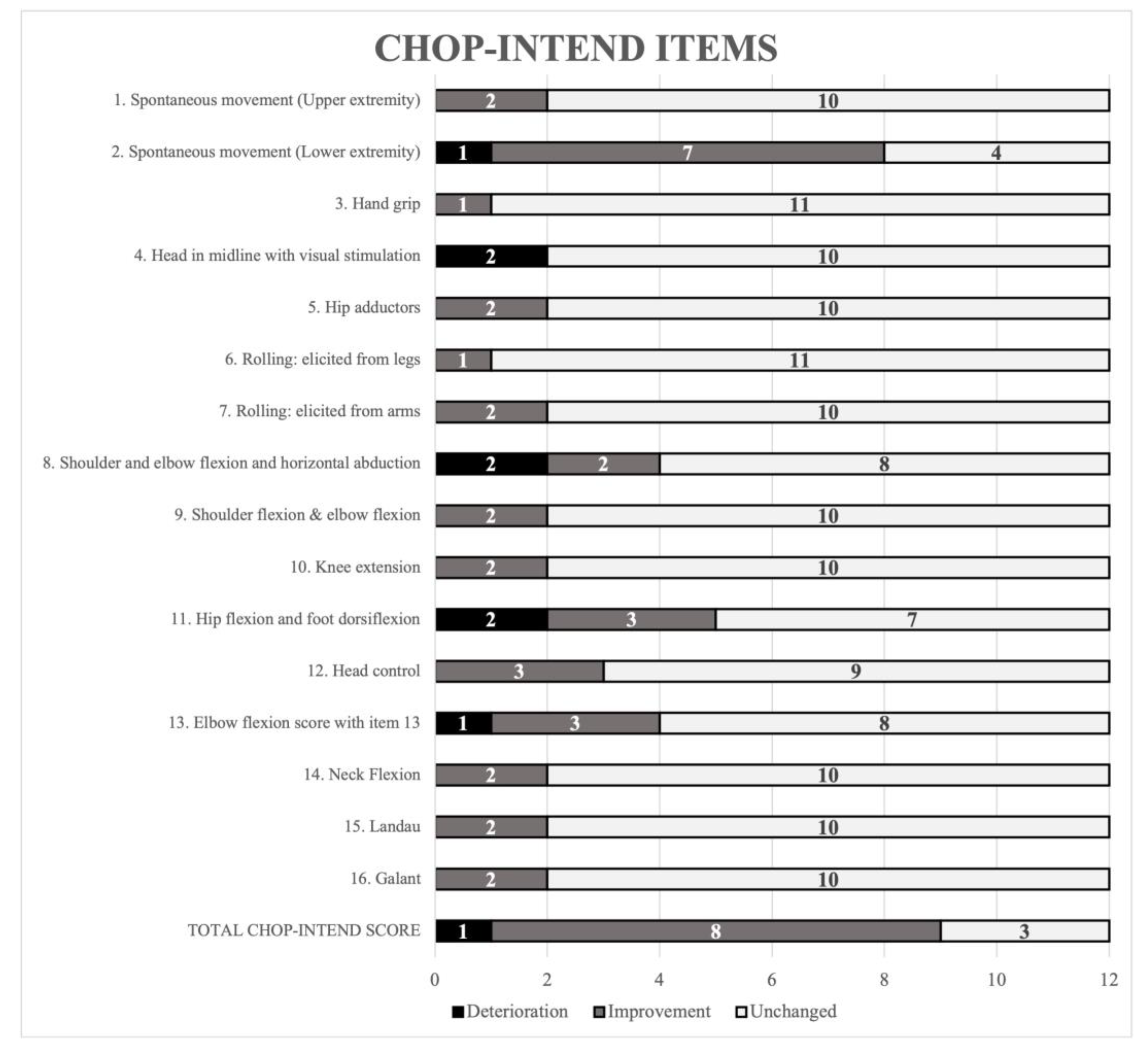

| Parameters | All Participants n = 40 | Non-Sitters Group n = 12 | Sitters Group n = 28 |
|---|---|---|---|
| Age (years): median Q1–Q3 | 7.50 5.00–12.00 | 7.50 2.00–12.25 | 7.50 5.00–12.00 |
| Age at the start of treatment (years): median Q1–Q3 | 5.00 3.00–11.00 | 6.00 0.00–10.25 | 5.00 3.00–11.00 |
| sex: female male | 22 18 | 7 5 | 15 13 |
| SMA type: SMA1 SMA2 SMA3 | 14 18 8 | 12 - - | 2 18 8 |
| SMN2 copy number: 2 copies 3 copies 4 copies | 13 21 6 | 10 1 1 | 3 20 5 |
| therapy used: Nusinersen Risdiplam Gene therapy | 19 19 2 | 3 7 2 | 18 10 - |
| child’s functional level recumbent sits unassisted rolls over crawls stands with assistance stands unassisted walks with assistance walks unassisted | 9 8 9 4 3 2 1 4 | 9 2 1 - - - - - | - 6 8 4 3 2 1 4 |
| rehabilitation intensity: 1–3 times a week 4–6 times a week every day | 6 17 17 | 1 5 6 | 5 12 11 |
| the duration between the start of treatment to the first examination (months): median Q1–Q3 | 15 7–32 | 13 6–21 | 18 10–36 |
| the duration between the diagnosis to the start of the treatment (months): median Q1–Q3 | 48 25–111 | 78 1–122 | 48 27–97 |
| follow-up duration median | 5 months | 5 months | 5 months |
| Parameter | Total n = 40 | Non-Sitters n = 12 | Sitters n = 28 | |
|---|---|---|---|---|
| Goniometer measurement | ||||
| HF-L | Examination I | 123 (113–130); 100–140 | 123 (110–128); 100–130 | 123 (118–133); 105–140 |
| Examination II | 130 (115–130); 95–145 | 130 (115–130); 95–140 | 130 (115–133); 105–145 | |
| significance | z = 1.47 p = 0.142 | z = 1.27 p = 0.203 | z = 0.80 p = 0.421 | |
| HF-R | Examination I | 120 (112–130); 90–145 | 115 (105–120); 90–125 | 120 (115–133); 105–145 |
| Examination II | 128 (110–133); 90–145 | 123 (105–130); 90–140 | 128 (113–138); 90–145 | |
| significance | z = 1.65 p = 0.098 | z = 1.53 p = 0.126 | z = 1.29 p = 0.196 | |
| KF-L | Examination I | 145 (138–153); 110–160 | 140 (130–143); 110–155 | 148 (140–155); 120–160) |
| Examination II | 145 (140–155); 115–160 | 140 (130–148); 115–155 | 150 (140–155); 120–160 | |
| significance | z = 2.13 p = 0.033 | z = 1.86 p = 0.063 | z = 1.29 p = 0.196 | |
| KF-R | Examination I | 150 (138–153); 120–160 | 138 (130–148); 120–150 | 150 (140–155); 120–160 |
| Examination II | 150 (140–155); 110–160 | 140 (130–150); 110–155 | 150 (140–155); 120–160) | |
| significance | z = 0.08 p = 0.936 | z = 0.71 p = 0.477 | z = 0.76 p = 0.445 | |
| Scoliometer measurement | ||||
| SATR-U | Examination I Examination II significance | 5 (5–5); 0–15 5 (0–5); 0–15 z = 1.48 p = 0.139 | 5 (5–8); 5–15 5 (5–8); 0–15 z = 0.53 p = 0.593 | 5 (0–5) 0–10 3 (0–5); 0–10 z = 1.47 p = 0.142 |
| SATR-L | Examination I Examination II significance | 5 (3–8); 0–20 5 (0–5); 0–20 z = 2.93 p = 0.003 | 8 (5–10); 5–20 5 (3–8); 0–20 z = 2.37 p = 0.018 | 5 (0–5); 0–15 5 (0–5); 0–10 z = 1.83 p = 0.068 |
| PO-ANGLE | Examination I Examination II significance | 5 (5–10); 0–30 5 (5–10); 0–30 z = 0.63 p = 0.529 | 5 (5–5); 0–20 5 (3–5); 0–5 z = 0.00 p = 1.000 | 5 (5–10); 0–30 5 (5–13); 0–30 z = 0.73 p = 0.463 |
| Plurimeter measurement | ||||
| CR-L | Examination I | 78 (70–80); 40–90 | 70 (58–85); 40–90 | 80 (70–80); 40–90 |
| Examination II | 80 (70–85); 40–90 | 80 (60–85); 40–90 | 80 (70–85); 50–90 | |
| significance | z = 1.95 p = 0.051 | z = 1.26 p = 0.208 | z = 1.53 p = 0.126 | |
| CR-R | Examination I | 70 (60–80); 20–85 | 70 (50–80); 20–85 | 70 (60–78); 30–85 |
| Examination II | 70 (68–80); 15–90 | 70 (63–80); 15–85 | 73 (70–80); 40–90 | |
| significance | z = 2.49 p = 0.013 | z = 0.28 p = 0.779 | z = 2.66 p = 0.008 | |
| HE-L | Examination I | 15 (−10–30) −60–45 | 13 (0–28); −30–40 | 15 (−10–30); −60–45 |
| Examination II | 10 (−5–25); −45–40 | 10 (−3–25); −25–40 | 10 (−8–28); −45–40 | |
| significance | z = 1.12 p = 0.264 | z = 1.13 p = 0.260 | z = 0.64 p = 0.520 | |
| HE-R | Examination I | 15 (−3–30) −50–45 | 13 (0–33); −20–35 | 18 (−5–28); −50–45 |
| Examination II | 10 (−10–30); −40–45 | 10 (−8–28); −30–40 | 10 (−13–30); −40–45 | |
| significance | z = 0.10 p = 0.318 | z = 0.42 p = 0.674 | z = 0.88 p = 0.378 | |
| Parameters | All Participants n = 40 | Non-Sitters Group n = 12 | Sitters Group n = 28 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Range | Improvement | No Change | Worsening | Normal Range | Improvement | No Change | Worsening | Normal Range | Improvement | No Change | Worsening | |
| HF-L | 8 | 18 | 3 | 11 | 2 | 7 | 0 | 3 | 6 | 11 | 3 | 8 |
| HF-R | 4 | 20 | 3 | 13 | 0 | 8 | 0 | 4 | 4 | 12 | 3 | 9 |
| KF-L | 7 | 17 | 10 | 6 | 1 | 6 | 4 | 1 | 6 | 11 | 6 | 5 |
| KF-R | 7 | 11 | 14 | 8 | 0 | 6 | 3 | 3 | 7 | 5 | 11 | 5 |
| SATR-U | 10 | 7 | 21 | 2 | 0 | 2 | 9 | 1 | 10 | 5 | 12 | 1 |
| SATR-L | 11 | 11 | 18 | 0 | 0 | 7 | 5 | 0 | 11 | 4 | 13 | 0 |
| PO-ANGLE | 5 | 3 | 19 | 5 | 0 | 1 | 2 | 1 | 5 | 2 | 17 | 4 |
| CR-L | 4 | 18 | 9 | 9 | 3 | 4 | 3 | 2 | 1 | 14 | 6 | 7 |
| CR-R | 0 | 18 | 11 | 11 | 0 | 3 | 4 | 5 | 0 | 15 | 7 | 6 |
| HE-L | 1 | 12 | 6 | 21 | 1 | 3 | 2 | 6 | 0 | 9 | 4 | 15 |
| HE-R | 1 | 14 | 8 | 17 | 1 | 4 | 3 | 4 | 0 | 10 | 5 | 13 |
| Non-Sitters Group, n = 12 | ||||||
| Contracture, examination I | Both hips, both knees, YES, n = 10 | Both hips, both knees, NO, n = 2 | ||||
| CHOP I SUM | 15 (2–37) | 32; 52 | ||||
| CHOP II SUM | 16 (5–35) | 59; 60 | ||||
| difference between examinations I and II | −4 to +6 | −8 to 27 | ||||
| Significance of the difference between the I and II tests, in relation to the presence of contracture | z = −2.06; p = 0.039 | |||||
| Sitters group, n = 28 | ||||||
| contracture, examination I | Both hips | Both hips | Left knee | Right knee | ||
| YES, n = 18 | NO, n = 10 | YES, n = 22 | NO, n = 6 | YES, n = 23 | NO, n = 5 | |
| HFMSE I SUM | 18 (5–54) | 39 (25–59) | 22 (14–35) | 33 (25–61) | 23 (14–36) | 26 (25–41) |
| HFMSE II SUM | 20 (6–54) | 38 (30–59) | 25 (14–35) | 39 (29–63) | 25 (14–38) | 34 (29–44) |
| difference between examinations I and II | −4 to +9 | −4 to +13 | −4 to +13 | +1 to +9 | −4 to +13 | +1 to +9 |
| Significance of the difference between the I and II tests, in relation to the presence of contracture | z = −1.65 p = 0.010 | z = 2.18 p = 0.030 | z = 2.12 p = 0.034 | |||
| Parameters | Rho Value= |
|---|---|
| Non-Sitters Group | |
| HF-L and total scale score, examination II | 0.736 |
| HF-L and SATR-U, examination II | −0.580 |
| HF-L and HE-L, examination II | 0.588 |
| HF-L and PO-ANGLE, examination I | −0.668 |
| HF-R and PO-ANGLE, examination I | −0.682 |
| Sitters group | |
| HF-L and total scale score, examination II | 0.528 |
| HF-R and total scale score, examination II | 0.581 |
| HF-L and CR-R, examination II | 0.427 |
| HF-L and SATR-U, examination I | −0.460 |
| HF-R and SATR-U, examination I | −0.387 |
| HF-L and SATR-L, examination I | −0.424 |
| HF-R a SATR-L, examination I | −0.407 |
| HF-L and HE-L, examination II | 0.413 |
| HF-R and HE-L, examination II | 0.433 |
| HF-L and HE-R, examination II | 0.433 |
| HF-R and HE-R, examination II | 0.435 |
| HF-R and PO-ANGLE, examination I | −0.384 |
| HF-L and PO-ANGLE, examination II | −0.498 |
| HF-R and PO-ANGLE, examination II | −0.0534 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieniaszewska, A.; Sobieska, M.; Gajewska, E. Prospective Analysis of Functional and Structural Changes in Patients with Spinal Muscular Atrophy—A Pilot Study. Biomedicines 2022, 10, 3187. https://doi.org/10.3390/biomedicines10123187
Bieniaszewska A, Sobieska M, Gajewska E. Prospective Analysis of Functional and Structural Changes in Patients with Spinal Muscular Atrophy—A Pilot Study. Biomedicines. 2022; 10(12):3187. https://doi.org/10.3390/biomedicines10123187
Chicago/Turabian StyleBieniaszewska, Aleksandra, Magdalena Sobieska, and Ewa Gajewska. 2022. "Prospective Analysis of Functional and Structural Changes in Patients with Spinal Muscular Atrophy—A Pilot Study" Biomedicines 10, no. 12: 3187. https://doi.org/10.3390/biomedicines10123187
APA StyleBieniaszewska, A., Sobieska, M., & Gajewska, E. (2022). Prospective Analysis of Functional and Structural Changes in Patients with Spinal Muscular Atrophy—A Pilot Study. Biomedicines, 10(12), 3187. https://doi.org/10.3390/biomedicines10123187







