Polyelectrolyte Coating of Ferumoxytol Differentially Impacts the Labeling of Inflammatory and Steady-State Dendritic Cell Subtypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyelectrolyte Coating of Ferumoxytol
2.2. Dendritic Cell Culture
2.3. Flow Cytometry
2.4. MNP Labeling of DCs
2.5. Cell Viability Assessment after MNP Labeling
2.6. Quantification of Cellular Iron Content
2.7. Prussian Blue Staining of Intracellular Iron
2.8. Transmission Electron Microscopy
2.9. Statistical Analysis
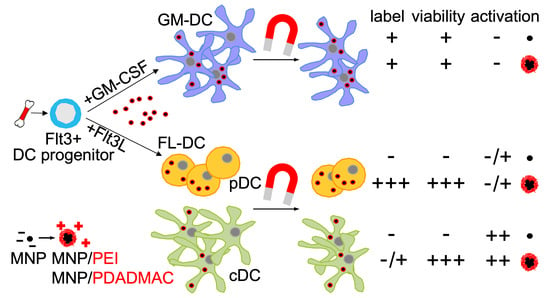
3. Results
3.1. Characterization of PE-Coated Ferumoxytol MNPs
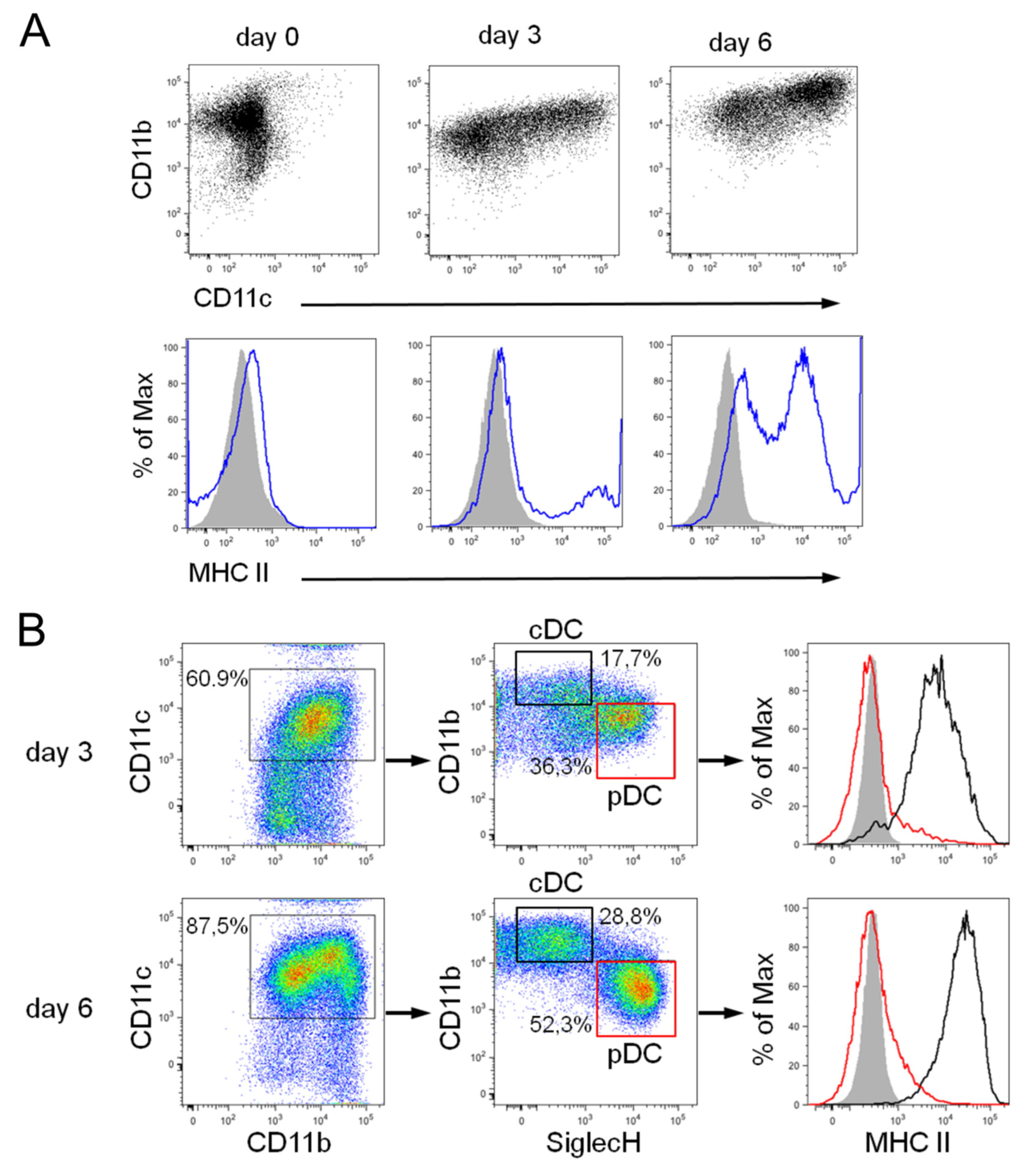
3.2. Differentiation of Steady-State and Inflammatory DC Subsets
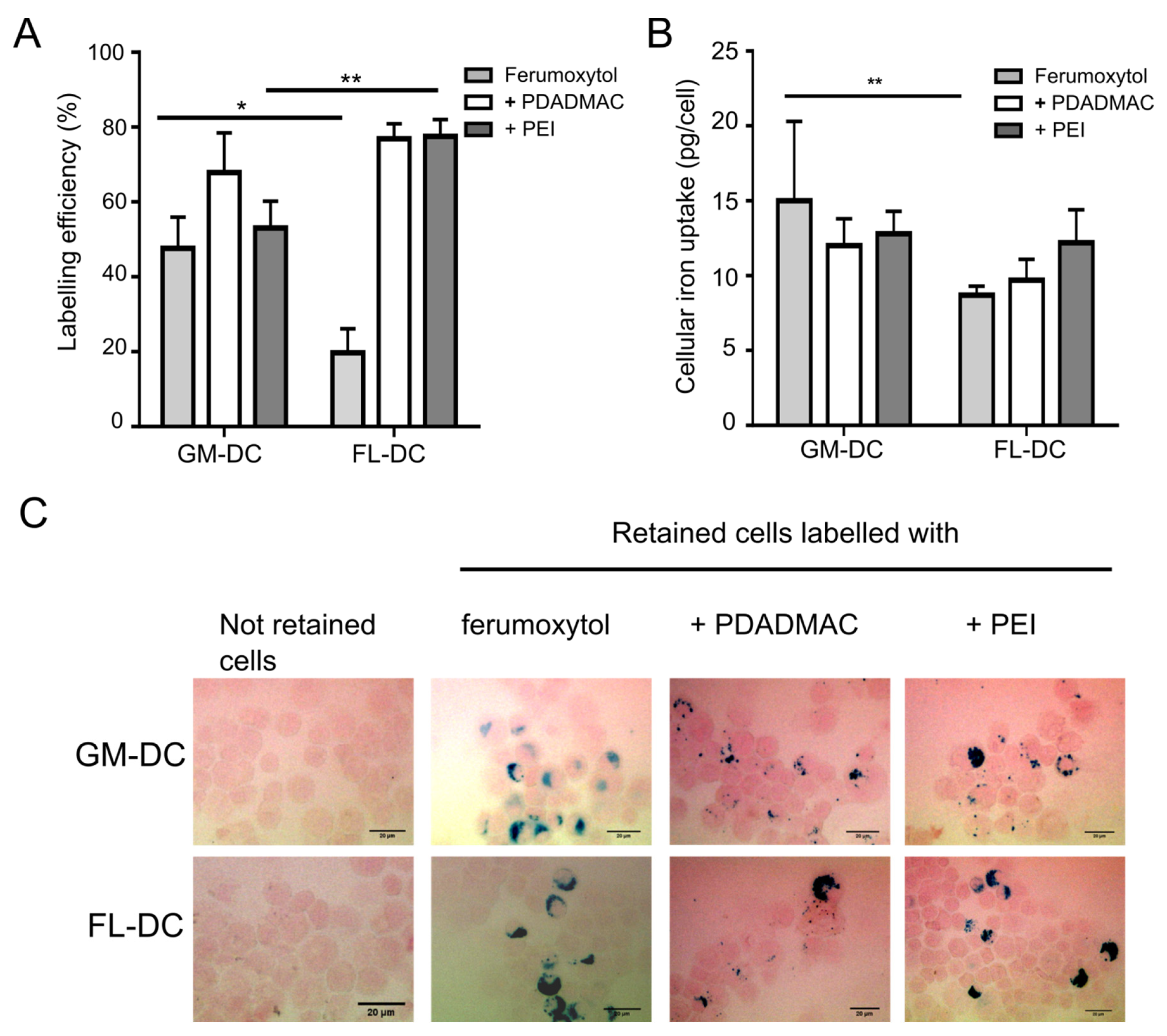
3.3. Labeling of DCs with Uncoated and PE-Coated Ferumoxytol
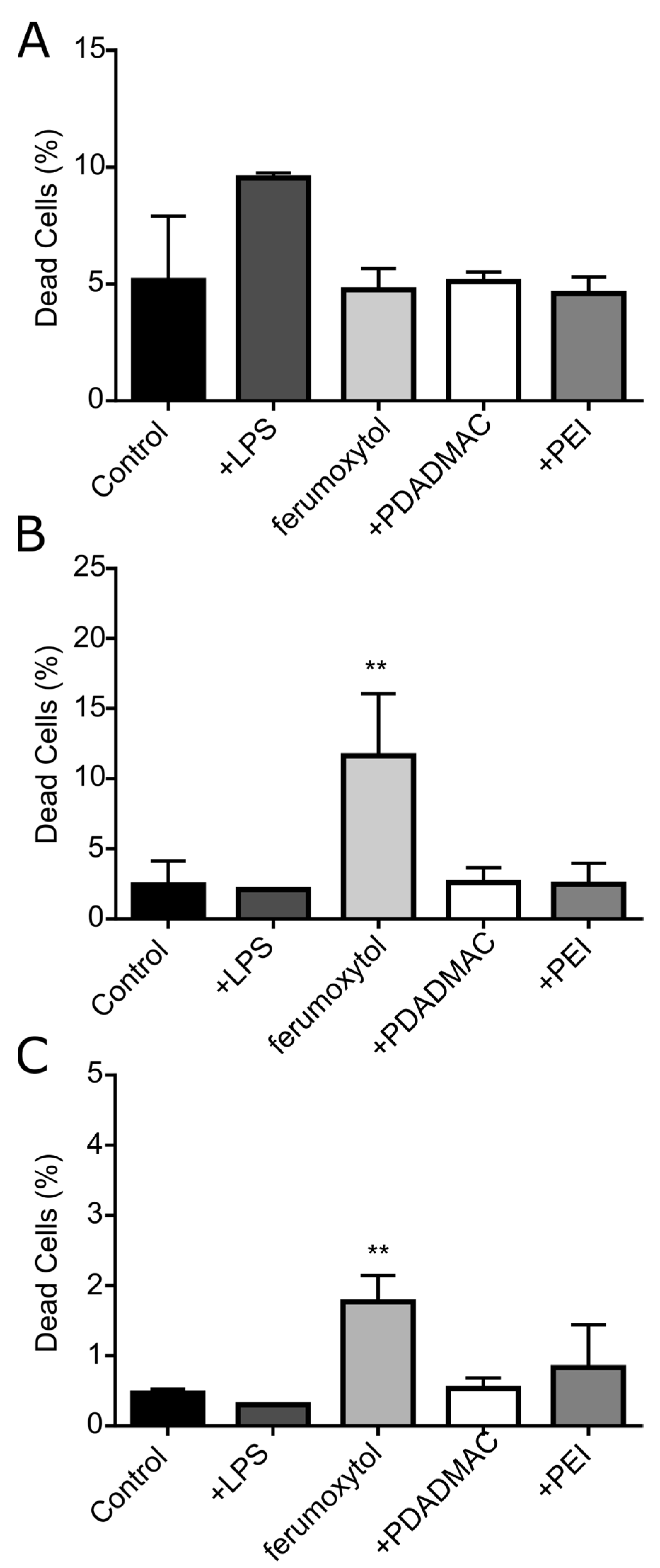
3.4. Cytotoxicity Assessment of Uncoated and PE-Coated MNPs upon Uptake into DCs
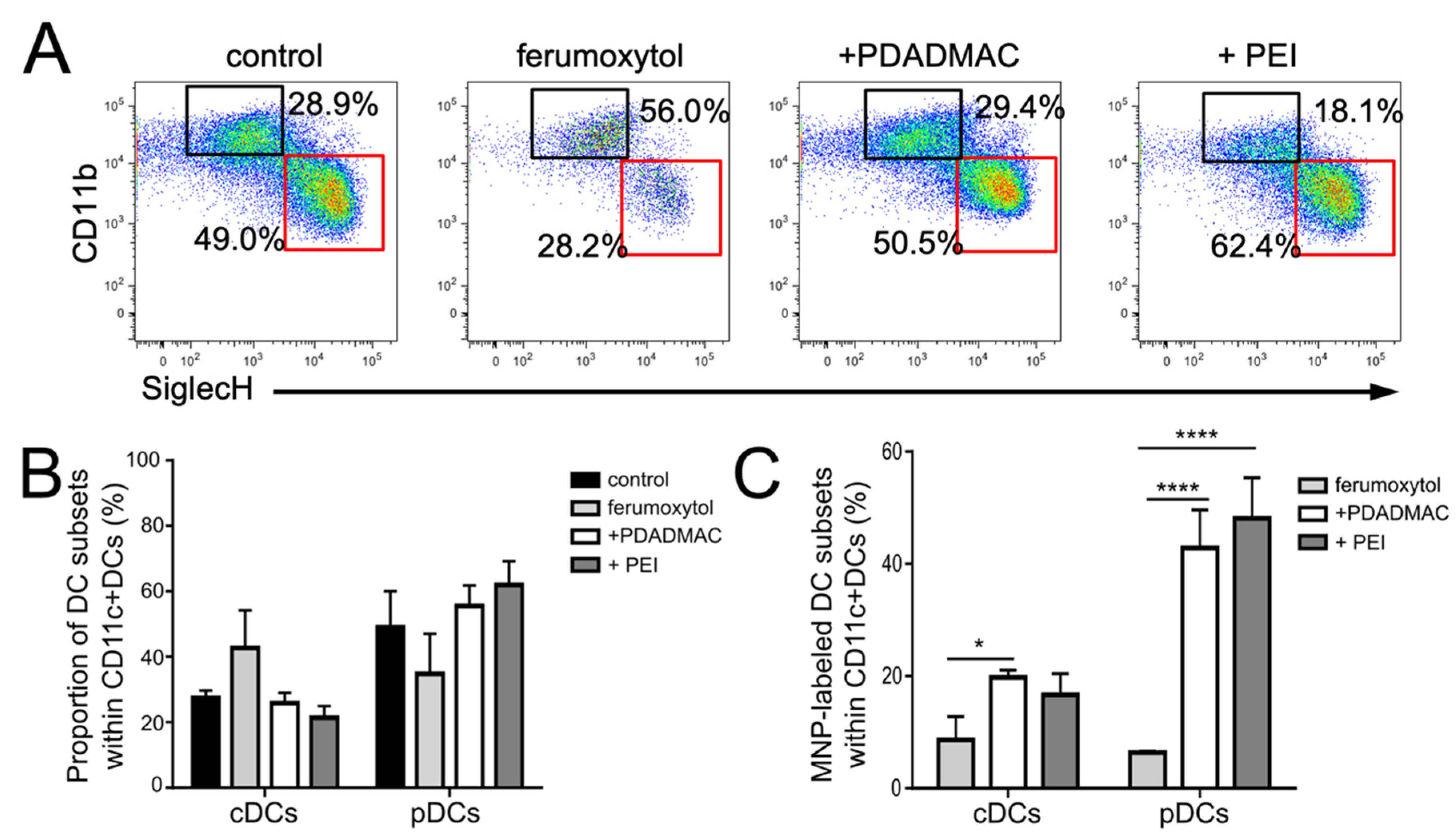
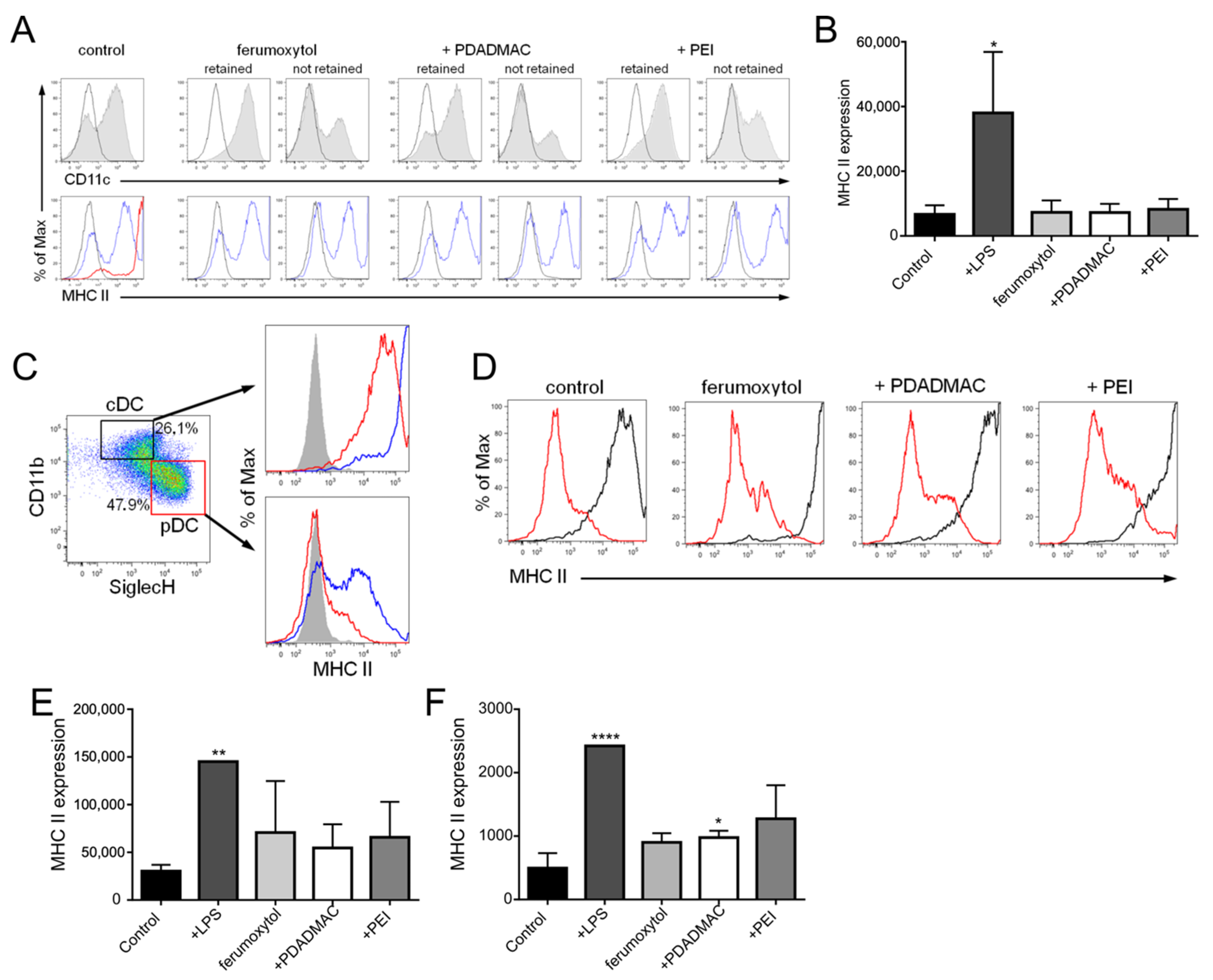
3.5. Immunophenotypic Differences in Steady-State and Inflammatory DCs upon MNP Labeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Beriou, G.; Moreau, A.; Cuturi, M.C. Tolerogenic dendritic cells: Applications for solid organ transplantation. Curr. Opin. Organ Transplant. 2012, 17, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, C.M.U.U.; Isaacs, J.D. Tolerogenic dendritic cell therapy for rheumatoid arthritis: Where are we now? Clin. Exp. Immunol. 2013, 172, 148–157. [Google Scholar] [CrossRef]
- Sprooten, J.; Ceusters, J.; Coosemans, A.; Agostinis, P.; de Vleeschouwer, S.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; Garg, A.D. Trial watch: Dendritic cell vaccination for cancer immunotherapy. Oncoimmunology 2019, 8, 1638212. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Bulte, J.W.M. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 2013, 13, 755–763. [Google Scholar] [CrossRef]
- de Vries, I.J.M.; Lesterhuis, W.J.; Barentsz, J.O.; Verdijk, P.; van Krieken, J.H.; Boerman, O.C.; Oyen, W.J.G.; Bonenkamp, J.J.; Boezeman, J.B.; Adema, G.J.; et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 2005, 23, 1407–1413. [Google Scholar] [CrossRef]
- Schwarz, S.; Fernandes, F.; Sanroman, L.; Hodenius, M.; Lang, C.; Himmelreich, U.; Schmitz-Rode, T.; Schueler, D.; Hoehn, M.; Zenke, M.; et al. Synthetic and biogenic magnetite nanoparticles for tracking of stem cells and dendritic cells. J. Magn. Magn. Mater. 2009, 321, 1533–1538. [Google Scholar] [CrossRef]
- Dekaban, G.A.; Hamilton, A.M.; Fink, C.A.; Au, B.; de Chickera, S.N.; Ribot, E.J.; Foster, P.J. Tracking and evaluation of dendritic cell migration by cellular magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 469–483. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, C.; Zhu, W.; Xia, C.; Wang, D.; Zhang, H.; Wu, J.; Lin, G.; Wu, B.; Gong, Q.; et al. Superparamagnetic MRI probes for in vivo tracking of dendritic cell migration with a clinical 3T scanner. Biomaterials 2015, 58, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, W.; Procissi, D.; Li, K.; Sheu, A.Y.; Gordon, A.C.; Guo, Y.; Khazaie, K.; Huan, Y.; Han, G. Antigen-loaded dendritic cell migration: MR imaging in a pancreatic carcinoma model. Radiology 2015, 274, 192–200. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Tranexamic acid. Drugs 2012, 72, 585–617. [Google Scholar] [CrossRef]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Muldoon, L.L.; Varallyay, C.; Markwardt, S.; Jones, R.E.; Neuwelt, E.A. In vivo leukocyte labeling with intravenous ferumoxides/protamine sulfate complex and in vitro characterization for cellular magnetic resonance imaging. Am. J. Physiol. Physiol. 2007, 293, C1698–C1708. [Google Scholar] [CrossRef]
- Celikkin, N.; Jakubcova, L.; Zenke, M.; Hoss, M.; Wong, J.E.J.E.; Hieronymus, T.; Jakubcová, L.; Zenke, M.; Hoss, M.; Wong, J.E.J.E.; et al. Polyelectrolyte coating of ferumoxytol nanoparticles for labeling of dendritic cells. J. Magn. Magn. Mater. 2015, 380, 39–45. [Google Scholar] [CrossRef]
- Shortman, K.; Naik, S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007, 7, 19–30. [Google Scholar] [CrossRef]
- Schwarz, S.; Wong, J.E.; Bornemann, J.; Hodenius, M.; Himmelreich, U.; Richtering, W.; Hoehn, M.; Zenke, M.; Hieronymus, T. Polyelectrolyte coating of iron oxide nanoparticles for MRI-based cell tracking. Nanomedicine 2012, 8, 682–691. [Google Scholar] [CrossRef]
- Wong, J.E.; Richtering, W. Surface Modification of Thermoresponsive Microgels via Layer-by-Layer Assembly of Polyelectrolyte Multilayers. Progr. Colloid Polym. Sci. 2006, 133, 45–51. [Google Scholar]
- Wong, J.E.; Gaharwar, A.K.; Müller-Schulte, D.; Bahadur, D.; Richtering, W.; Gaharwar, A.K.; Müller-Schulte, D.; Bahadur, D.; Richtering, W. Layer-by-layer assembly of a magnetic nanoparticle shell on a thermoresponsive microgel core. J. Magn. Magn. Mater. 2007, 311, 219–223. [Google Scholar] [CrossRef]
- Hieronymus, T.; Gust, T.C.; Kirsch, R.D.; Jorgas, T.; Blendinger, G.; Goncharenko, M.; Supplitt, K.; Rose-John, S.; Müller, A.M.; Zenke, M.; et al. Progressive and Controlled Development of Mouse Dendritic Cells from Flt3+CD11b+ Progenitors In Vitro. J. Immunol. 2005, 174, 2552–2562. [Google Scholar] [CrossRef] [PubMed]
- Felker, P.; Seré, K.; Lin, Q.; Becker, C.; Hristov, M.; Hieronymus, T.; Zenke, M. TGF-beta1 accelerates dendritic cell differentiation from common dendritic cell progenitors and directs subset specification toward conventional dendritic cells. J. Immunol. 2010, 185, 5326–5335. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.A.; Kingston, D.; Boddupalli, S.; Manz, M.G. Instructive cytokine signals in dendritic cell lineage commitment. Immunol. Rev. 2010, 234, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.H.; Sathe, P.; Park, H.-Y.; Metcalf, D.; Proietto, A.I.; Dakic, A.; Carotta, S.; O’Keeffe, M.; Bahlo, M.; Papenfuss, A. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007, 8, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Onai, N.; Obata-Onai, A.; Schmid, M.A.; Ohteki, T.; Jarrossay, D.; Manz, M.G. Identification of clonogenic common Flt3+ M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007, 8, 1207–1216. [Google Scholar] [CrossRef]
- Merad, M.; Manz, M.G. Dendritic cell homeostasis. Blood J. Am. Soc. Hematol. 2009, 113, 3418–3427. [Google Scholar]
- Thu, M.S.; Bryant, L.H.; Coppola, T.; Jordan, E.K.; Budde, M.D.; Lewis, B.K.; Chaudhry, A.; Ren, J.; Varma, N.R.S.; Arbab, A.S.; et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat. Med. 2012, 18, 463–467. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Khurana, A.; Nejadnik, H.; Chapelin, F.; Lenkov, O.; Gawande, R.; Lee, S.; Gupta, S.N.; Aflakian, N.; Derugin, N.; Messing, S. Ferumoxytol: A new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine 2013, 8, 1969–1983. [Google Scholar] [CrossRef]
- Moon, H.; Park, H.E.; Kang, J.; Lee, H.; Cheong, C.; Lim, Y.T.; Ihm, S.-H.; Seung, K.-B.; Jaffer, F.A.; Narula, J. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation 2012, 125, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Stirrat, C.G.; Alam, S.R.; MacGillivray, T.J.; Gray, C.D.; Dweck, M.R.; Dibb, K.; Spath, N.; Payne, J.R.; Prasad, S.K.; Gardner, R.S. Ferumoxytol-enhanced magnetic resonance imaging in acute myocarditis. Heart 2018, 104, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.H.K.; Heger, L.; Heidkamp, G.F.; Baranska, A.; Lühr, J.J.; Hoffmann, A.; Dudziak, D. Direct delivery of antigens to dendritic cells via antibodies specific for endocytic receptors as a promising strategy for future therapies. Vaccines 2016, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Shao, K.; Ellestad, K.K.; Yang, Y.; Santamaria, P. Nanoparticles for immune stimulation against infection, cancer, and autoimmunity. ACS Nano 2018, 12, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Noh, Y.-W.; Kang, T.H.; Kim, J.-E.; Kim, S.; Um, S.H.; Oh, D.-B.; Park, Y.-M.; Lim, Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials 2017, 130, 56–66. [Google Scholar] [CrossRef]
- Bookstaver, M.L.; Hess, K.L.; Jewell, C.M. Self-assembly of immune signals improves codelivery to antigen presenting cells and accelerates signal internalization, processing kinetics, and immune activation. Small 2018, 14, 1802202. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celikkin, N.; Wong, J.E.; Zenke, M.; Hieronymus, T. Polyelectrolyte Coating of Ferumoxytol Differentially Impacts the Labeling of Inflammatory and Steady-State Dendritic Cell Subtypes. Biomedicines 2022, 10, 3137. https://doi.org/10.3390/biomedicines10123137
Celikkin N, Wong JE, Zenke M, Hieronymus T. Polyelectrolyte Coating of Ferumoxytol Differentially Impacts the Labeling of Inflammatory and Steady-State Dendritic Cell Subtypes. Biomedicines. 2022; 10(12):3137. https://doi.org/10.3390/biomedicines10123137
Chicago/Turabian StyleCelikkin, Nehar, John E. Wong, Martin Zenke, and Thomas Hieronymus. 2022. "Polyelectrolyte Coating of Ferumoxytol Differentially Impacts the Labeling of Inflammatory and Steady-State Dendritic Cell Subtypes" Biomedicines 10, no. 12: 3137. https://doi.org/10.3390/biomedicines10123137
APA StyleCelikkin, N., Wong, J. E., Zenke, M., & Hieronymus, T. (2022). Polyelectrolyte Coating of Ferumoxytol Differentially Impacts the Labeling of Inflammatory and Steady-State Dendritic Cell Subtypes. Biomedicines, 10(12), 3137. https://doi.org/10.3390/biomedicines10123137