Abstract
Background: Lafora disease (LD) is a neurodegenerative condition characterized by the accumulation of polyglucosan bodies (PBs) throughout the brain. Alongside metabolic and molecular alterations, neuroinflammation has emerged as another key histopathological feature of LD. Methods: To investigate the role of astrocytes and microglia in LD, we performed a systematic review according to the PRISMA statement. PubMed, Scopus, and Web-of-Science database searches were performed independently by two reviewers. Results: Thirty-five studies analyzing the relationship of astrocytes and microglia with LD and/or the effects of anti-inflammatory treatments in LD animal models were identified and included in the review. Although LD has long been dominated by a neuronocentric view, a growing body of evidence suggests a role of glial cells in the disease, starting with the finding that these cells accumulate PBs. We discuss the potential meaning of glial PB accumulations, the likely factors activating glial cells, and the possible contribution of glial cells to LD neurodegeneration and epilepsy. Conclusions: Given the evidence for the role of neuroinflammation in LD, future studies should consider glial cells as a potential therapeutic target for modifying/delaying LD progression; however, it should be kept in mind that these cells can potentially assume multiple reactive phenotypes, which could influence the therapeutic response.
1. Introduction
Lafora disease (LD) is a severe neurodegenerative condition belonging to the group of progressive myoclonic epilepsies (PME). It is a rare genetic disorder linked to mutations in the EPM2A [1] and EPM2B genes [2], which respectively encode laforin and malin, components of a functional complex [3]. LD’s histopathological hallmark is the accumulation of polyglucosan bodies (PBs) scattered throughout the brain [4,5]. Despite numerous advances, the pathogenesis of LD remains unclear, and the functions of laforin and malin and their role in neurodegeneration are only partially understood. In addition to alterations in glycogen metabolism, animal models of LD revealed alterations in several metabolic and molecular pathways [6,7] that may influence each other and play a role in the disease [6,7]. These include, in particular, the alteration of autophagy [8,9,10,11,12,13,14] and the ubiquitin–proteasome system [15,16,17], the impairment of the heat shock response [16,18], increased oxidative stress, and mitochondrial dysfunction [19,20].
In the recent years, neuroinflammation has also emerged as a key histopathological feature in animal models of LD [14,21,22,23]. Although, neuroinflammation begins as a defensive response [24], sustained inflammatory responses that involve microglia and astrocytes are detrimental and can contribute to neurodegeneration [25,26]. Astrocytes and microglia perform numerous housekeeping functions in the brain [27]. However, these cells also show highly variable phenotypes, depending on the environmental stimuli received and the brain region in which they are located, and in certain situations they can therefore become a source of damage. Neurotoxic and protective phenotypes have been documented in each of these cell types [25,28]; this distinction, however, is now thought to be too simplistic, as both microglial and astrocytic cells can assume multiple reactive phenotypes, even within the same disease [29,30]. Proinflammatory microglia can exert their damaging effects by producing many inflammatory mediators, including tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), nitric oxide synthase (NOS), and reactive oxygen species (ROS) [31]. Furthermore, they upregulate several lipid-metabolism-related genes, phagosome-related genes, and cathepsins [32] and downregulate genes expressed by homeostatic microglia [33]. Some authors have referred to these CNS-damaging microglia, present in many neurodegenerative diseases, as ‘disease-associated microglia’ (DAM) [34,35]. On the contrary, protective microglia produce anti-inflammatory cytokines such as interleukins 4, 10, and 13 (IL-4, IL-10, and IL-13) and transforming growth factor beta (TGF)-β); upregulate markers such as arginase-1 (ARG-1), found in inflammatory zone 1 (FIZZ-1), CD206 pattern recognition receptor, also known as mannose receptor C type 1, and chitinase 3-like 3 (YM-1) phagocytosis genes [32]; and promote repair mechanisms [31]. Similar to these microglia, proinflammatory reactive astrocytes lose their homeostatic functions, in this case upregulating genes such as glial fibrillary acidic protein (GFAP) and complement cascade genes and releasing proinflammatory factors (e.g., IL-1β, TNF-α, and NO), damaging nervous tissue [28,36]. In contrast, neuroprotective astrocytes upregulate many neurotrophic factors and thrombospondins [36] and can release anti-inflammatory cytokines such as IL-4, IL-10, and TGF-β [37].
In this review we systematically examined studies investigating the roles of glial cells in neuroinflammation in LD. Several studies have claimed a contribution of astrocytes and microglia in LD, starting with the evidence that these cells also accumulate PBs [38,39,40,41]. With these premises, we tried to understand the roles of astrocytes and microglia in LD, the significance of PB accumulations in glial cells, potential triggers that activate astrocytes and microglia, and the possible contribution of glia to neurodegeneration and to the epileptic phenotype of LD.
2. Materials and Methods
A systematic literature review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [42]. The PubMed, Scopus, and Web of Science databases were searched from their start date to October 2022 (12 October 2022). The reference lists of all included studies were also searched for additional relevant citations. The authors used the following search strategy: (Lafora disease) AND ((glia) OR (microglia) OR (astrocyt*) OR (gliosis) OR (cytokin*) OR (inflammat*)). The search included only original studies. We used the research tool “Zotero” (https://www.zotero.org/download/, accessed on 12 October 2022) to collect all the results in a single library. Abstracts were retrieved using our search strategy, and duplicates were removed. The full text of all potentially eligible articles and their Supplementary Information were obtained and independently assessed by two authors (S.D.V. and M.M.). We resolved any ambiguities about eligibility through discussion. Studies were included if they reported information on (1) astrocytes, microglia, and/or neuroinflammation in LD or (2) the effects of therapeutic strategies on astrocytes, microglia, and/or neuroinflammation in LD. The exclusion criteria were as follows: (a) duplicates; (b) studies that did not relate to the objective of the article; (c) articles written in a language other than English or Italian; (d) reviews and meeting and workshop abstracts; and (e) books. The data were extracted manually by two authors and were summarized in tables. Extrapolated data concerned the neuropathological features (the presence or absence of LBs, microgliosis, astrogliosis, and inflammatory cytokines) and the phenotypical features (the presence or absence of epileptic manifestations, motor impairment, and cognitive impairment) of animal models of LD. Whenever possible, the same data were analyzed at various developmental stages and after any therapeutic interventions. We extracted data on the type of treatment (the inhibition of glycogen synthesis or alternative strategies), the start and duration of treatment, the treatment effect on neuropathological features (LBs and neuroinflammation), and clinical features (epileptic manifestations, motor impairment, and cognitive impairment). Data on the start and duration of treatment were described as continuous variables, and those on the treatment effect were described as categorical variables (neuropathological features: no effect, rescue, reduction, or increase; epileptic manifestations: no effect, rescue, reduction, or increase; and motor and cognitive impairment: no effect, improved, or worsened). The studies were grouped in tables according to the main topics covered: the presence or absence of neuroinflammation at different stages of the disease and the neuropathological and clinical effects of treatments inhibiting glycogen synthesis or alternative strategies. For the narrative review, the following main topic paragraphs were identified: PB accumulation in glial cells, neuroinflammation in animal models of LD, glial cell activation factors in LD, and the contribution of glial cells to neurodegeneration and the epileptic phenotype of the disease. The PRISMA checklist can be found in Supplementary Material. The systematic review protocol was registered on PROSPERO with the following code: 367094.
3. Results
3.1. Database Search Process
The PRISMA flow chart of the review process is presented in Figure 1. The search on PubMed, Scopus, and Web of Science provided a total of 176 records. After the correction of duplicates, 101 records remained. Of these, 39 were excluded because they dealt with a topic different than the topic of interest, 4 were excluded because they were written in a language other than English or Italian, a single manuscript could not be found, 26 were excluded because they were reviews, meeting abstracts, or editorial comments, and 1 was excluded because it was a book. Five additional studies were selected by checking the references of the identified relevant papers. A total of 35 studies were then identified for inclusion in the current review [10,12,14,18,21,22,23,38,39,40,41,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
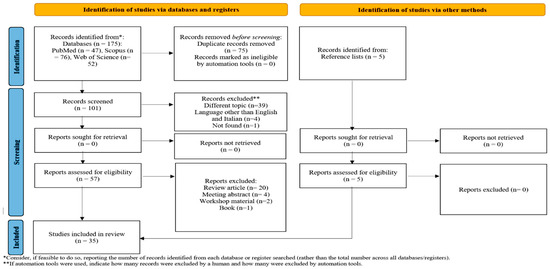
Figure 1.
PRISMA flow chart of the review process.
3.2. Identification of PBs in Glial Cells
The PBs found in LD have long been assumed to accumulate only in neurons [10], which seems paradoxical given that glycogen metabolism in the brain occurs mainly in glial cells [67]. In fact, postmortem histopathological studies on the brains of individuals [45,48] and dogs with LD [55,57] have shown the presence of PBs in both neurons and glial cells, including astrocytes [48], as well as some PBs in extracellular locations [48]. Using a malin-deficient mouse model (Epm2b−/−), Valles-Ortega and colleagues [41] showed the presence of PBs in both astrocytes and neurons and that astrocytic PBs form earlier than the neuronal ones. However, while neurons degenerate as they accumulate PBs, astrocytes remain viable, suggesting that neurons are more sensitive than astrocytes to cell death induced by glycogen overload [68]. A subsequent study performed in Epm2a−/− and Epm2b−/− mice confirmed that PBs colocalize in neurons, astrocytes, and microglial cells [40] and that the formation of astrocytic PBs precedes that of neuronal PBs, as is the case of other glycogen storage diseases [69]. Interestingly, the PBs that accumulate in astrocytes are different from those found in neurons [38]. Research in malin KO mice has identified corpora-amylacea-like bodies (CALs), which are different from the neuronal Lafora bodies (nLBs) found in astrocytes; however, glycogen synthesis is required for the formation of both [70]. These two types of PBs differ in both location (astrocytic CALs are found predominantly in the hippocampus, while nLBs are mainly in the cerebral cortex) and constitution [38]. In particular, CALs, unlike nLBs, contain neo-epitopes recognized by natural IgM [38], which suggests that they may be eliminated by mechanisms of natural immunity, in particular by macrophages, after being extruded from the CNS or in the CNS itself in the case of blood–brain-barrier disruption [71]. The protein p62, which plays a role in secretory autophagy, seems to be involved in this elimination process [38]. Furthermore, this protein also seems to be important for PB formation; in fact, its deletion in malin KO mice altered the morphology of brain PBs, making them more toxic and worsening neuroinflammation and susceptibility to kainate-induced epilepsy [39].
3.3. Neuroinflammation as a New Hallmark of LD
Alongside the accumulation of PBs in both neuronal and glial cells, neuroinflammation has emerged as another key histopathological feature in LD animal models [21,22]. It appears in the very early stages and worsens over time, which suggests a role in the progression of the disease [21,22]. Most of the available studies on this topic have been conducted in mouse models and investigated neuroinflammation through immunostaining studies, in order to investigate astrogliosis and microgliosis, and through the analysis of mRNA and protein levels of inflammatory mediators [21,22]. A transcriptomic analysis on the brains of laforin- and malin-deficient mice at different ages (3 to 16 months) revealed that most LD mouse models already show reactive astrogliosis and microgliosis at around 3 months of age, together with the upregulation of inflammatory mediators belonging mainly to the class of inflammatory chemokines, such as ccl4, ccl5, and cxcl10. Over time, a progressive upregulation of genes involved in immune and inflammatory responses occurs, 60% of which encode proteins specific to microglia and 26% of which encode proteins shared by microglia and other cell types (astrocytes and endothelial cells) [21]. In particular, there is an upregulation of inflammatory cytokines (TNF-a, IL-1β, and IL-1a) [18,22,39,62,63,66]; complement factors (C1q, C1ql1, C3, C3ar1, and C4b) [22,39,51,56]; chemokines (cxcl10, ccl2, ccl3, ccl4, ccl5, ccl12, etc.) [21,22,39,44,51,56,63]; other mediators of immune responses, such as hepatic lipocalin-2 (Lcn2) [21,44,51,56]; cyclooxygenage-2 (COX-2)-producing prostaglandins [18,22,62]; and iNOS-producing free radicals [39]. All these mediators could contribute to neurodegeneration [72]. Table 1 summarizes the existing data on neuroinflammation in LD mouse models.

Table 1.
Neuroinflammation in mouse models of LD.
Our analysis of data from transcriptomic [21] and PCR analyses [18,22,39,62,63,66] performed in mouse models of LD revealed the upregulation of several genes characteristically expressed in proinflammatory microglia [33,73,74], such as lipid-metabolism- and phagosome-related genes (Cst7, Lyz2, Clec7a, Axl, and Itgax), cathepsins (Ctss and Ctsz), major histocompatibility complex (MHC-II)-related genes (H2-D1 and H2-K1), proinflammatory genes (Tlr2, Il1b, and TNFa), and Trem2, a receptor required for DAM activation [75]. One of these studies [39], performed in a 12-month-old Epm2b KO mouse model, also showed the upregulation of several anti-inflammatory genes such as IL-10, IL-10ra, TGF-β, S100A-10, IL-13, IL-4, ARG-1, and CD206, which are characteristic of glia polarized towards a protective phenotype. ARG-1 and IL10ra upregulation was also found in an RNA-Seq analysis performed on Epm2a−/− and Epm2b−/− mice at 16 months of age [21]. Another study, instead, did not confirm the upregulation of Arg-1 in a malin KO mouse model [63].
The presence of astrogliosis and microgliosis was also confirmed by histopathological studies on brain sections from dogs with LD [49,50].
The early activation of glial cells has also been observed in a laforin-deficient zebrafish model [14], while the upregulation of gfap also confirmed astrocyte involvement in epm2a−/− zebrafish larvae [14]. Furthermore, the analysis of inflammatory and glia-specific genes suggests that differently polarized glial populations may coexist in the zebrafish LD model. The upregulation of tnfa, cox2b, and il1b and genes such as csfr1a suggests the presence of proinflammatory activated cells [76,77]; in the case of csfr1a, in particular, this upregulation may, through a positive feedback mechanism, help to support the DAM signature by interacting with trem2 signaling [76]. Finally, the upregulation of il10 suggests that at least some microglial cells may also be activated in an anti-inflammatory manner in the LD zebrafish model [78].
3.4. What Triggers Glial Cell Activation?
The question of what triggers the activation of glial cells in LD is still debated. It is very likely that multiple factors (neuronal death, polyglucosan accumulation, altered autophagy, etc.) play a part, especially considering that these are very active cells that are capable of responding to minimal changes in their environment [79,80].
The inhibition of glycogen synthesis has been shown to have positive effects on neuroinflammation, suggesting a possible role of PB accumulation in glial cell activation. Table 2 summarizes the neuropathological and clinical effects of glycogen synthesis inhibition in several mouse models of LD [10,43,44,51,56,58,59,60,63,64]. Interestingly, the inhibition of astrocytic glycogen synthesis appears to be sufficient to rescue astrogliosis and microgliosis as well as the impairment of autophagy and metabolic alterations observed in LD mice [51]. These data suggest that astrocytic PBs may be responsible for the activation of both astrocytes and microglia. However, to date there are no studies targeting microglial PBs.

Table 2.
Summary of studies describing the effects of the inhibition of glycogen synthesis in animal models of LD.
Positive effects on neuroinflammation were also obtained using drugs with anti-inflammatory actions or acting on other molecular/metabolic pathways found to be impaired in LD [18,23,62]. Table 3 summarizes the neuropathological and clinical effects of the administration of these drugs in animal models of LD. These data seem to confirm the hypothesis that different factors may contribute to the development of neuroinflammation. Autophagy stimulators [46,62], chaperones [46], antioxidants [46,61], and drugs that increase the heat shock response [18], often without any action on PBs, have also been shown to reduce or rescue neuroinflammation in LD murine models. In many cases, however, it is difficult to determine how much the reduction in neuroinflammation depends on a direct anti-inflammatory action or a specific activation of chaperone pathways. For example, dexamethasone has a known anti-inflammatory action but also acts by increasing the heat shock response [18]. Moreover, metformin, which promotes autophagy through the activation of AMP-activated protein kinase (AMPK) and acts as a neuroprotective agent in several neurodegenerative diseases [81], and 4-PBA, a chemical chaperone that sequesters misfolded and aggregated proteins associated with several human neurodegenerative diseases [82], also reduced PBs and the susceptibility to induced seizures [46,47]. Cannabidiol, on the other hand, had no effect on neuroinflammation or seizure susceptibility but reduced cognitive impairment in a mouse model of LD [66].

Table 3.
Summary of studies describing alternative therapeutic strategies to the inhibition of glycogen synthesis in animal models of LD.
Interesting results emerged from the larval zebrafish model of LD [14]. Up to 5-day-old laforin-deficient zebrafish larvae do not develop PBs but show several metabolic/histopathological alterations, including altered autophagy and neuroinflammation, and are already epileptic, suggesting a role of alternative factors to PBs in neuroinflammation in the epileptic phenotype, at least in zebrafish [14].
3.5. Contribution of Glial Cells to Neuronal Dysfunction and Death
Albeit variable in degree, LD mice exhibit neurodegeneration with neuronal loss and the activation of astrocytes and microglia [10,40,41,46,83]. However, the mechanisms underlying the neuronal death in LD remain unknown. Some studies in mouse models of LD support a role for PBs [68,84,85,86]. On the contrary, an increase in PB-independent apoptosis death was observed in the laforin-deficient zebrafish model [14]. A form of cell death called ‘dark cell death’, which precedes the formation of PBs in neurons, has also been described in some mouse models of LD [83,87]. Many degenerated neurons furthermore do not show visible PBs [83,87]. Taken together, these data suggest that different mechanisms of neuronal death may coexist in LD. As suggested by a recent study showing that neurodegeneration may be linked to the accumulation of PBs in astrocytes [51], glial cells could also play a role. In particular, astrocytic PBs appear to be responsible for proinflammatory glial cell activation, triggering a persistent inflammatory reaction [51]. Proinflammatory reactive glial cells could participate in neuronal death both because they lose important homeostatic functions and because they gain neurotoxic properties [28,88,89].
One of the most important homeostatic roles of astrocytes is the clearance of the K+ and glutamate released during neuronal activity, which they perform through the Kir4.1 channel and the EAAT2 transporter [90,91]. If these processes are impaired, neuronal excitability increases to the point of excitotoxicity, leading to cell death [92,93]. Indeed, different mechanisms may impair K+ and glutamate clearance by astrocytes in LD. One is the accumulation of glycogen in astrocytes, given that these processes depend on glycogenolysis [38,40,69,94,95]. Second, inflammatory cytokines (e.g., TNFα and IL-6) released by proinflammatory glial cells reduce glutamate uptake by EAAT2 transporters and promote its astrocytic release [96,97,98]. A third mechanism demonstrated in cellular [52] and mouse models [53] of LD is the functional reduction in the astrocytic EEAT2 transporter [52,53] linked to its altered ubiquitination and recycling due to the deficiency of the laforin/malin complex [54].
Another important glial cell function is the trophic support provided to neurons through the release of several growth factors (e.g., BDNF and NGF) [36,88,99]. Proinflammatory reactive glial cells stop releasing neurotrophins [36,88], and even though reduced levels of neurotrophins (e.g., BDNF and NGF) were demonstrated in the cerebral cortex of a laforin-deficient mouse model [100], the possibility that proinflammatory glia contribute to neuronal death in this way cannot be ruled out [36,88].
Alongside this loss of their homeostatic functions, glial cells activated in a proinflammatory sense may acquire neurotoxic properties [101]. Proinflammatory glial cells release greater amounts of free radicals and conversely produce fewer antioxidant agents [102,103,104], thereby contributing to the increased oxidative stress and the reduction in detoxifying enzymes observed in mouse models of LD [20,105]. Proinflammatory astrocytes and microglia also release a multitude of mediators that are toxic to neurons. Ccl4 and Ccl5 are mainly involved in the infiltration and activation of immune cells [106], including microglia [107], and have been implicated in the progression of several neurodegenerative diseases [108,109]. Cxcl10, produced predominantly by astrocytes, recruits microglia [110] and, by binding to chemokine, CXC motif, receptor 3 on neurons, can induce neuronal dysfunction and death [111,112]. Lcn2, secreted by proinflammatory astrocytes in a mouse model of LD, is selectively toxic to neurons but harmless to glial cells [113]. In general, the increase in chemokines observed in LD animal models (Cxcl10, Ccl2, Ccl4, Ccl5, etc.) may favor the infiltration of peripheral immune cells such as monocytes into the brain and promote the persistence of neuroinflammation [114]. As in other neurodegenerative diseases [115], it is possible that in LD the increased expression of local chemokines induces the infiltration of peripheral immune cells that contribute to the persistence if neuroinflammation.
Proinflammatory cytokines (e.g., IL6, IL1B, and TNFa) released by both proinflammatory microglia and astrocytes [101] can cause synaptic dysfunction, neuronal death, and the inhibition of neurogenesis [116]. In particular, it has been shown that TNF released by inflamed microglia, in conjunction with IL-1α and C1q, drives the damaging activation of astrocytes, leading to a final toxic effect on neurons in various neurodegenerative disorders [117]. Since elevated TNF- α, IL-1α, and C1q levels were also found in LD animal models, a role of this molecular pathway in the neurodegeneration of LD cannot be excluded.
The observation of the upregulation of the complement system, in particular of the components C1q [39] and C3 factors [39,44,51,56], in mouse models of LD suggests that the inappropriate phagocytosis of synapses and neurons by reactive astrocytes and microglia may contribute to the progression of LD [118]. C1q produced mainly by reactive microglia and C3 produced by reactive astrocytes [119] induce neuronal dysfunction and phagocytosis [76,119,120,121]. Trem2 receptors, which are expressed by reactive microglia and are upregulated in mouse models of LD [21,39], may also be involved in in the phagocytic process [76].
COX-2, increased in several models of LD, contributes to neuroinflammation and possibly neurodegeneration through the production of prostanoids [122]. Although positive effects of a COX-2 inhibitor, ibuprofen, were recently described in a mouse model of LD [123], a high dose of ibuprofen was used, and in humans, chronic treatment with high doses of ibuprofen is associated with health risks [123]. However, it might be worth testing drugs that selectively inhibit COX-2 and are associated with fewer systemic side effects.
It is also important to remember that immune cells, including microglia, play an important role in the regulation and distribution of brain iron. In addition, inflammatory cytokines such as TNF-α and IL-6 induce the expression of iron transport receptors and promote iron accumulation in neurons and microglia, which may contribute to neuroinflammation [124]. Therefore, it would be of interest to test the possible accumulation of iron in LD animal models.
Data from the studies of the mouse and zebrafish models of LD included in this review suggest that a portion of glial cells may exhibit an anti-inflammatory phenotype that, contrary to what has been seen so far, could have a neuroprotective effect. To date, however, this hypothesis has not been fully investigated.
3.6. Glial Contribution to LD Epileptic Phenotype
The mechanisms underlying LD epilepsy remain unknown. Since, over time, several authors have argued that it may have a multifactorial origin, in this review we focus on the possible contribution of glial cells to epileptogenesis in LD.
In animal models of LD, all drugs [18,47,61,62] and therapeutic strategies (glycogen synthesis inhibition) [10,59,60,64] found to reduce neuroinflammation also resulted in a reduction/rescue of the epileptic phenotype (Table 2 and Table 3), suggesting the existence of a causal relationship between seizure susceptibility and neuroinflammation, and thus a role for proinflammatory activated glial cells in the development of the LD epileptic phenotype. In support of this, cannabidiol, which did not improve neuroinflammation, had no effect on the epileptic phenotype [66]. Furthermore the elimination of astrocytic glycogen synthesis results in the rescue of neuroinflammation but not of the epileptic phenotype, which according to the authors, is related to the accumulation of PBs within neurons and not astrocytes [51].
Overall, the literature seems to support a role for neuroinflammation in LD epilepsy, be it driven by PBs or other mechanisms (impaired autophagy, oxidative stress, reduced response to thermal stress, etc.). Neuroinflammation and epilepsy are known to influence each other [125,126]. Data obtained in mouse models of LD show that neuroinflammation precedes the development of the epileptic phenotype, suggesting that activated glial cells may play a causative role in its development. However, epileptic activity may then, in turn, aggravate the inflammatory phenotype, thus establishing a positive feedback loop that amplifies the neuronal damage.
The mechanisms by which glial cells may contribute to LD epilepsy are multiple. First, impaired K+ and glutamate clearance, as seen above, can lead to neuronal hyperexcitability and contribute to epileptogenesis. In this regard, in addition to the aforementioned data on EAAT2 [52,53,54], we recently observed the upregulation of the astrocytic Kir4.1 channel in a new laforin-deficient zebrafish model [14]. The downregulation of this channel has generally been described in inflammatory states, but impaired function of Kir4.1 has also been associated with autism–epilepsy phenotypes in humans [127], and we therefore cannot exclude a possible role for it in the epileptic phenotype observed in epm2a KO larvae. Second, proinflammatory glial cells produce numerous inflammatory mediators that may contribute to the development of epileptic activity [125]. Many of the inflammatory signaling pathways that have been implicated in epileptogenesis are upregulated in animal models of LD and could be effective targets for seizure treatment. Third, proinflammatory glial cells may also contribute to LD epileptogenesis by increasing the production of oxygen and nitrogen radicals [128,129]. Indeed, in mouse models of LD, increased oxidative stress [20,105] and the upregulation of iNOS [39], which reactive glial cells can produce in large quantities, were observed.
4. Discussion
This paper systematically reviews published data dealing specifically with the role of astrocytes and microglia in neuroinflammation in LD. Although LD has long been approached from a “neuronocentric” perspective, there is growing evidence suggesting that glial cells also play a part in its disease mechanisms, in line with what is seen in most neurological [29,130] and neurodegenerative conditions [131].
The first important observation emerging from this data review concerns the discovery of PBs in glial cells [38,39,40,41,51], although the significance of glycogen accumulations in non-neuronal cells remains to be established. In particular, the literature data only allow speculation on astrocytic PBs, as microglial PBs have not yet been studied in detail. However, studies exploring the inhibition of glycogen synthesis support a pathogenic and detrimental role for astrocytic PBs in neuroinflammation, autophagic alterations, and metabolic changes [51]. The accumulation of astrocytic PBs seems to be capable, on its own, of triggering astrocyte and microglia activation [51]. Proinflammatory activated glial cells secrete several inflammatory mediators and upregulate genes typically involved in glial-damaging phenotypes. Therefore, glial cells, by losing their homeostatic functions, may contribute to the neurodegeneration and epilepsy occurring in LD. On the other hand, the observation—in line with what occurs in normal aging and in other neurodegenerative diseases [132]—that astrocytes in LD accumulate CALs [38,39,51] (also termed “wasteosomes”) [132,133], supports the suggestion that they may be waste containers that are produced to clean up the brain [132,133]. It is thought that they may be secreted by the cells [38,71] and eliminated by macrophagic phagocytosis once they reach the cervical lymph nodes through the meningeal lymphatic system [134]. On this basis, it seems possible that the difference in mortality between astrocytes and neurons accumulating PBs in LD may be due to a difference in toxicity between astrocytic CALs and neuronal PBs. However, a protective role for neuronal PBs was also suggested by observing that alterations of PB morphology, such as that caused by the deletion of p62, increase the toxicity of these cells and thus the susceptibility to epilepsy [39].
The drivers of the proinflammatory polarization of glial cells are only partially known and are potentially numerous. Whereas the accumulation of PBs in astrocytes has been shown to activate both astrocytes and microglia in a proinflammatory manner, pharmacological studies in LD mouse models have suggested that neuroinflammation may also depend on other pathways, such as impaired autophagy [62], increased oxidative stress [61], and a reduced heat stress response [18]. Similar results have also been obtained in a new zebrafish model of LD [14]. It should be noted that zebrafish have no star-shaped astrocytes, only radial glia [135] whose functions have yet to be precisely defined. However, they seem to perform several functions of classical mammalian glia, such as neurogenesis functions and homeostatic roles in neural circuits and brain barriers [135]. Therefore, the possible roles of glial cells in LD and neurodegenerative diseases in general, as well as the differences in the roles of these cells between teleost fish and mammals, remain to be elucidated.
In short, the role of microglia in LD is still underexplored, and inferences regarding this type of cell in LD are limited by the lack of targeted studies. Although PBs have also been observed in this cell type, we do not know whether they accumulate glycogen autonomously or whether they phagocytize it from outside. Certainly, characterizing microglial glycogen inclusions (e.g., CALs, LBs, or other forms) could help to clarify some issues. The finding of mediators and markers of anti-inflammatory pathways in mouse LD models [21,39] suggests that at least some microglial cells may be engaged in protective functions, for example, to eliminate cells containing PBs. The observation of hexb gene upregulation in a zebrafish model of LD [14] suggests that the dysregulation of lysosomal function and autophagy in microglia may contribute to the neuropathology of LD [8,9,10,11,12,13]. On the other hand, the results of analyses of inflammatory markers [18,22,39,62,63,66] and RNA-Seq analyses indicate the possible presence in LD, as in other neurodegenerative diseases, of disease-associated microglia. However, there is still a need for the targeted characterization of glial phenotypes in LD.
5. Conclusions
Although the recent literature is revealing knowledge gaps, and many questions remain unanswered, glial cells are clearly involved in LD. Future studies should be designed to address glial cells as a potential therapeutic target, with the aim to arrest the progression of LD. In this context, the variability in the polarization of astrocytes and microglia observed within the same disease could make treatment with anti-inflammatory drugs difficult and not entirely effective. For this reason, it is important to characterize the phenotypes of these cells in more detail and possibly combine strategies aimed at enhancing the anti-inflammatory/protective processes. Although animal models remain the ideal platforms to continue studying the disease and for drug screening, it would also be useful to study microglial and astrocytic activation in humans through fluid [136,137,138,139] and imaging biomarkers [137,140,141].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines10123103/s1, The PRISMA checklist of the review process.
Author Contributions
Conceptualization, S.D.V.; methodology, S.D.V. and M.M.; software, S.D.V. and M.M.; formal analysis, S.D.V. and M.M.; investigation, S.D.V. and M.M.; data curation, S.D.V. and M.M.; writing—original draft preparation, S.D.V.; writing—review and editing, S.D.V., M.M. and F.M.S.; visualization, S.D.V., M.M. and F.M.S.; supervision, F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by grants from the Telethon Foundation (http://www.telethon.it/en, accessed on 31 October 2022) (grant GGP20011 to M.M. and grant GSA22B005 to S.D.V.), the Italian Ministry of Health (Ricerca Corrente 5 × 1000 to F.M.S. and Ricerca Finalizzata 2018 Starting Grant SG-2018-12367839 to M.M.), and the Tuscany Region (Bando Ricerca Salute 2018, DEM-AGING to F.M.S.). M.M. is the holder of the Telethon Career Award.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Additional information on the data may be requested from the corresponding author.
Acknowledgments
The authors are grateful to Catherine J. Wrenn for expert revision and editorial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Minassian, B.A.; Lee, J.R.; Herbrick, J.A.; Huizenga, J.; Soder, S.; Mungall, A.J.; Dunham, I.; Gardner, R.; Fong, C.Y.; Carpenter, S.; et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat. Genet. 1998, 20, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.M.; Young, E.J.; Ianzano, L.; Munteanu, I.; Zhao, X.; Christopoulos, C.C.; Avanzini, G.; Elia, M.; Ackerley, C.A.; Jovic, N.J.; et al. Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat. Genet. 2003, 35, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Towler, M.C.; Hardie, D.G.; Knecht, E.; Sanz, P. The laforin-malin complex, involved in Lafora disease, promotes the incorporation of K63-linked ubiquitin chains into AMP-activated protein kinase beta subunits. Mol. Biol. Cell 2010, 21, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.A.; Nitschke, S.; Steup, M.; Minassian, B.A.; Nitschke, F. Pathogenesis of Lafora Disease: Transition of Soluble Glycogen to Insoluble Polyglucosan. Int. J. Mol. Sci. 2017, 18, 1743. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.; Tiberia, E.; Striano, P.; Genton, P.; Carpenter, S.; Ackerley, C.A.; Minassian, B.A. Lafora disease. Epileptic Disord. 2016, 18, 38–62. [Google Scholar] [CrossRef]
- Mitra, S.; Gumusgoz, E.; Minassian, B.A. Lafora disease: Current biology and therapeutic approaches. Rev. Neurol. 2022, 178, 315–325. [Google Scholar] [CrossRef]
- Parihar, R.; Rai, A.; Ganesh, S. Lafora disease: From genotype to phenotype. J. Genet. 2018, 97, 611–624. [Google Scholar] [CrossRef]
- Aguado, C.; Sarkar, S.; Korolchuk, V.I.; Criado, O.; Vernia, S.; Boya, P.; Sanz, P.; de Córdoba, S.R.; Knecht, E.; Rubinsztein, D.C. Laforin, the most common protein mutated in Lafora disease, regulates autophagy. Hum. Mol. Genet. 2010, 19, 2867–2876. [Google Scholar] [CrossRef]
- Criado, O.; Aguado, C.; Gayarre, J.; Duran-Trio, L.; Garcia-Cabrero, A.M.; Vernia, S.; San Millán, B.; Heredia, M.; Romá-Mateo, C.; Mouron, S.; et al. Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum. Mol. Genet. 2012, 21, 1521–1533. [Google Scholar] [CrossRef]
- Duran, J.; Gruart, A.; García-Rocha, M.; Delgado-García, J.M.; Guinovart, J.J. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum. Mol. Genet. 2014, 23, 3147–3156. [Google Scholar] [CrossRef]
- Knecht, E.; Criado-García, O.; Aguado, C.; Gayarre, J.; Duran-Trio, L.; Garcia-Cabrero, A.M.; Vernia, S.; San Millán, B.; Heredia, M.; Romá-Mateo, C.; et al. Malin knockout mice support a primary role of autophagy in the pathogenesis of Lafora disease. Autophagy 2012, 8, 701–703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puri, R.; Suzuki, T.; Yamakawa, K.; Ganesh, S. Dysfunctions in endosomal-lysosomal and autophagy pathways underlie neuropathology in a mouse model for Lafora disease. Hum. Mol. Genet. 2012, 21, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.; Maity, R.; Sharma, J.; Dey, P.; Shankar, S.K.; Satishchandra, P.; Jana, N.R. Sequestration of chaperones and proteasome into Lafora bodies and proteasomal dysfunction induced by Lafora disease-associated mutations of malin. Hum. Mol. Genet. 2010, 19, 4726–4734. [Google Scholar] [CrossRef] [PubMed]
- Della Vecchia, S.; Ogi, A.; Licitra, R.; Abramo, F.; Nardi, G.; Mero, S.; Landi, S.; Battini, R.; Sicca, F.; Ratto, G.M.; et al. Trehalose Treatment in Zebrafish Model of Lafora Disease. Int. J. Mol. Sci. 2022, 23, 6874. [Google Scholar] [CrossRef]
- Garyali, P.; Siwach, P.; Singh, P.K.; Puri, R.; Mittal, S.; Sengupta, S.; Parihar, R.; Ganesh, S. The malin-laforin complex suppresses the cellular toxicity of misfolded proteins by promoting their degradation through the ubiquitin-proteasome system. Hum. Mol. Genet. 2009, 18, 688–700. [Google Scholar] [CrossRef]
- Mittal, S.; Dubey, D.; Yamakawa, K.; Ganesh, S. Lafora disease proteins malin and laforin are recruited to aggresomes in response to proteasomal impairment. Hum. Mol. Genet. 2007, 16, 753–762. [Google Scholar] [CrossRef]
- Vernia, S.; Rubio, T.; Heredia, M.; Rodríguez de Córdoba, S.; Sanz, P. Increased endoplasmic reticulum stress and decreased proteasomal function in lafora disease models lacking the phosphatase laforin. PLoS ONE 2009, 4, e5907. [Google Scholar] [CrossRef]
- Sinha, P.; Verma, B.; Ganesh, S. Dexamethasone-induced activation of heat shock response ameliorates seizure susceptibility and neuroinflammation in mouse models of Lafora disease. Exp. Neurol. 2021, 340, 113656. [Google Scholar] [CrossRef]
- Lahuerta, M.; Aguado, C.; Sánchez-Martín, P.; Sanz, P.; Knecht, E. Degradation of altered mitochondria by autophagy is impaired in Lafora disease. FEBS J. 2018, 285, 2071–2090. [Google Scholar] [CrossRef]
- Romá-Mateo, C.; Aguado, C.; García-Giménez, J.L.; Knecht, E.; Sanz, P.; Pallardó, F.V. Oxidative stress, a new hallmark in the pathophysiology of Lafora progressive myoclonus epilepsy. Free Radic. Biol. Med. 2015, 88, 30–41. [Google Scholar] [CrossRef]
- Lahuerta, M.; Gonzalez, D.; Aguado, C.; Fathinajafabadi, A.; García-Giménez, J.L.; Moreno-Estellés, M.; Romá-Mateo, C.; Knecht, E.; Pallardó, F.V.; Sanz, P. Reactive Glia-Derived Neuroinflammation: A Novel Hallmark in Lafora Progressive Myoclonus Epilepsy That Progresses with Age. Mol. Neurobiol. 2020, 57, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- López-González, I.; Viana, R.; Sanz, P.; Ferrer, I. Inflammation in Lafora Disease: Evolution with Disease Progression in Laforin and Malin Knock-out Mouse Models. Mol. Neurobiol. 2017, 54, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Mollá, B.; Heredia, M.; Sanz, P. Modulators of Neuroinflammation Have a Beneficial Effect in a Lafora Disease Mouse Model. Mol. Neurobiol. 2021, 58, 2508–2522. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Russo, M.V.; McGavern, D.B. Inflammatory neuroprotection following traumatic brain injury. Science 2016, 353, 783–785. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V.; Li, B.; Scuderi, C. Astrocytes: The Housekeepers and Guardians of the CNS. Adv. Neurobiol. 2021, 26, 21–53. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- De Biase, L.M.; Schuebel, K.E.; Fusfeld, Z.H.; Jair, K.; Hawes, I.A.; Cimbro, R.; Zhang, H.Y.; Liu, Q.R.; Shen, H.; Xi, Z.X.; et al. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 2017, 95, 341–356.e6. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, S.; Dammer, E.B.; Raza, S.A.; Rathakrishnan, P.; Xiao, H.; Gao, T.; Duong, D.M.; Pennington, M.W.; Lah, J.J.; Seyfried, N.T.; et al. Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G. Defining activation states of microglia in human brain tissue: An unresolved issue for Alzheimer’s disease. Neuroimmunol. Neuroinflamm. 2020, 7, 194–214. [Google Scholar] [CrossRef]
- Crotti, A.; Ransohoff, R.M. Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity 2016, 44, 505–515. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Oksanen, M.; Lehtonen, S.; Jaronen, M.; Goldsteins, G.; Hämäläinen, R.H.; Koistinaho, J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell. Mol. Life Sci. 2019, 76, 2739–2760. [Google Scholar] [CrossRef]
- Augé, E.; Pelegrí, C.; Manich, G.; Cabezón, I.; Guinovart, J.J.; Duran, J.; Vilaplana, J. Astrocytes and neurons produce distinct types of polyglucosan bodies in Lafora disease. Glia 2018, 66, 2094–2107. [Google Scholar] [CrossRef]
- Pellegrini, P.; Hervera, A.; Varea, O.; Brewer, M.K.; López-Soldado, I.; Guitart, A.; Aguilera, M.; Prats, N.; Del Río, J.A.; Guinovart, J.J.; et al. Lack of p62 Impairs Glycogen Aggregation and Exacerbates Pathology in a Mouse Model of Myoclonic Epilepsy of Lafora. Mol. Neurobiol. 2022, 59, 1214–1229. [Google Scholar] [CrossRef]
- Rubio-Villena, C.; Viana, R.; Bonet, J.; Garcia-Gimeno, M.A.; Casado, M.; Heredia, M.; Sanz, P. Astrocytes: New players in progressive myoclonus epilepsy of Lafora type. Hum. Mol. Genet. 2018, 27, 1290–1300. [Google Scholar] [CrossRef]
- Valles-Ortega, J.; Duran, J.; Garcia-Rocha, M.; Bosch, C.; Saez, I.; Pujadas, L.; Serafin, A.; Cañas, X.; Soriano, E.; Delgado-García, J.M.; et al. Neurodegeneration and functional impairments associated with glycogen synthase accumulation in a mouse model of Lafora disease. EMBO Mol. Med. 2011, 3, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Gumusgoz, E.; Guisso, D.R.; Kasiri, S.; Wu, J.; Dear, M.; Verhalen, B.; Nitschke, S.; Mitra, S.; Nitschke, F.; Minassian, B.A. Targeting Gys1 with AAV-SaCas9 Decreases Pathogenic Polyglucosan Bodies and Neuroinflammation in Adult Polyglucosan Body and Lafora Disease Mouse Models. Neurotherapeutics 2021, 18, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, S.; Nitschke, S.; Grossman, T.R.; Kordasiewicz, H.; Wang, P.; Zhao, X.; Guisso, D.R.; Kasiri, S.; Nitschke, F.; Minassian, B.A. Gys1 antisense therapy rescues neuropathological bases of murine Lafora disease. Brain 2021, 144, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.N.; Satishchandra, P.; Asha, T.; Shankar, S.K. Lafora’s disease in south India: A clinical, electrophysiologic, and pathologic study. Epilepsia 1993, 34, 476–487. [Google Scholar] [CrossRef]
- Berthier, A.; Payá, M.; García-Cabrero, A.M.; Ballester, M.I.; Heredia, M.; Serratosa, J.M.; Sánchez, M.P.; Sanz, P. Pharmacological Interventions to Ameliorate Neuropathological Symptoms in a Mouse Model of Lafora Disease. Mol. Neurobiol. 2016, 53, 1296–1309. [Google Scholar] [CrossRef]
- Sánchez-Elexpuru, G.; Serratosa, J.M.; Sanz, P.; Sánchez, M.P. 4-Phenylbutyric acid and metformin decrease sensitivity to pentylenetetrazol-induced seizures in a malin knockout model of Lafora disease. Neuroreport 2017, 28, 268–271. [Google Scholar] [CrossRef]
- Schwarz, G.A.; Yanoff, M. Lafora’s disease. Distinct clinico-pathologic form of unverricht’s syndrome. Arch. Neurol. 1965, 12, 172–188. [Google Scholar] [CrossRef]
- Chambers, J.K.; Thongtharb, A.; Shiga, T.; Azakami, D.; Saito, M.; Sato, M.; Morozumi, M.; Nakayama, H.; Uchida, K. Accumulation of Laforin and Other Related Proteins in Canine Lafora Disease With EPM2B Repeat Expansion. Vet. Pathol. 2018, 55, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, M.; Antinori, L.; Serra, G.D.; Bertolini, G.; Rosati, M. Clinical features, imaging characteristics, genetic investigation and histopathologic findings in a Chihuahua dog with Lafora disease. Vet. Rec. Case Rep. 2021, 9, e206. [Google Scholar] [CrossRef]
- Duran, J.; Hervera, A.; Markussen, K.H.; Varea, O.; López-Soldado, I.; Sun, R.C.; Del Río, J.A.; Gentry, M.S.; Guinovart, J.J. Astrocytic glycogen accumulation drives the pathophysiology of neurodegeneration in Lafora disease. Brain 2021, 144, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Ballester, C.; Berthier, A.; Viana, R.; Sanz, P. Homeostasis of the astrocytic glutamate transporter GLT-1 is altered in mouse models of Lafora disease. Biochim. Biophys. Acta 2016, 1862, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Ballester, C.; Santana, N.; Perez-Jimenez, E.; Viana, R.; Artigas, F.; Sanz, P. In vivo glutamate clearance defects in a mouse model of Lafora disease. Exp. Neurol. 2019, 320, 112959. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, E.; Viana, R.; Muñoz-Ballester, C.; Vendrell-Tornero, C.; Moll-Diaz, R.; Garcia-Gimeno, M.A.; Sanz, P. Endocytosis of the glutamate transporter 1 is regulated by laforin and malin: Implications in Lafora disease. Glia 2021, 69, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Hegreberg, G.A.; Padgett, G.A. Inherited progressive epilepsy of the dog with comparisons to Lafora’s disease of man. Fed. Proc. 1976, 35, 1202–1205. [Google Scholar]
- Israelian, L.; Nitschke, S.; Wang, P.; Zhao, X.; Perri, A.M.; Lee, J.; Verhalen, B.; Nitschke, F.; Minassian, B.A. Ppp1r3d deficiency preferentially inhibits neuronal and cardiac Lafora body formation in a mouse model of the fatal epilepsy Lafora disease. J. Neurochem. 2021, 157, 1897–1910. [Google Scholar] [CrossRef]
- Jian, Z.; Alley, M.R.; Cayzer, J.; Swinney, G.R. Lafora’s disease in an epileptic Basset hound. N. Z. Vet. J. 1990, 38, 75–79. [Google Scholar] [CrossRef]
- Nitschke, S.; Chown, E.E.; Zhao, X.; Gabrielian, S.; Petković, S.; Guisso, D.R.; Perri, A.M.; Wang, P.; Ahonen, S.; Nitschke, F.; et al. An inducible glycogen synthase-1 knockout halts but does not reverse Lafora disease progression in mice. J. Biol. Chem. 2021, 296, 100150. [Google Scholar] [CrossRef]
- Pederson, B.A.; Turnbull, J.; Epp, J.R.; Weaver, S.A.; Zhao, X.; Pencea, N.; Roach, P.J.; Frankland, P.W.; Ackerley, C.A.; Minassian, B.A. Inhibiting glycogen synthesis prevents Lafora disease in a mouse model. Ann. Neurol. 2013, 74, 297–300. [Google Scholar] [CrossRef]
- Rai, A.; Mishra, R.; Ganesh, S. Suppression of leptin signaling reduces polyglucosan inclusions and seizure susceptibility in a mouse model for Lafora disease. Hum. Mol. Gen. 2017, 26, 4778–4785. [Google Scholar] [CrossRef]
- Sánchez-Elexpuru, G.; Serratosa, J.M.; Sánchez, M.P. Sodium selenate treatment improves symptoms and seizure susceptibility in a malin-deficient mouse model of Lafora disease. Epilepsia 2017, 58, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Verma, B.; Ganesh, S. Trehalose Ameliorates Seizure Susceptibility in Lafora Disease Mouse Models by Suppressing Neuroinflammation and Endoplasmic Reticulum Stress. Mol. Neurobiol. 2021, 58, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Varea, O.; Duran, J.; Aguilera, M.; Prats, N.; Guinovart, J.J. Suppression of glycogen synthesis as a treatment for Lafora disease: Establishing the window of opportunity. Neurobiol. Dis. 2021, 147, 105173. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.; DePaoli-Roach, A.A.; Zhao, X.; Cortez, M.A.; Pencea, N.; Tiberia, E.; Piliguian, M.; Roach, P.J.; Wang, P.; Ackerley, C.A.; et al. PTG depletion removes Lafora bodies and rescues the fatal epilepsy of Lafora disease. PLoS Genet. 2011, 7, e1002037. [Google Scholar] [CrossRef] [PubMed]
- Gumusgoz, E.; Kasiri, S.; Guisso, D.R.; Wu, J.; Dear, M.; Verhalen, B.; Minassian, B.A. AAV-Mediated Artificial miRNA Reduces Pathogenic Polyglucosan Bodies and Neuroinflammation in Adult Polyglucosan Body and Lafora Disease Mouse Models. Neurotherapeutic 2022, 19, 982–993. [Google Scholar] [CrossRef]
- Aso, E.; Andrés-Benito, P.; Grau-Escolano, J.; Caltana, L.; Brusco, A.; Sanz, P.; Ferrer, I. Cannabidiol-Enriched Extract Reduced the Cognitive Impairment but Not the Epileptic Seizures in a Lafora Disease Animal Model. Cannabis Cannabinoid Res. 2020, 5, 150–163. [Google Scholar] [CrossRef]
- Bak, L.K.; Walls, A.B.; Schousboe, A.; Waagepetersen, H.S. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef]
- Vilchez, D.; Ros, S.; Cifuentes, D.; Pujadas, L.; Vallès, J.; García-Fojeda, B.; Criado-García, O.; Fernández-Sánchez, E.; Medraño-Fernández, I.; Domínguez, J.; et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007, 10, 1407–1413. [Google Scholar] [CrossRef]
- DiNuzzo, M.; Mangia, S.; Maraviglia, B.; Giove, F. Does abnormal glycogen structure contribute to increased susceptibility to seizures in epilepsy? Metab. Brain Dis. 2015, 30, 307–316. [Google Scholar] [CrossRef]
- Sinadinos, C.; Valles-Ortega, J.; Boulan, L.; Solsona, E.; Tevy, M.F.; Marquez, M.; Duran, J.; Lopez-Iglesias, C.; Calbó, J.; Blasco, E.; et al. Neuronal glycogen synthesis contributes to physiological aging. Aging Cell 2014, 13, 935–945. [Google Scholar] [CrossRef]
- Augé, E.; Cabezón, I.; Pelegrí, C.; Vilaplana, J. New perspectives on corpora amylacea in the human brain. Sci. Rep. 2017, 7, 41807. [Google Scholar] [CrossRef]
- Peterson, P.K.; Toborek, M. Neuroinflammation and Neurodegeneration, 1st ed.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Benusa, S.D.; George, N.M.; Dupree, J.L. Microglial heterogeneity: Distinct cell types or differential functional adaptation? Neuroimmunol. Neuroinflamm. 2020, 7, 248–263. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Colonna, M. The identity and function of microglia in neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Candlish, M.; Hefendehl, J.K. Microglia Phenotypes Converge in Aging and Neurodegenerative Disease. Front. Neurol. 2021, 12, 660720. [Google Scholar] [CrossRef]
- Cunningham, C.; Dunne, A.; Lopez-Rodriguez, A.B. Astrocytes: Heterogeneous and Dynamic Phenotypes in Neurodegeneration and Innate Immunity. Neuroscientist 2019, 25, 455–474. [Google Scholar] [CrossRef]
- Wiley, J.C.; Meabon, J.S.; Frankowski, H.; Smith, E.A.; Schecterson, L.C.; Bothwell, M.; Ladiges, W.C. Phenylbutyric acid rescues endoplasmic reticulum stress-induced suppression of APP proteolysis and prevents apoptosis in neuronal cells. PLoS ONE 2010, 5, e9135. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Pérez-Mediavilla, A.; Frechilla, D.; Del Río, J.; García-Osta, A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 2009, 34, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Delgado-Escueta, A.V.; Sakamoto, T.; Avila, M.R.; Machado-Salas, J.; Hoshii, Y.; Akagi, T.; Gomi, H.; Suzuki, T.; Amano, K.; et al. Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum. Mol. Genet. 2002, 11, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.; Tevy, M.F.; Garcia-Rocha, M.; Calbó, J.; Milán, M.; Guinovart, J.J. Deleterious effects of neuronal accumulation of glycogen in flies and mice. EMBO Mol. Med. 2012, 4, 719–729. [Google Scholar] [CrossRef]
- Gentry, M.S.; Dixon, J.E.; Worby, C.A. Lafora disease: Insights into neurodegeneration from plant metabolism. Trends Biochem. Sci. 2009, 34, 628–639. [Google Scholar] [CrossRef][Green Version]
- Gentry, M.S.; Guinovart, J.J.; Minassian, B.A.; Roach, P.J.; Serratosa, J.M. Lafora disease offers a unique window into neuronal glycogen metabolism. J. Biol. Chem. 2018, 293, 7117–7125. [Google Scholar] [CrossRef]
- Machado-Salas, J.; Avila-Costa, M.R.; Guevara, P.; Guevara, J.; Durón, R.M.; Bai, D.; Tanaka, M.; Yamakawa, K.; Delgado-Escueta, A.V. Ontogeny of Lafora bodies and neurocytoskeleton changes in Laforin-deficient mice. Exp. Neurol. 2012, 236, 131–140. [Google Scholar] [CrossRef][Green Version]
- Luo, X.G.; Chen, S.D. The changing phenotype of microglia from homeostasis to disease. Transl. Neurodegener. 2012, 1, 9. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Nwaobi, S.E.; Cuddapah, V.A.; Patterson, K.C.; Randolph, A.C.; Olsen, M.L. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 2016, 132, 1–21. [Google Scholar] [CrossRef]
- Amédée, T.; Robert, A.; Coles, J.A. Potassium homeostasis and glial energy metabolism. Glia 1997, 21, 46–55. [Google Scholar] [CrossRef]
- Coulter, D.A.; Eid, T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 2012, 60, 1215–1226. [Google Scholar] [CrossRef]
- DiNuzzo, M.; Mangia, S.; Maraviglia, B.; Giove, F. Physiological bases of the K+ and the glutamate/GABA hypotheses of epilepsy. Epilepsy Res. 2014, 108, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, D.; Xue, Z.; Gu, L.; Hertz, L.; Peng, L. Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: Potential implications for K+ homeostasis and glycogen usage in brain. Neurochem. Res. 2013, 38, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.; Serratosa, J.M. Neuroinflammation and progressive myoclonus epilepsies: From basic science to therapeutic opportunities. Expert Rev. Mol. Med. 2020, 22, e4. [Google Scholar] [CrossRef]
- Clark, I.A.; Vissel, B. Excess cerebral TNF causing glutamate excitotoxicity rationalizes treatment of neurodegenerative diseases and neurogenic pain by anti-TNF agents. J. Neuroinflamm. 2016, 13, 236. [Google Scholar] [CrossRef]
- Bedner, P.; Steinhäuser, C. TNFα-Driven Astrocyte Purinergic Signaling during Epileptogenesis. Trends Mol. Med. 2019, 25, 70–72. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Ortolano, S.; Vieitez, I.; Agis-Balboa, R.C.; Spuch, C. Loss of GABAergic cortical neurons underlies the neuropathology of Lafora disease. Mol. Brain 2014, 7, 7. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Benarroch, E.E. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology 2013, 81, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Ben Haim, L.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Romá-Mateo, C.; Aguado, C.; García-Giménez, J.L.; Ibáñez-Cabellos, J.S.; Seco-Cervera, M.; Pallardó, F.V.; Knecht, E.; Sanz, P. Increased oxidative stress and impaired antioxidant response in Lafora disease. Free Radic. Biol. Med. 2014, 75 (Suppl. 1), S47. [Google Scholar] [CrossRef][Green Version]
- Ren, M.; Guo, Q.; Guo, L.; Lenz, M.; Qian, F.; Koenen, R.R.; Xu, H.; Schilling, A.B.; Weber, C.; Ye, R.D.; et al. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme. EMBO Rep. 2010, 29, 3952–3966. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Schuchman, E.H.; Jin, H.K.; Bae, J.S. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid β ameliorates Alzheimer’s disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells 2012, 30, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Szczuciński, A.; Losy, J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol. Scand. 2007, 115, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998, 53, 31–37. [Google Scholar] [CrossRef]
- Rappert, A.; Biber, K.; Nolte, C.; Lipp, M.; Schubel, A.; Lu, B.; Gerard, N.P.; Gerard, C.; Boddeke, H.W.; Kettenmann, H. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl- current and chemotaxis in murine microglia. J. Immunol. Res. 2002, 168, 3221–3226. [Google Scholar] [CrossRef]
- Sui, Y.; Stehno-Bittel, L.; Li, S.; Loganathan, R.; Dhillon, N.K.; Pinson, D.; Nath, A.; Kolson, D.; Narayan, O.; Buch, S. CXCL10-induced cell death in neurons: Role of calcium dysregulation. Eur. J. Neurosci. 2006, 23, 957–964. [Google Scholar] [CrossRef]
- Nelson, T.E.; Gruol, D.L. The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. J. Neuroimmunol. 2004, 156, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Huang, C.; Tong, J.; Qiu, G.; Huang, B.; Wu, Q.; Li, F.; Xu, Z.; Bowser, R.; Xia, X.G.; et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl. Acad. Sci. USA 2013, 110, 4069–4074. [Google Scholar] [CrossRef] [PubMed]
- Cronk, J.C.; Filiano, A.J.; Louveau, A.; Marin, I.; Marsh, R.; Ji, E.; Goldman, D.H.; Smirnov, I.; Geraci, N.; Acton, S.; et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 2018, 215, 1627–1647. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Boddu, R.; Abdelmotilib, H.A.; Sokratian, A.; Kelly, K.; Liu, Z.; Bryant, N.; Chandra, S.; Carlisle, S.M.; Lefkowitz, E.J.; et al. Pathological α-synuclein recruits LRRK2 expressing pro-inflammatory monocytes to the brain. Mol. Neurodegener. 2022, 17, 7. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Lian, H.; Litvinchuk, A.; Chiang, A.C.; Aithmitti, N.; Jankowsky, J.L.; Zheng, H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J. Neurosci. 2016, 36, 577–589. [Google Scholar] [CrossRef]
- Wu, T.; Dejanovic, B.; Gandham, V.D.; Gogineni, A.; Edmonds, R.; Schauer, S.; Srinivasan, K.; Huntley, M.A.; Wang, Y.; Wang, T.M.; et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep. 2019, 28, 2111–2123.e6. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Sil, S.; Ghosh, T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer’s Disease. J. Neuroimmunol. 2016, 291, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Markussen, K.H.; Macedo, J.; Machío, M.; Dolce, A.; Goldberg, Y.P.; Vander Kooi, C.W.; Gentry, M.S. The 6th International Lafora Epilepsy Workshop: Advances in the search for a cure. Epilepsy Behav. 2021, 119, 107975. [Google Scholar] [CrossRef] [PubMed]
- Panther, E.J.; Zelmanovich, R.; Hernandez, J.; Dioso, E.R.; Foster, D.; Lucke-Wold, B. Ferritin and Neurotoxicity: A Contributor to Deleterious Outcomes for Subarachnoid Hemorrhage. Eur. Neurol. 2022, 85, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.; Garcia-Gimeno, M.A. Reactive Glia Inflammatory Signaling Pathways and Epilepsy. Int. J. Mol. Sci. 2020, 21, 4096. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef]
- Sicca, F.; Ambrosini, E.; Marchese, M.; Sforna, L.; Servettini, I.; Valvo, G.; Brignone, M.S.; Lanciotti, A.; Moro, F.; Grottesi, A.; et al. Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy. Sci. Rep. 2016, 6, 34325. [Google Scholar] [CrossRef]
- Aguiar, C.C.; Almeida, A.B.; Araújo, P.V.; de Abreu, R.N.; Chaves, E.M.; do Vale, O.C.; Macêdo, D.S.; Woods, D.J.; Fonteles, M.M.; Vasconcelos, S.M. Oxidative stress and epilepsy: Literature review. Oxidative Med. Cell. Longev. 2012, 2012, 795259. [Google Scholar] [CrossRef]
- Terrone, G.; Balosso, S.; Pauletti, A.; Ravizza, T.; Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 2020, 167, 107742. [Google Scholar] [CrossRef]
- Ricci, G.; Volpi, L.; Pasquali, L.; Petrozzi, L.; Siciliano, G. Astrocyte-neuron interactions in neurological disorders. J. Biol. Phys. 2009, 35, 317–336. [Google Scholar] [CrossRef]
- Franklin, H.; Clarke, B.E.; Patani, R. Astrocytes and microglia in neurodegenerative diseases: Lessons from human in vitro models. Prog. Neurobiol. 2021, 200, 101973. [Google Scholar] [CrossRef]
- Riba, M.; Del Valle, J.; Augé, E.; Vilaplana, J.; Pelegrí, C. From Corpora amylacea to wasteosomes: History and perspectives. Ageing Res. Rev. 2021, 72, 101484. [Google Scholar] [CrossRef] [PubMed]
- Riba, M.; Augé, E.; Campo-Sabariz, J.; Moral-Anter, D.; Molina-Porcel, L.; Ximelis, T.; Ferrer, R.; Martín-Venegas, R.; Pelegrí, C.; Vilaplana, J. Corpora amylacea act as containers that remove waste products from the brain. Proc. Natl. Acad. Sci. USA 2019, 116, 26038–26048. [Google Scholar] [CrossRef] [PubMed]
- Riba, M.; Augé, E.; Tena, I.; Del Valle, J.; Molina-Porcel, L.; Ximelis, T.; Vilaplana, J.; Pelegrí, C. Corpora amylacea in the Human Brain Exhibit Neoepitopes of a Carbohydrate Nature. Front. Immunol. 2021, 12, 618193. [Google Scholar] [CrossRef] [PubMed]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional, structural, and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Khrestian, M.; Dyne, E.; Shao, Y.; Pillai, J.A.; Rao, S.M.; Bemiller, S.M.; Lamb, B.; Fernandez, H.H.; Leverenz, J.B. Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease. J. Neuroimmunol. 2018, 319, 19–27. [Google Scholar] [CrossRef]
- Carter, S.F.; Herholz, K.; Rosa-Neto, P.; Pellerin, L.; Nordberg, A.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol. Med. 2019, 25, 77–95. [Google Scholar] [CrossRef]
- Kwon, H.S.; Lee, E.H.; Park, H.H.; Jin, J.H.; Choi, H.; Lee, K.Y.; Lee, Y.J.; Lee, J.H.; de Oliveira, F.; Kim, H.Y.; et al. Early increment of soluble triggering receptor expressed on myeloid cells 2 in plasma might be a predictor of poor outcome after ischemic stroke. J. Clin. Neurosci. 2020, 73, 215–218. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Kleinberger, G.; Araque Caballero, M.Á.; Brendel, M.; Rominger, A.; Alcolea, D.; Fortea, J.; Lleó, A.; Blesa, R.; Gispert, J.D.; et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 2016, 8, 466–476. [Google Scholar] [CrossRef]
- Scarf, A.M.; Kassiou, M. The translocator protein. J. Nucl. Med. 2011, 52, 677–680. [Google Scholar] [CrossRef]
- Malpetti, M.; Kievit, R.A.; Passamonti, L.; Jones, P.S.; Tsvetanov, K.A.; Rittman, T.; Mak, E.; Nicastro, N.; Bevan-Jones, W.R.; Su, L.; et al. Microglial activation and tau burden predict cognitive decline in Alzheimer’s disease. Brain 2020, 143, 1588–1602. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).