Potential Antioxidant Multitherapy against Complications Occurring in Sepsis
Abstract
1. Introduction
2. Oxidative Stress and Sepsis
2.1. Normal Response to Infection and Development of Sepsis
2.2. Endothelial Dysfunction
2.3. Systemic Complications in Sepsis
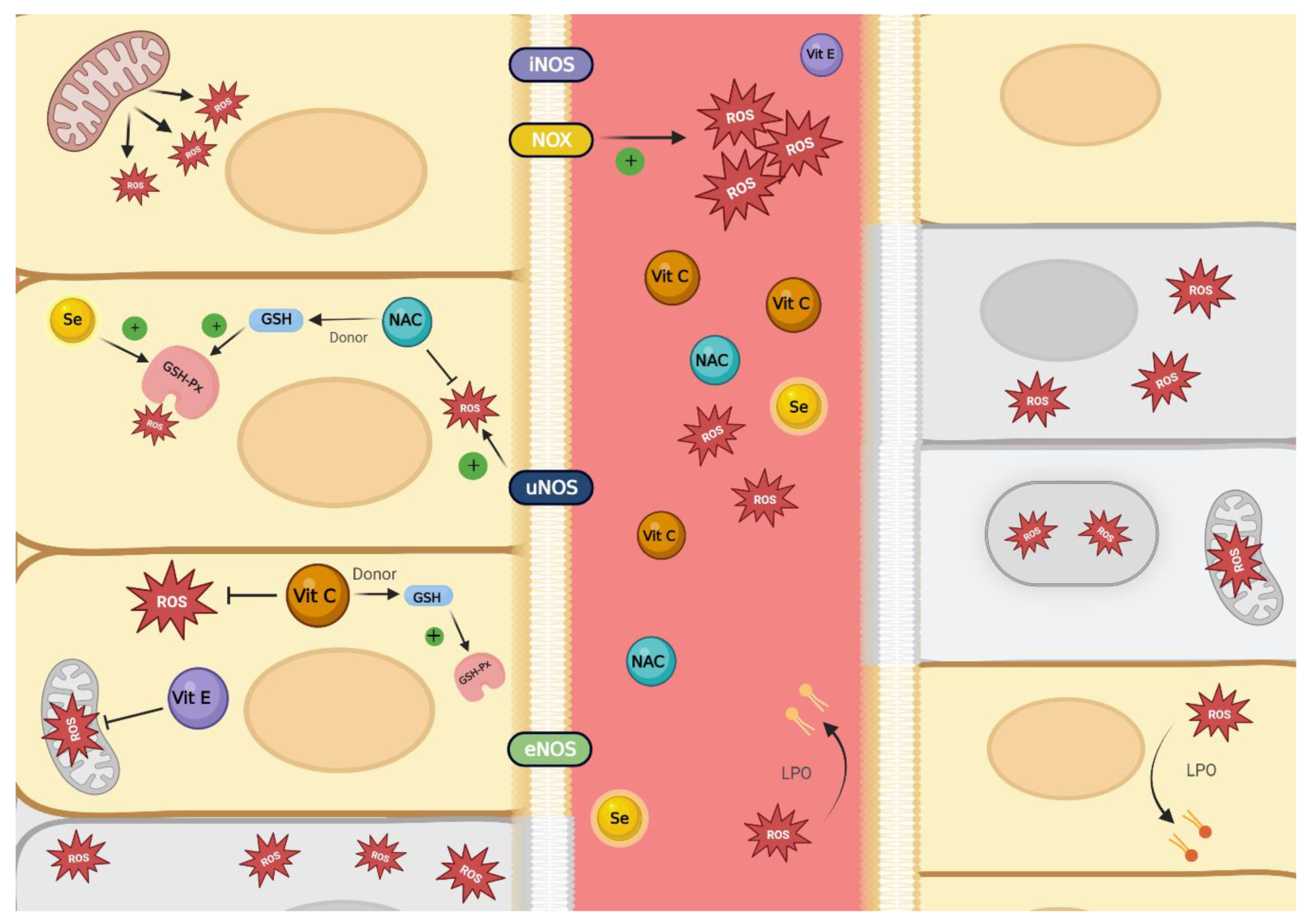
2.4. Oxidative Stress Definition and ROS Sources
2.5. Antioxidant Defense System
3. Antioxidant Treatments in Sepsis
3.1. Vitamin C
3.2. Selenium
3.3. N-acetylcysteine
3.4. Vitamin E
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Legrand, M. Epidemiology of sepsis and septic shock. Curr. Opin. Anaesthesiol. 2021, 34, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; De Simone, G.; Boccia, G.; De Caro, F.; Pagliano, P. Sepsis and septic shock: New definitions, new diagnostic and therapeutic approaches. J. Glob. Antimicrob. Resist. 2017, 10, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Merdji, H.; Schini-Kerth, V.; Meziani, F.; Toti, F. Long-term cardiovascular complications following sepsis: Is senescence the missing link? Ann. Intensive Care 2021, 11, 166. [Google Scholar] [CrossRef]
- Grebenchikov, O.A.; Kuzovlev, A.N. Long-Term Outcomes after Sepsis. Biochemistry 2021, 86, 563–567. [Google Scholar] [CrossRef]
- Forceville, X.; Vitoux, D.; Gauzit, R.; Combes, A.; Lahilaire, P.; Chappuis, P. Selenium, systemic immune response syndrome, sepsis and outcome in critically ill patients. Crit. Care Med. 1998, 26, 1536–1544. [Google Scholar] [CrossRef]
- Fowler, A.; Truwit, J.; Hite, R.; Morris, P.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure. JAMA 2019, 322, 1261. [Google Scholar] [CrossRef]
- Aisa-Alvarez, A.; Soto, M.E.; Guarner-Lans, V.; Camarena-Alejo, G.; Franco-Granillo, J.; Martínez-Rodríguez, E.A.; Gamboa Ávila, R.; Manzano Pech, L.; Pérez-Torres, I. Usefulness of Antioxidants as Adjuvant Therapy for Septic Shock: A Randomized Clinical Trial. Medicina 2020, 56, 619. [Google Scholar] [CrossRef]
- Spies, C.D.; Reinhart, K.; Witt, I.; Meier-Hellmann, A.; Hannemann, L.; Bredle, D.L.; Schaffartzik, W. Influence of N-acetylcysteine on indirect indicators of tissue oxygenation in septic shock patients: Results from a prospective, randomized, double-blind study. Crit. Care Med. 1994, 22, 1738–1746. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef]
- Taeb, A.M.; Hooper, M.H.; Marik, P.E. Sepsis: Current Definition, Pathophysiology, Diagnosis, and Management. Nutr. Clin. Pract. 2017, 32, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003, 101, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef]
- Nolt, B.; Tu, F.; Wang, X.; Ha, T.; Winter, R.; Williams, D.L.; Li, C. Lactate and Immunosuppression in Sepsis. Shock 2018, 49, 120–125. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528. [Google Scholar] [CrossRef]
- Levi, M. Pathogenesis and treatment of disseminated intravascular coagulation in the septic patient. J. Crit. Care 2001, 16, 167–177. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef]
- Neri, M.; Riezzo, I.; Pomara, C.; Schiavone, S.; Turillazzi, E. Oxidative-Nitrosative Stress and Myocardial Dysfunctions in Sepsis: Evidence from the Literature and Postmortem Observations. Mediat. Inflamm. 2016, 2016, 3423450. [Google Scholar] [CrossRef]
- Walley, K.R. Sepsis-induced myocardial dysfunction. Curr. Opin. Crit. Care 2018, 24, 292–299. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Wan, L.; Egi, M.; May, C.N.; Bellomo, R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006, 69, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Takasu, O.; Gaut, J.P.; Watanabe, E. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.R.; Glassford, N.; Bellomo, R. Acute kidney injury after cardiac arrest. Resuscitation 2012, 83, 721–727. [Google Scholar] [CrossRef]
- Fay, K.T.; Ford, M.L.; Coopersmith, C.M. The intestinal microenvironment in sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863 Pt B, 2574–2583. [Google Scholar] [CrossRef]
- Haussner, F.; Chakraborty, S.; Halbgebauer, R.; Huber-Lang, M. Challenge to the Intestinal Mucosa During Sepsis. Front. Immunol. 2019, 10, 891. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E.J.r.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef]
- Torres, L.K.; Pickkers, P.; van der Poll, T. Sepsis-Induced Immunosuppression. Annu. Rev. Physiol. 2022, 84, 157–181. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox. Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Aguayo, R.; Ramos, C.; Puentes, Á.; Gajardo, A.; Panieri, E.; Rojas-Solé, C.; Lillo-Moya, J.; Saso, L. Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Molecules 2021, 26, 5702. [Google Scholar] [CrossRef] [PubMed]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Gutterman, D.D. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid. Redox Signal. 2011, 15, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- González-Montero, J.; Brito, R.; Gajardo, A.I.; Rodrigo, R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J. Cardiol. 2018, 10, 74–86. [Google Scholar] [CrossRef]
- Ramos, M.F.P.; Monteiro de Barros, A.D.C.M.; Razvickas, C.V.; Borges, F.T.; Schor, N. Xanthine oxidase inhibitors and sepsis. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418772210. [Google Scholar] [CrossRef]
- Wang, X.; Han, M.; Bao, J.; Tu, W.; Dai, Z. A superoxide anion biosensor based on direct electron transfer of superoxide dismutase on sodium alginate sol-gel film and its application to monitoring of living cells. Anal. Chim. Acta 2012, 717, 61–66. [Google Scholar] [CrossRef]
- Nathan, A.T.; Singer, M. The oxygen trail: Tissue oxygenation. Br. Med. Bull. 1999, 55, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Galley, H.F.; Webster, N.R. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003, 90, 221–232. [Google Scholar] [CrossRef]
- Mantzarlis, K.; Tsolaki, V.; Zakynthinos, E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 5985209. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: Molecular mechanisms and potential clinical applications. Clin. Sci. 2013, 124, 1–15. [Google Scholar] [CrossRef]
- Ismy, J.; Syukri, M.; Emril, D.R.; Sekarwana, N.; Ismy, J.; Suhanda, R. The Effect of Superoxide Dismutase on Inhibition of Acute Kidney Injury Induced by Sepsis Based on Kidney Tissue Histology and Murine Sepsis Score. Sci. World J. 2021, 2021, 1827296. [Google Scholar] [CrossRef]
- Manzanares, W.; Biestro, A.; Galusso, F. Serum selenium and glutathione peroxidase-3 activity: Biomarkers of systemic inflammation in the critically ill? Intensive Care Med. 2009, 35, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Chen, Y.L.; Chu, Y.W.; Chien, D.S. Comparison of glutathione peroxidase-3 protein expression and enzyme bioactivity in normal subjects and patients with sepsis. Clin. Chim. Acta 2019, 489, 77–182. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.O.; Meissner, K.; Mayes, L.M.; Bartels, K. Vitamin C in sepsis. Curr. Opin. Anaesthesiol. 2018, 31, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kashiouris, M.G.; L’Heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients 2020, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Tyml, K. Vitamin C and Microvascular Dysfunction in Systemic Inflammation. Antioxidants 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A., 3rd; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Medical Respiratory Intensive Care Unit Nursing, Fisher BJ, Natarajan, R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 31, 12–32. [Google Scholar] [CrossRef]
- Borrelli, E.; Roux-Lombard, P.; Grau, G.E.; Girardin, E.; Ricou, B.; Dayer, J.; Suter, P.M. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996, 24, 392–397. [Google Scholar] [CrossRef]
- Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalil, H. Effect of high-dose ascorbic acid on vasopressor requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100. [Google Scholar] [CrossRef]
- El Driny, W.A.; Esmat, I.M.; Shaheen, S.M.; Sabri, N.A. Efficacy of High-Dose Vitamin C Infusion on Outcomes in Sepsis Requiring Mechanical Ventilation: A Double-Blind Randomized Controlled Trial. Anesthesiol. Res. Pract. 2022, 2022, 4057215. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, W.A.; van Zanten, A.R. Antioxidant Vitamins and Trace Elements in Critical Illness. Nutr. Clin. Pract. 2016, 31, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Hawker, F.H.; Stewart, P.M.; Snitch, P.J. Effects of acute illness on selenium homeostasis. Crit. Care Med. 1990, 18, 442–446. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Taylor, B.E.; McClave, S.A.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.; Cresci, G.A.; et al. Society of Critical Care Medicine; American Society of Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit. Care Med. 2016, 44, 390–438. [Google Scholar] [CrossRef]
- Angstwurm, M.W.; Engelmann, L.; Zimmermann, T.; Lehmann, C.; Spes, C.H.; Abel, P.; Strauss, R.; Meier-Hellmann, A.; Insel, R.; Radke, J.; et al. Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit. Care Med. 2007, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kočan, L.; Vašková, J.; Vaško, L.; Simonová, J.; Simon, R.; Firment, J. Selenium adjuvant therapy in septic patients selected according to Carrico index. Clin. Biochem. 2014, 47, 44–50. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Q.; Liu, B. The impact of selenium administration on severe sepsis or septic shock: A meta-analysis of randomized controlled trials. Afr. Health Sci. 2021, 21, 277–285. [Google Scholar] [CrossRef]
- Coles, L.D.; Tuite, P.J.; Öz, G. Repeated-Dose Oral N-Acetylcysteine in Parkinson’s Disease: Pharmacokinetics and Effect on Brain Glutathione and Oxidative Stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain. Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; González-Montero, J.; Sotomayor, C.G. Novel Combined Antioxidant Strategy against Hypertension, Acute Myocardial Infarction and Postoperative Atrial Fibrillation. Biomedicines 2021, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Noguchi, N. Antioxidant action of vitamin E in vivo as assessed from its reaction products with multiple biological oxidants. Free Radic. Res. 2021, 55, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.U.; Lehmann, L.E.; Schewe, J.C.; Thiele, J.T.; Schröder, S.; Book, M.; Hoeft, A.; Stüber, F. Low serum alpha-tocopherol and selenium are associated with accelerated apoptosis in severe sepsis. Biofactors 2008, 33, 107–119. [Google Scholar] [CrossRef]
- Basu, S.; Eriksson, M. Vitamin E in relation to lipid peroxidation in experimental septic shock. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 195–199. [Google Scholar] [CrossRef]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert. Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- Peake, S.L.; Moran, J.L.; Leppard, P.I. N-acetyl-L-cysteine depresses cardiac performance in patients with septic shock. Crit. Care Med. 1996, 24, 1302–1310. [Google Scholar] [CrossRef]
- McCune, T.R.; Toepp, A.J.; Sheehan, B.E.; Sherani, M.S.K.; Petr, S.T.; Dodani, S. High dose intravenous vitamin C treatment in Sepsis: Associations with acute kidney injury and mortality. BMC Nephrol. 2021, 22, 387. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Shadvar, K.; Sanaie, S.; Hadipoor, M.R.; Pourmoghaddam, M.A.; Saghaleini, S.H. Effect of Vitamin C on mortality of critically ill patients with severe pneumonia in intensive care unit: A preliminary study. BMC Infect. Dis. 2021, 21, 616. [Google Scholar] [CrossRef]
- Spapen, H.D.; Diltoer, M.W.; Nguyen, D.N.; Hendrickx, I.; Huyghens, L.P. Effects of N-acetylcysteine on microalbuminuria and organ failure in acute severe sepsis: Results of a pilot study. Chest 2005, 127, 1413–1419. [Google Scholar] [CrossRef]
- Valenta, J.; Brodska, H.; Drabek, T.; Hendl, J.; Kazda, A. High-dose selenium substitution in sepsis: A prospective randomized clinical trial. Intensive Care Med. 2011, 37, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Woth, G.; Nagy, B.; Mérei, Á.; Ernyey, B.; Vincze, R.; Kaurics, Z.; Lantos, J.; Bogár, L.; Mühl, D. The effect of Na-selenite treatment on the oxidative stress-antioxidants balance of multiple organ failure. J. Crit. Care 2014, 29, 883. [Google Scholar] [CrossRef] [PubMed]
- Bloos, F.; Trips, E.; Nierhaus, A.; Briegel, J.; Heyland, D.K.; Jaschinski, U.; Moerer, O.; Weyland, A.; Marx, G.; Gründling, M.; et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients with Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, F.; Masse, M.H.; Menard, J.; Sprague, S.; Pinto, R.; Heyland, D.K.; Cook, D.J.; Battista, M.C.; Day, A.G.; Guyatt, G.H.; et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N. Engl. J. Med. 2022, 386, 2387–2398. [Google Scholar] [CrossRef]
- Chelkeba, L.; Ahmadi, A.; Abdollahi, M. The effect of parenteral selenium on outcomes of mechanically ventilated patients following sepsis: A prospective randomized clinical trial. Ann. Intensive Care 2015, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Srinath, J.; Feuerecker, M.; Crucian, B.; Briegel, J.; Boulesteix, A.-L.; Kaufmann, I.; Choukèr, A. Immune Function Testing in Sepsis Patients Receiving Sodium Selenite. J. Crit. Care 2019, 52, 208–212. [Google Scholar] [CrossRef]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef]
- Carlson, D.; Maass, D.L.; White, D.J.; Tan, J.; Horton, J.W. Antioxidant Vitamin Therapy Alters Sepsis-Related Apoptotic Myocardial Activity and Inflammatory Responses. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2779–H2789. [Google Scholar] [CrossRef]
- Galvão, A.M.; Wanderley, M.S.O.; Silva, R.A.; Filho, C.A.M.; Melo-Junior, M.R.; Silva, L.A.; Streck, E.L.; Dornelas de Andrade, A.F.; Souza Maia, M.B.; Barbosa de Castro, C.M.M. Intratracheal co-administration of antioxidants and ceftriaxone reduces pulmonary injury and mortality rate in an experimental model of sepsis. Respirology 2014, 19, 1080–1087. [Google Scholar] [CrossRef]
- Galley, H.F.; Howdle, P.D.; Walker, B.E.; Webster, N.R. The effects of intravenous antioxidants in patients with septic shock. Free Radic. Biol. Med. 1997, 23, 768–774. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant | Study Details | n | Main Findings | Ref. | |
|---|---|---|---|---|---|
| Intervention Control | |||||
| Ascorbate | Vitamin C intravenous infusion in patients with sepsis and ARDS for less than 24 h. | 84 | 83 | There were no significant differences in organ dysfunction scores or markers in inflammation and vascular injury between both groups. | [7] |
| Intravenous vitamin C in patients with sepsis receiving vasopressor therapy. | 435 | 437 | The risk of death or persistent organ dysfunction at 28 days was higher in the Vitamin C group. | [74] | |
| Effect of high-dose ascorbic acid on vasopressor drug requirement in surgical patients with septic shock. | 14 | 14 | The mean dose of epinephrine and duration of norepinephrine administration was significantly lower in the ascorbic acid group. | [50] | |
| Effects of vitamin C therapy on acute kidney injury (AKI) and mortality among septic patients. | 212 | 1178 | The occurrence of AKI in ICU patients was significantly higher in the vitamin C group, with no protective benefit against mortality. | [68] | |
| Effect of Vitamin C on mortality of critically ill patients with severe pneumonia in intensive care unit. | 40 | 40 | Duration of mechanical ventilation and vasopressor use were significantly lower in the vitamin C group. | [69] | |
| Effect of orally administered vitamin C adjuvant treatment on septic patients with multi organ failure. | 18 | 21 | The vitamin C group had decreased C reactive protein levels and the nitrate/nitrite ratio. | [8] | |
| NAC | Continuous N-acetylcysteine infusion adjuvant to standard sepsis therapy. | 10 | 10 | NAC infusion was associated with a depression in cardiovascular performance. No differences in hospital mortality rate. | [67] |
| Continuous N-acetylcysteine infusion adjuvant to standard sepsis therapy. | 18 | 17 | Worsening of organ failure and particularly cardiovascular failure in the NAC treatment group. | [70] | |
| Effect of orally administered N-acetylcysteine adjuvant treatment on septic patients with multi organ failure. | 20 | 21 | The NAC administered group had a decrease in procalcitonin levels and increased antioxidant capacity. | [8] | |
| Selenium | Sodium-selenite in patients with severe sepsis to reduce oxidative stress. | 21 | 19 | Serum selenium levels increased, and ROS decreased significantly. However, there was no significant difference in the survival rate and SOFA score between both groups. | [72] |
| High-dose intravenous administration of sodium-selenite in patients with severe sepsis or septic shock. | 543 | 546 | There was no statistical difference in the 28-day mortality between both groups. | [73] | |
| High-dose supplementation of sodium-selenite as an adjuvant therapy in patients with severe sepsis or septic shock. | 92 | 97 | The adjuvant therapy of sodium-selenite reduced the mortality rate. | [56] | |
| High dose compared with standard dose (control group) of selenium in patients with SIRS/sepsis with SOFA >5. | 75 | 75 | High dose selenium substitution increased serum levels of selenium and GSH-Px levels but did not reduce mortality. | [71] | |
| Parenteral selenium in mechanically ventilated patients with sepsis or septic shock. | 29 | 25 | The 28-day mortality was not significantly different. The activity of GSH-Px increased. Additionally, selenium supplementation was associated with reduced occurrence of ventilator-associated pneumonia. | [75] | |
| Immune function testing in sepsis patients receiving sodium selenite | 40 | 36 | An overall dampening in cytokine release was observed in the selenium group. | [76] | |
| Vitamin E | Effect of orally administered vitamin E adjuvant treatment on septic patients with multi organ failure. | 18 | 21 | The group supplemented with vitamin E had decreased levels of procalcitonin. | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abelli, J.; Méndez-Valdés, G.; Gómez-Hevia, F.; Bragato, M.C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Potential Antioxidant Multitherapy against Complications Occurring in Sepsis. Biomedicines 2022, 10, 3088. https://doi.org/10.3390/biomedicines10123088
Abelli J, Méndez-Valdés G, Gómez-Hevia F, Bragato MC, Chichiarelli S, Saso L, Rodrigo R. Potential Antioxidant Multitherapy against Complications Occurring in Sepsis. Biomedicines. 2022; 10(12):3088. https://doi.org/10.3390/biomedicines10123088
Chicago/Turabian StyleAbelli, Joaquin, Gabriel Méndez-Valdés, Francisca Gómez-Hevia, Maria Chiara Bragato, Silvia Chichiarelli, Luciano Saso, and Ramón Rodrigo. 2022. "Potential Antioxidant Multitherapy against Complications Occurring in Sepsis" Biomedicines 10, no. 12: 3088. https://doi.org/10.3390/biomedicines10123088
APA StyleAbelli, J., Méndez-Valdés, G., Gómez-Hevia, F., Bragato, M. C., Chichiarelli, S., Saso, L., & Rodrigo, R. (2022). Potential Antioxidant Multitherapy against Complications Occurring in Sepsis. Biomedicines, 10(12), 3088. https://doi.org/10.3390/biomedicines10123088









