Pixel-Level Clustering of Hematoxylin–Eosin-Stained Sections of Mouse and Human Biliary Tract Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse BTC Tissue Images
2.2. Human Cholangiocarcinoma Tissue Images
2.3. Clustering-Based Analysis of H&E Images
2.4. Entropy
3. Results
3.1. Image Comparison for Mouse BTC Tumor and Human Cholangiocarcinoma Tissue
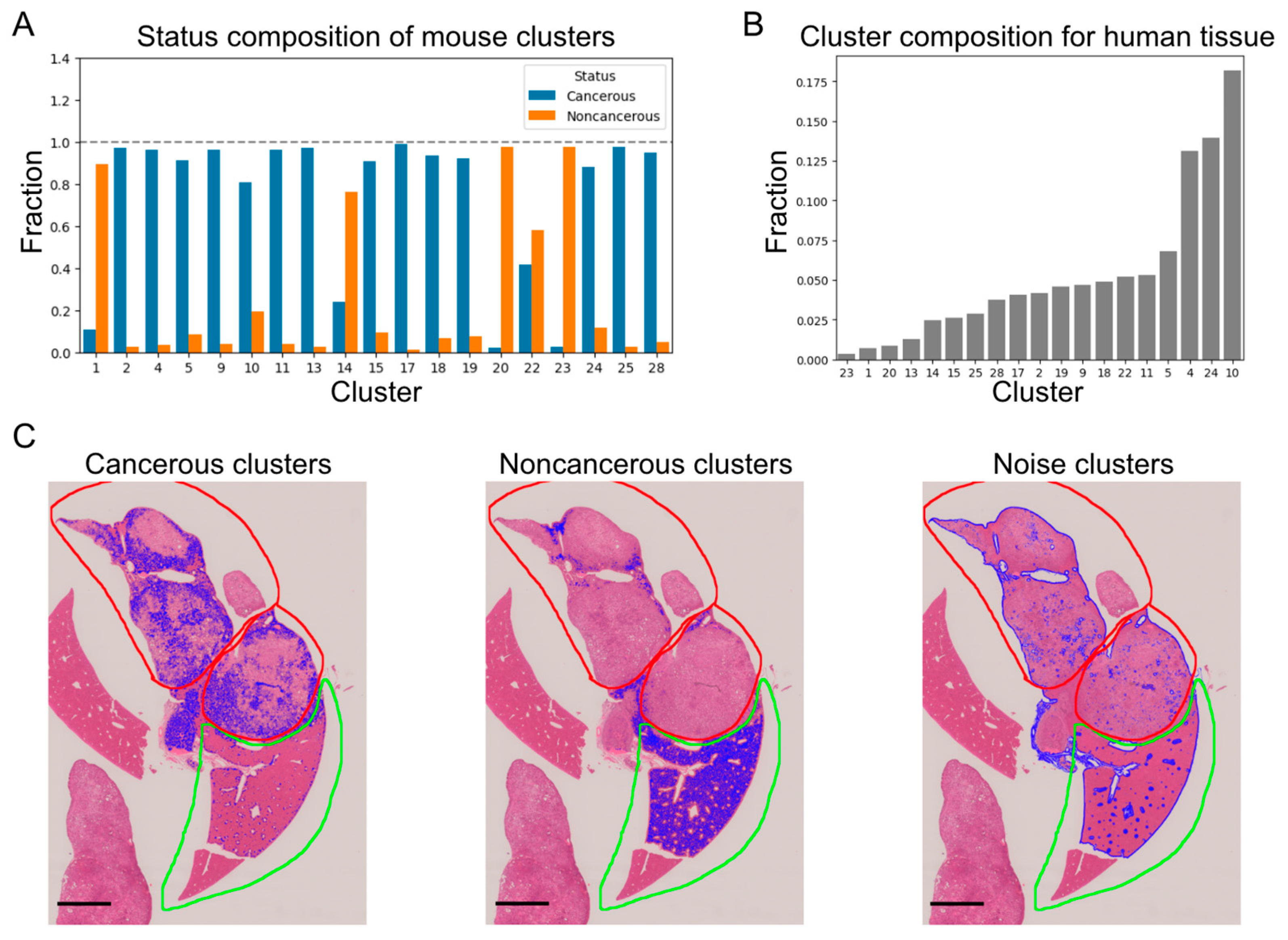
3.2. Pixel-Level Clustering
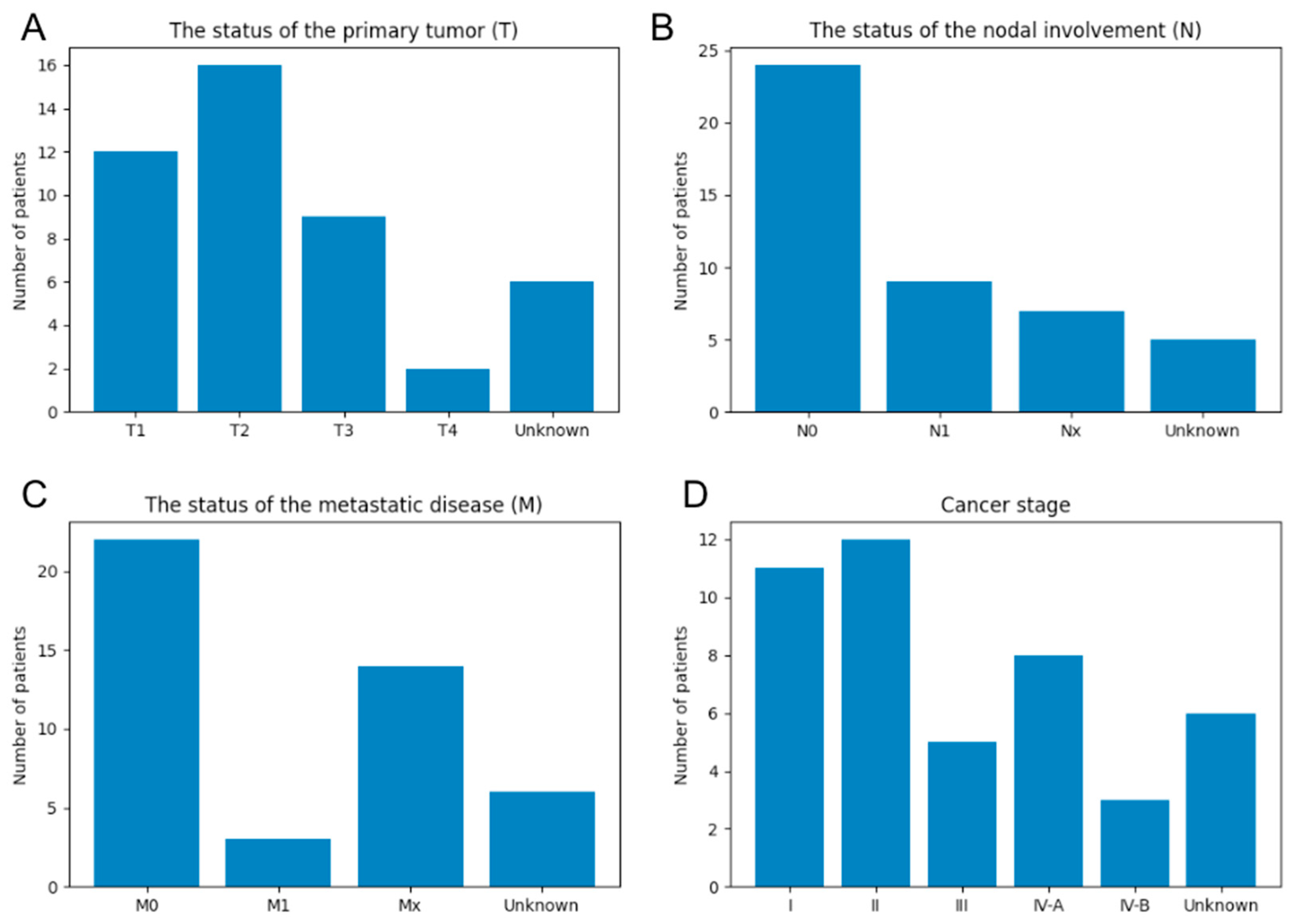
3.3. Relation between Image Features and Clinical Information
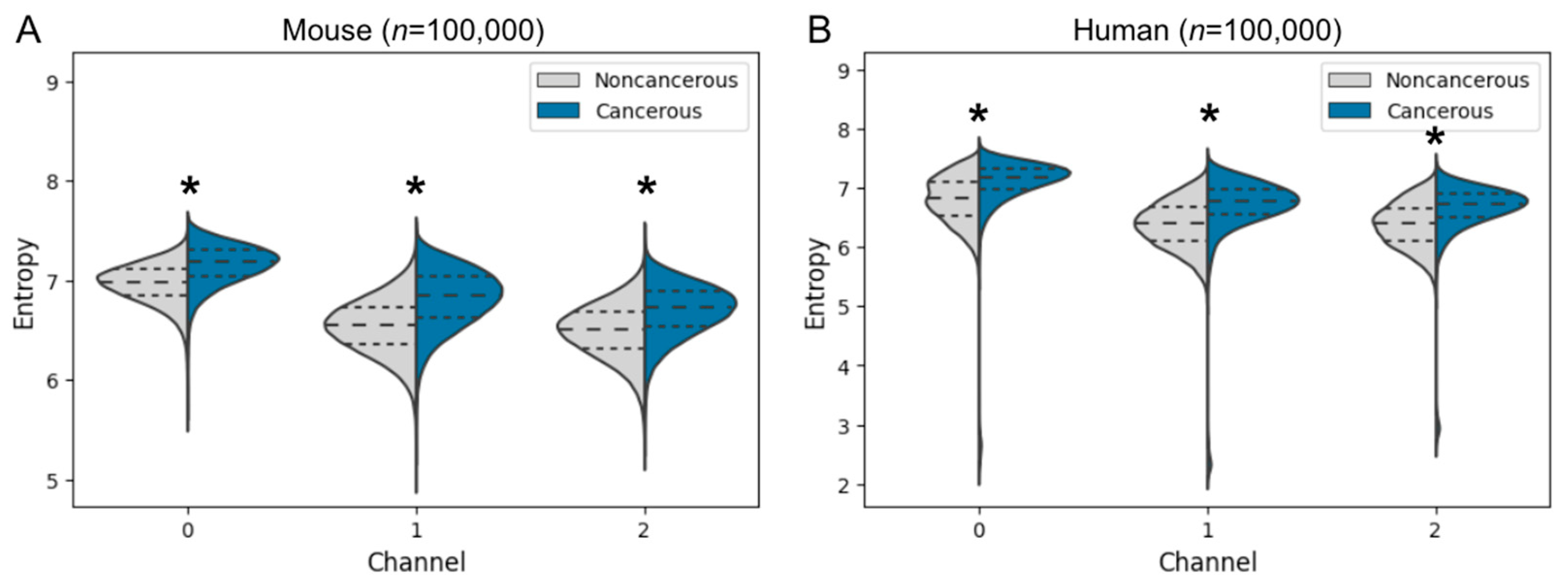
3.3.1. Entropy Comparison of Cancer and Noncancer Regions in Mice and Humans
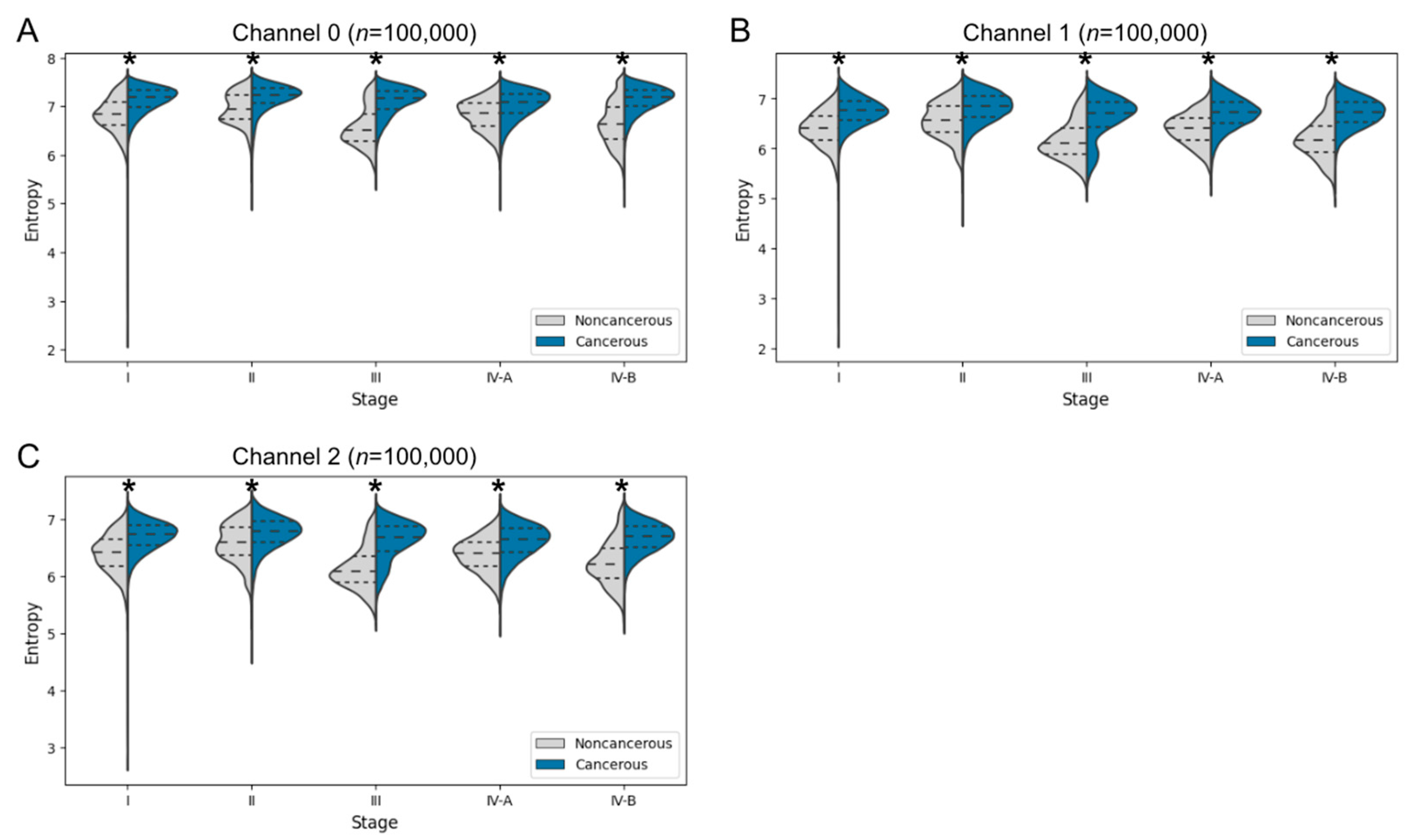
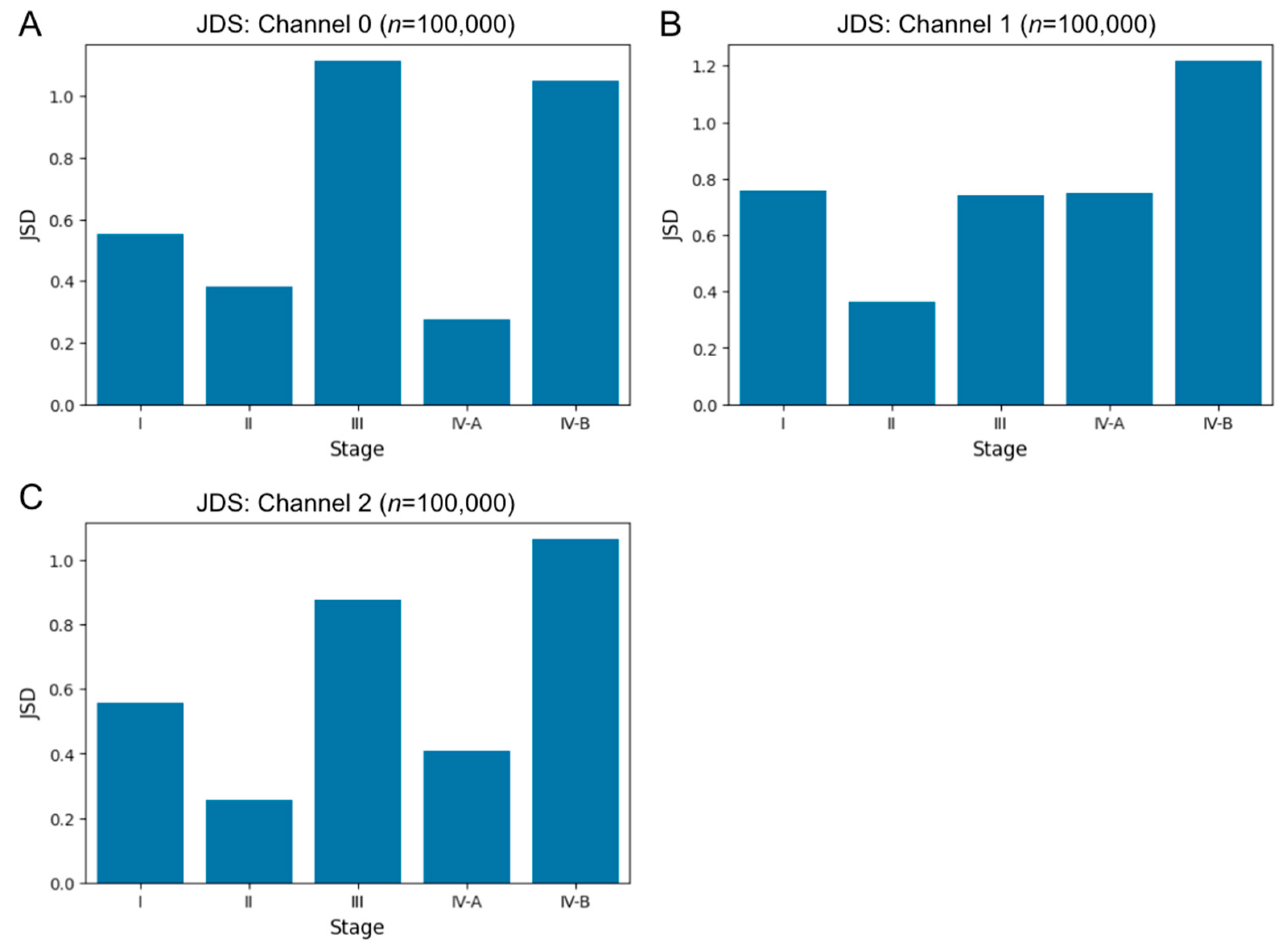
3.3.2. Entropy Comparison of Cancer and Noncancer Regions at Each Cancer Stage in Human Specimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Patel, T. Worldwide Trends in Mortality from Biliary Tract Malignancies. BMC Cancer 2002, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination Gemcitabine plus S-1 versus Gemcitabine plus Cisplatin for Advanced/Recurrent Biliary Tract Cancer: The FUGA-BT (JCOG1113) Randomized Phase III Clinical Trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized Phase III Study of Gemcitabine, Cisplatin plus S-1 versus Gemcitabine, Cisplatin for Advanced Biliary Tract Cancer (KHBO1401-MITSUBA). J. Hepato-Biliary Pancreat. Sci. 2022. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board and International Agency for Research on Cancer. Digestive System Tumours. In World Health Organization Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Kanel, G.C.; Korula, J. Atlas of Liver Pathology. In Atlases in Diagnostic Surgical Pathology, 2nd ed.; Elsevier Saunders: Philadelphia, PA, USA, 2005. [Google Scholar]
- Nakanuma, Y.; Uesaka, K.; Kakuda, Y.; Sugino, T.; Kubota, K.; Furukawa, T.; Fukumura, Y.; Isayama, H.; Terada, T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. J. Clin. Med. 2020, 9, 3991. [Google Scholar] [CrossRef]
- Sugihara, E.; Saya, H. Complexity of Cancer Stem Cells. Int. J. Cancer 2013, 132, 1249–1259. [Google Scholar] [CrossRef]
- Semba, T.; Sato, R.; Kasuga, A.; Suina, K.; Shibata, T.; Kohno, T.; Suzuki, M.; Saya, H.; Arima, Y. Lung Adenocarcinoma Mouse Models Based on Orthotopic Transplantation of Syngeneic Tumor-Initiating Cells Expressing EpCAM, SCA-1, and Ly6d. Cancers 2020, 12, 3805. [Google Scholar] [CrossRef]
- Kasuga, A.; Semba, T.; Sato, R.; Nobusue, H.; Sugihara, E.; Takaishi, H.; Kanai, T.; Saya, H.; Arima, Y. Oncogenic KRAS-Expressing Organoids with Biliary Epithelial Stem Cell Properties Give Rise to Biliary Tract Cancer in Mice. Cancer Sci. 2021, 112, 1822–1838. [Google Scholar] [CrossRef]
- Abdelsamea, M.M.; Zidan, U.; Senousy, Z.; Gaber, M.M.; Rakha, E.; Ilyas, M. A Survey on Artificial Intelligence in Histopathology Image Analysis. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2022, 12, e1474. [Google Scholar] [CrossRef]
- Chan, L.; Hosseini, M.; Rowsell, C.; Plataniotis, K.; Damaskinos, S. HistoSegNet: Semantic Segmentation of Histological Tissue Type in Whole Slide Images. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision, Seoul, Republic of Korea, 27 October–2 November 2019; pp. 10661–10670. [Google Scholar] [CrossRef]
- Rakhlin, A.; Shvets, A.; Iglovikov, V.; Kalinin, A.A. Deep Convolutional Neural Networks for Breast Cancer Histology Image Analysis. BioRxiv 2018. [Google Scholar] [CrossRef]
- van der Walt, S.; Schönberger, J.L.; Nunez-Iglesias, J.; Boulogne, F.; Warner, J.D.; Yager, N.; Gouillart, E.; Yu, T. Contributors, scikit-image Scikit-Image: Image Processing in Python. PeerJ 2014, 2, e453. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Carloni, R.; Rizzo, A.; Ricci, A.D.; Federico, A.D.; Luca, R.D.; Guven, D.C.; Yalcin, S.; Brandi, G. Targeting Tumor Microenvironment for Cholangiocarcinoma: Opportunities for Precision Medicine. Transl. Oncol. 2022, 25, 101514. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Kashyap, A.; Rapsomaniki, M.A.; Barros, V.; Fomitcheva-Khartchenko, A.; Martinelli, A.L.; Rodriguez, A.F.; Gabrani, M.; Rosen-Zvi, M.; Kaigala, G. Quantification of Tumor Heterogeneity: From Data Acquisition to Metric Generation. Trends Biotechnol. 2021, 40, 647–676. [Google Scholar] [CrossRef]
- Kullback, S.; Leibler, R.A. On Information and Sufficiency. Ann. Math. Stat. 1951, 22, 79–86. [Google Scholar] [CrossRef]
- Lin, J. Divergence Measures Based on the Shannon Entropy. IEEE Trans. Inf. Theory 1991, 37, 145–151. [Google Scholar] [CrossRef]
- Heindl, A.; Nawaz, S.; Yuan, Y. Mapping Spatial Heterogeneity in the Tumor Microenvironment: A New Era for Digital Pathology. Lab. Investig. 2015, 95, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study of Durvalumab in Combination with Gemcitabine plus Cisplatin (GemCis) in Patients (Pts) with Advanced Biliary Tract Cancer (BTC): TOPAZ-1. J. Clin. Orthod. 2022, 40, 378. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Ogawa, S. Clonal Expansion in Non-Cancer Tissues. Nat. Rev. Cancer 2021, 21, 239–256. [Google Scholar] [CrossRef]
- Cho, J.H.; Mall, U.; Bala, K.; Hariharan, B. PiCIE: Unsupervised Semantic Segmentation Using Invariance and Equivariance in Clustering. arXiv 2021, arXiv:2103.17070. [Google Scholar] [CrossRef]
- Dahal, P. Learning Embedding Space for Clustering From Deep Representations. In Proceedings of the 2018 IEEE International Conference on Big Data (Big Data), Seattle, WA, USA, 10–13 December 2018; pp. 3747–3755. [Google Scholar] [CrossRef]
- Caron, M.; Bojanowski, P.; Joulin, A.; Douze, M. Deep Clustering for Unsupervised Learning of Visual Features. arXiv 2018, arXiv:1807.05520. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, H.; Zheng, Z.; Chen, J.; Li, Z.; Bu, J.; Wu, J.; Wang, X.; Zhu, W.; Ester, M. A Comprehensive Survey on Deep Clustering: Taxonomy, Challenges, and Future Directions. arXiv 2022, arXiv:2206.07579. [Google Scholar] [CrossRef]
- Liu, C.C.; Greenwald, N.F.; Kong, A.; McCaffrey, E.F.; Leow, K.X.; Mrdjen, D.; Angelo, M. Robust Phenotyping of Highly Multiplexed Tissue Imaging Data Using Pixel-Level Clustering. BioRxiv 2022. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Fang, X.; Chen, S.; Zhang, X. Pixel-Level Clustering Reveals Intra-Tumor Heterogeneity in Non-Small Cell Lung Cancer. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 1536–1539. [Google Scholar] [CrossRef]
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S.; et al. An Image Is Worth 16x16 Words: Transformers for Image Recognition at Scale. arXiv 2020, arXiv:2010.11929. [Google Scholar] [CrossRef]
- Li, K.; Wang, Z.; Cheng, Z.; Yu, R.; Zhao, Y.; Song, G.; Yuan, L.; Chen, J. Dynamic Clustering Network for Unsupervised Semantic Segmentation. arXiv 2022, arXiv:2210.5944. [Google Scholar]


| Parameter | Value |
|---|---|
| Intensity | True |
| Edges | False |
| Sigma_min | 3 |
| Sigma_max | 7 |
| Multichannel | True |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, H.; Aimono, E.; Kasuga, A.; Tanaka, H.; Iwasaki, A.; Saya, H.; Arima, Y. Pixel-Level Clustering of Hematoxylin–Eosin-Stained Sections of Mouse and Human Biliary Tract Cancer. Biomedicines 2022, 10, 3133. https://doi.org/10.3390/biomedicines10123133
Inoue H, Aimono E, Kasuga A, Tanaka H, Iwasaki A, Saya H, Arima Y. Pixel-Level Clustering of Hematoxylin–Eosin-Stained Sections of Mouse and Human Biliary Tract Cancer. Biomedicines. 2022; 10(12):3133. https://doi.org/10.3390/biomedicines10123133
Chicago/Turabian StyleInoue, Haruki, Eriko Aimono, Akiyoshi Kasuga, Haruto Tanaka, Aika Iwasaki, Hideyuki Saya, and Yoshimi Arima. 2022. "Pixel-Level Clustering of Hematoxylin–Eosin-Stained Sections of Mouse and Human Biliary Tract Cancer" Biomedicines 10, no. 12: 3133. https://doi.org/10.3390/biomedicines10123133
APA StyleInoue, H., Aimono, E., Kasuga, A., Tanaka, H., Iwasaki, A., Saya, H., & Arima, Y. (2022). Pixel-Level Clustering of Hematoxylin–Eosin-Stained Sections of Mouse and Human Biliary Tract Cancer. Biomedicines, 10(12), 3133. https://doi.org/10.3390/biomedicines10123133






