The Long-Term Effects of Prenatal Hypoxia on Coronary Artery Function of the Male and Female Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model of Prenatal Hypoxia
2.2. Coronary Artery Vascular Function by Wire Myography
2.3. Molecular Assessment of Plasma ET-1 Levels with ELISA
2.4. Immunofluorescent Staining of the Main Left Coronary Artery for ET-1, ETA, ETB, eNOS, PGHS-1, and PGHS-2
2.5. Image Analysis of Immunofluorescence Staining
2.6. Statistical Analysis
3. Results
3.1. Coronary Artery Endothelium-Dependent and Endothelium-Independent Vasodilation Responses
3.1.1. Endothelium-Dependent Vasodilation Was Impaired in Prenatally Hypoxic Male and Female 4-Month-Old Offspring
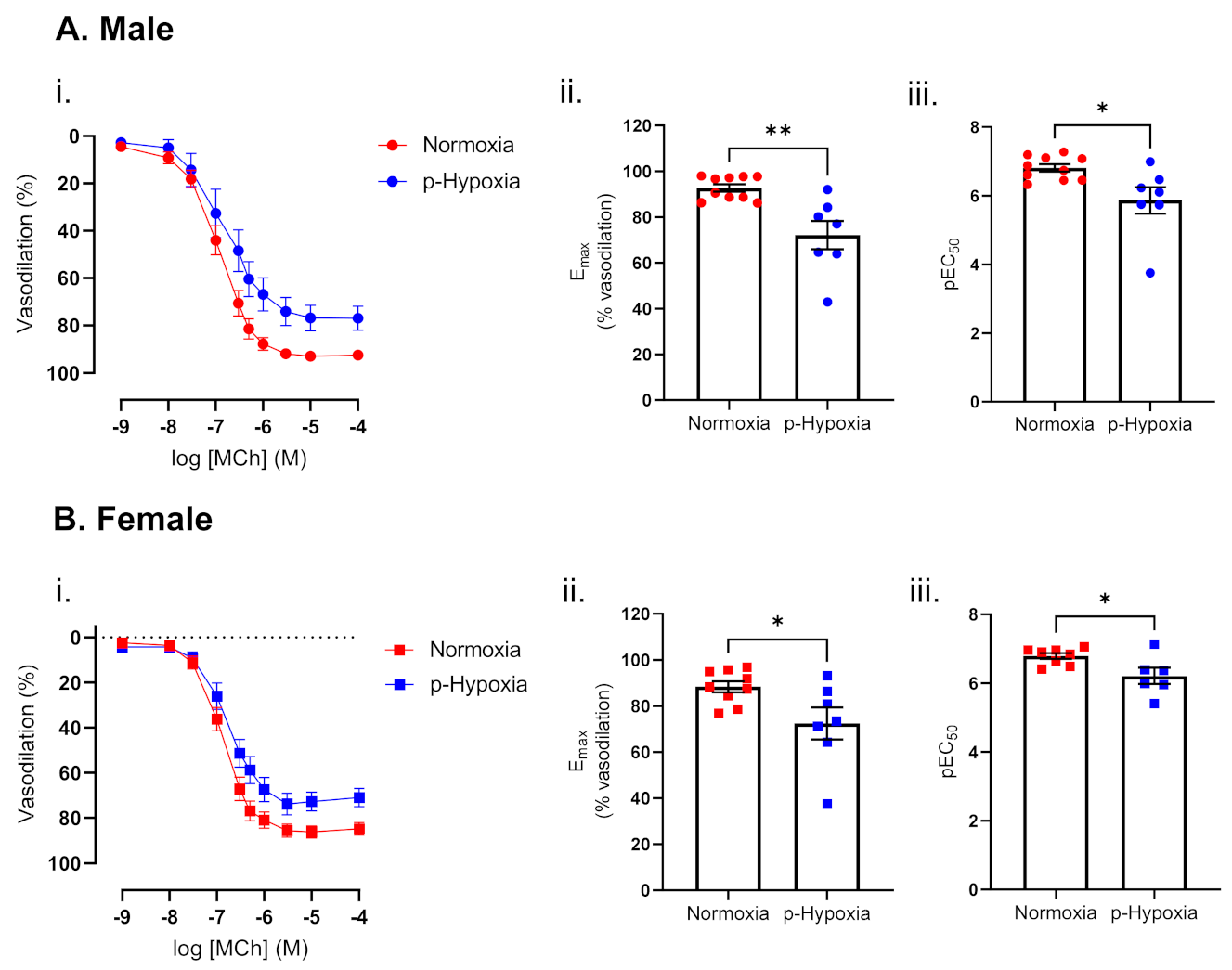
3.1.2. Impaired Endothelium-Dependent Vasodilation in Prenatally Hypoxic Male and Female 9.5-Month-Old Offspring
3.2. Mechanisms of Endothelium-Dependent Vasodilation in Male and Female 9.5-Moth-Old Offspring
3.2.1. The Nitric Oxide Synthase (NOS) Pathway Is a Major Contributor to Coronary Artery Endothelium-Dependent Vasodilation in Male and Female Offspring
3.2.2. Enhanced Contribution of the PGHS Pathway to Coronary Artery Endothelium-Dependent Vasodilation in Prenatally Hypoxic Offspring
3.2.3. The Contribution of Endothelium-Derived Hyperpolarization (EDH) to Endothelium-Dependent Vasodilation Is Enhanced in Coronary Arteries of Prenatally Hypoxia Male and Female Offspring
3.3. Coronary Artery Responses to ET-1 and the Contribution of ETA and ETB
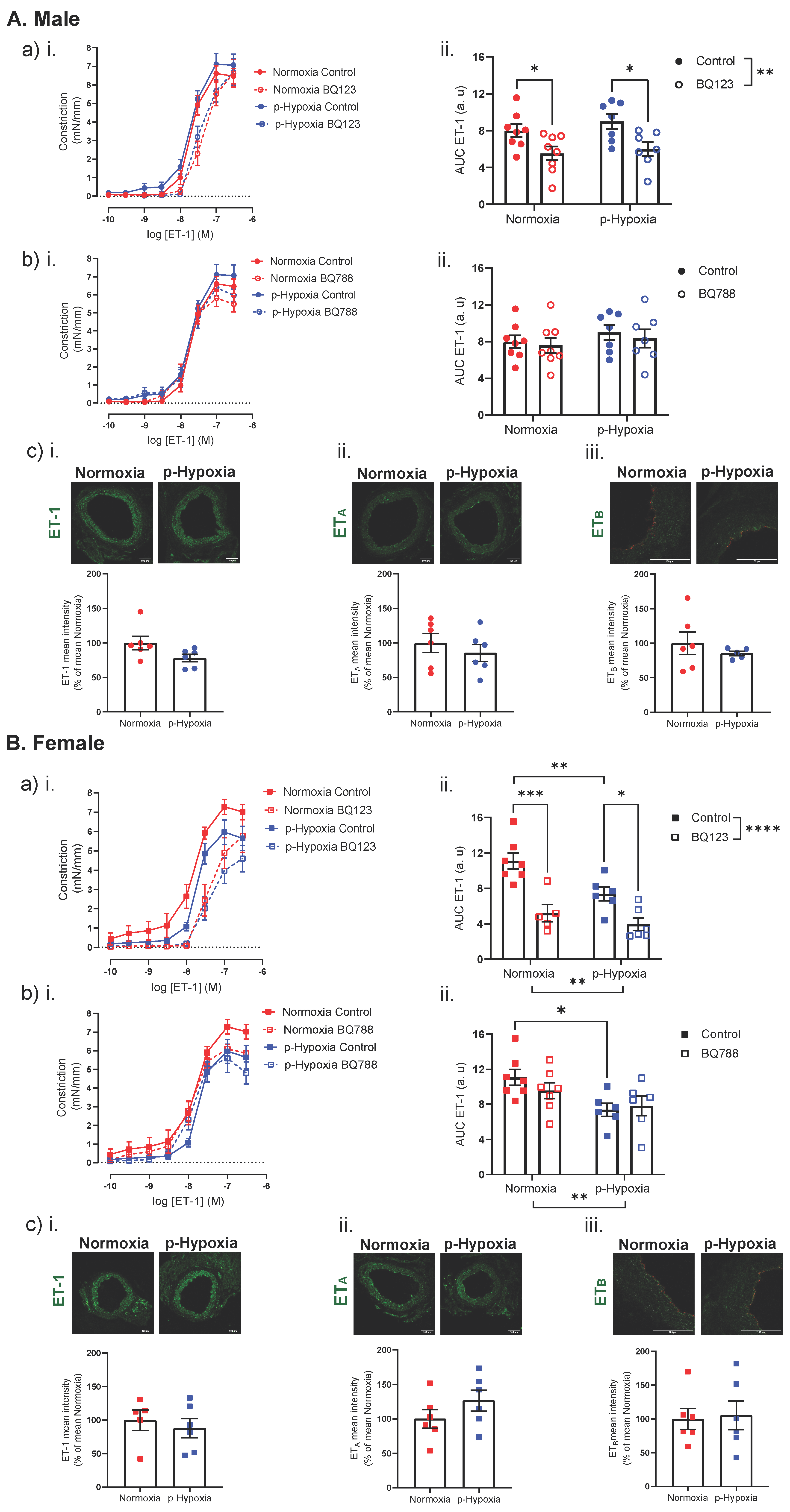
3.3.1. Increased Contribution of ETB Receptors to ET-1 Mediated Vasoconstriction in 4-Month-Old Female Offspring
3.3.2. An Impaired ET-1 Mediated Vasoconstriction in 9.5-Month-Old Female Offspring
4. Discussion
4.1. The Effect of Prenatal Hypoxia on Coronary Artery Endothelium-Dependent and Endothelium-Independent Vasodilation in Adult Male and Female Offspring
4.2. The Effect of Prenatal Hypoxia on Coronary Artery ET-1 System in Male and Female Offspring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheong, J.N.; Wlodek, M.E.; Moritz, K.M.; Cuffe, J.S. Programming of maternal and offspring disease: Impact of growth restriction, fetal sex and transmission across generations. J. Physiol. 2016, 594, 4727–4740. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Crimmins, S.; Telugu, B.; Turan, S. Intrauterine hypoxia: Clinical consequences and therapeutic perspectives. Res. Rep. Neonatol. 2015, 5, 79–89. [Google Scholar] [CrossRef]
- Giussani, D.A.; Davidge, S.T. Developmental programming of cardiovascular disease by prenatal hypoxia. J. Dev. Orig. Health Dis. 2013, 4, 328–337. [Google Scholar] [CrossRef]
- Bourque, S.L.; Gragasin, F.S.; Quon, A.L.; Mansour, Y.; Morton, J.S.; Davidge, S.T. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension 2013, 62, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Ream, M.; Ray, A.M.; Chandra, R.; Chikaraishi, D.M. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R583–R595. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wang, C. Prenatal hypoxia-induced epigenomic and transcriptomic reprogramming in rat fetal and adult offspring hearts. Sci. Data 2019, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacos, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Camm, E.J.; Niu, Y.; Richter, H.G.; Blanco, C.E.; Gottschalk, R.; Blake, E.Z.; Horder, K.A.; Thakor, A.S.; Hansell, J.A.; et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE 2012, 7, e31017. [Google Scholar] [CrossRef]
- Zhang, L. Prenatal hypoxia and cardiac programming. J. Soc. Gynecol. Investig. 2005, 12, 2–13. [Google Scholar] [CrossRef]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Stein, C.E.; Fall, C.H.; Kumaran, K.; Osmond, C.; Cox, V.; Barker, D.J. Fetal growth and coronary heart disease in south India. Lancet 1996, 348, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Forsen, T.; Eriksson, J.G.; Tuomilehto, J.; Osmond, C.; Barker, D.J. Growth in utero and during childhood among women who develop coronary heart disease: Longitudinal study. BMJ 1999, 319, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Leon, D.A.; Lithell, H.O.; Vagero, D.; Koupilova, I.; Mohsen, R.; Berglund, L.; Lithell, U.B.; McKeigue, P.M. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: Cohort study of 15 000 Swedish men and women born 1915-29. BMJ 1998, 317, 241–245. [Google Scholar] [CrossRef]
- Aburawi, E.H.; Malcus, P.; Thuring, A.; Fellman, V.; Pesonen, E. Coronary flow in neonates with impaired intrauterine growth. J. Am. Soc. Echocardiogr. 2012, 25, 313–318. [Google Scholar] [CrossRef]
- Baschat, A.A.; Gembruch, U.; Harman, C.R. Coronary blood flow in fetuses with intrauterine growth restriction. J. Perinat Med. 1998, 26, 143–156. [Google Scholar]
- Baschat, A.A.; Gembruch, U.; Reiss, I.; Gortner, L.; Diedrich, K. Demonstration of fetal coronary blood flow by Doppler ultrasound in relation to arterial and venous flow velocity waveforms and perinatal outcome--the ‘heart-sparing effect’. Ultrasound Obstet. Gynecol. 1997, 9, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Poore, K.R.; Boullin, J.P.; Khan, O.; Chau, R.; Hambidge, O.; Torrens, C.; Newman, J.P.; Poston, L.; Noakes, D.E.; et al. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc. Natl. Acad. Sci. USA 2007, 104, 9529–9533. [Google Scholar] [CrossRef]
- Bubb, K.J.; Cock, M.L.; Black, M.J.; Dodic, M.; Boon, W.M.; Parkington, H.C.; Harding, R.; Tare, M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J. Physiol. 2007, 578, 871–881. [Google Scholar] [CrossRef]
- Schipke, J.; Gonzalez-Tendero, A.; Cornejo, L.; Willfuhr, A.; Bijnens, B.; Crispi, F.; Muhlfeld, C.; Gratacos, E. Experimentally induced intrauterine growth restriction in rabbits leads to differential remodelling of left versus right ventricular myocardial microstructure. Histochem. Cell Biol. 2017, 148, 557–567. [Google Scholar] [CrossRef]
- Sutherland, M.R.; Ng, K.W.; Drenckhahn, J.D.; Wlodek, M.E.; Black, M.J. Impact of Intrauterine Growth Restriction on the Capillarization of the Early Postnatal Rat Heart. Anat. Rec. (Hoboken) 2019, 302, 1580–1586. [Google Scholar] [CrossRef]
- Tare, M.; Parkington, H.C.; Wallace, E.M.; Sutherland, A.E.; Lim, R.; Yawno, T.; Coleman, H.A.; Jenkin, G.; Miller, S.L. Maternal melatonin administration mitigates coronary stiffness and endothelial dysfunction, and improves heart resilience to insult in growth restricted lambs. J. Physiol. 2014, 592, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Canadilla, P.; de Vries, T.; Gonzalez-Tendero, A.; Bonnin, A.; Gratacos, E.; Crispi, F.; Bijnens, B.; Zhang, C. Structural coronary artery remodelling in the rabbit fetus as a result of intrauterine growth restriction. PLoS ONE 2019, 14, e0218192. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Yang, S.; Zhang, L. Prenatal cocaine exposure causes sex-dependent impairment in the myogenic reactivity of coronary arteries in adult offspring. Hypertension 2009, 54, 1123–1128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, X.; Qi, L.; Su, H.; He, Y.; Li, N.; Gao, Q.; Li, H.; Xu, T.; Lu, L.; Xu, Z.; et al. Prenatal hypoxia attenuated contraction of offspring coronary artery associated with decreased PKCbeta Ser(660) phosphorylation and intracellular calcium. Life Sci. 2020, 261, 118364. [Google Scholar] [CrossRef]
- Deussen, A.; Ohanyan, V.; Jannasch, A.; Yin, L.; Chilian, W. Mechanisms of metabolic coronary flow regulation. J. Mol. Cell Cardiol 2012, 52, 794–801. [Google Scholar] [CrossRef]
- Radico, F.; Zimarino, M.; Fulgenzi, F.; Ricci, F.; Di Nicola, M.; Jespersen, L.; Chang, S.M.; Humphries, K.H.; Marzilli, M.; De Caterina, R. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: A systematic review and meta-analysis. Eur. Heart J. 2018, 39, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Mensah, G.A. Healthy endothelium: The scientific basis for cardiovascular health promotion and chronic disease prevention. Vascul. Pharmacol. 2007, 46, 310–314. [Google Scholar] [CrossRef]
- Grover-Paez, F.; Zavalza-Gomez, A.B. Endothelial dysfunction and cardiovascular risk factors. Diabetes Res. Clin. Pract. 2009, 84, 1–10. [Google Scholar] [CrossRef]
- Brain, K.L.; Allison, B.J.; Niu, Y.; Cross, C.M.; Itani, N.; Kane, A.D.; Herrera, E.A.; Skeffington, K.L.; Botting, K.J.; Giussani, D.A. Intervention against hypertension in the next generation programmed by developmental hypoxia. PLoS Biol. 2019, 17, e2006552. [Google Scholar] [CrossRef]
- Garcia-Villalon, A.L.; Fernandez, N.; Monge, L.; Salcedo, A.; Dieguez, G. Effects of endothelin-1 on the relaxation of rat coronary arteries. J. Cardiovasc. Pharmacol. 2009, 54, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.E.; Greenwood, I.A.; Large, W.A. Tonic regulation of vascular tone by nitric oxide and chloride ions in rat isolated small coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2604–H2611. [Google Scholar] [CrossRef] [PubMed]
- Climent, B.; Moreno, L.; Martinez, P.; Contreras, C.; Sanchez, A.; Perez-Vizcaino, F.; Garcia-Sacristan, A.; Rivera, L.; Prieto, D. Upregulation of SK3 and IK1 channels contributes to the enhanced endothelial calcium signaling and the preserved coronary relaxation in obese Zucker rats. PLoS ONE 2014, 9, e109432. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, M.Q.; Khalil, R.A. Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012, 84, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A.; Burnett, J.C., Jr. Intact and altered endothelium in regulation of vasomotion. Circulation 1992, 86, III12–III19. [Google Scholar]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E., Jr.; Epstein, S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef]
- Vita, J.A.; Treasure, C.B.; Nabel, E.G.; McLenachan, J.M.; Fish, R.D.; Yeung, A.C.; Vekshtein, V.I.; Selwyn, A.P.; Ganz, P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation 1990, 81, 491–497. [Google Scholar] [CrossRef]
- Chong, A.Y.; Freestone, B.; Patel, J.; Lim, H.S.; Hughes, E.; Blann, A.D.; Lip, G.Y. Endothelial activation, dysfunction, and damage in congestive heart failure and the relation to brain natriuretic peptide and outcomes. Am. J. Cardiol. 2006, 97, 671–675. [Google Scholar] [CrossRef]
- Niu, Y.; Kane, A.D.; Lusby, C.M.; Allison, B.J.; Chua, Y.Y.; Kaandorp, J.J.; Nevin-Dolan, R.; Ashmore, T.J.; Blackmore, H.L.; Derks, J.B.; et al. Maternal Allopurinol Prevents Cardiac Dysfunction in Adult Male Offspring Programmed by Chronic Hypoxia During Pregnancy. Hypertension 2018, 72, 971–978. [Google Scholar] [CrossRef]
- Rueda-Clausen, C.F.; Morton, J.S.; Davidge, S.T. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc. Res. 2009, 81, 713–722. [Google Scholar] [CrossRef]
- Xu, Y.; Williams, S.J.; O’Brien, D.; Davidge, S.T. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006, 20, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: Role of protein kinase C epsilon. J. Pharmacol. Exp. Ther. 2009, 330, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Hasdai, D.; Gibbons, R.J.; Holmes, D.R., Jr.; Higano, S.T.; Lerman, A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation 1997, 96, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Quyyumi, A.A.; Dakak, N.; Andrews, N.P.; Husain, S.; Arora, S.; Gilligan, D.M.; Panza, J.A.; Cannon, R.O., 3rd. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J. Clin. Invest. 1995, 95, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.; Schrader, J. Control of coronary vascular tone by nitric oxide. Circ. Res. 1990, 66, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the role of nitric oxide and its clinical applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef]
- Garcia, V.; Sessa, W.C. Endothelial NOS: Perspective and recent developments. Br. J. Pharmacol 2019, 176, 189–196. [Google Scholar] [CrossRef]
- Berges, A.; Van Nassauw, L.; Timmermans, J.P.; Vrints, C. Role of nitric oxide during coronary endothelial dysfunction after myocardial infarction. Eur. J. Pharmacol. 2005, 516, 60–70. [Google Scholar] [CrossRef]
- Chen, C.; Ochoa, L.N.; Kagan, A.; Chai, H.; Liang, Z.; Lin, P.H.; Yao, Q. Lysophosphatidic acid causes endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells. Atherosclerosis 2012, 222, 74–83. [Google Scholar] [CrossRef]
- Ramaswami, G.; Chai, H.; Yao, Q.; Lin, P.H.; Lumsden, A.B.; Chen, C. Curcumin blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Vasc Surg 2004, 40, 1216–1222. [Google Scholar] [CrossRef]
- Davidge, S.T. Prostaglandin H synthase and vascular function. Circ. Res. 2001, 89, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef] [PubMed]
- Loftin, C.D.; Trivedi, D.B.; Tiano, H.F.; Clark, J.A.; Lee, C.A.; Epstein, J.A.; Morham, S.G.; Breyer, M.D.; Nguyen, M.; Hawkins, B.M.; et al. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc. Natl. Acad. Sci. USA 2001, 98, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Norwood, V.F.; Morham, S.G.; Smithies, O. Postnatal development and progression of renal dysplasia in cyclooxygenase-2 null mice. Kidney Int. 2000, 58, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Baserga, M.; Hale, M.A.; Wang, Z.M.; Yu, X.; Callaway, C.W.; McKnight, R.A.; Lane, R.H. Uteroplacental insufficiency alters nephrogenesis and downregulates cyclooxygenase-2 expression in a model of IUGR with adult-onset hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1943–R1955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellinsworth, D.C.; Sandow, S.L.; Shukla, N.; Liu, Y.; Jeremy, J.Y.; Gutterman, D.D. Endothelium-Derived Hyperpolarization and Coronary Vasodilation: Diverse and Integrated Roles of Epoxyeicosatrienoic Acids, Hydrogen Peroxide, and Gap Junctions. Microcirculation 2016, 23, 15–32. [Google Scholar] [CrossRef]
- Edwards, G.; Feletou, M.; Weston, A.H. Endothelium-derived hyperpolarising factors and associated pathways: A synopsis. Pflugers Arch. 2010, 459, 863–879. [Google Scholar] [CrossRef]
- Sandow, S.L.; Neylon, C.B.; Chen, M.X.; Garland, C.J. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: Possible relationship to vasodilator function? J. Anat. 2006, 209, 689–698. [Google Scholar] [CrossRef]
- Socha, M.J.; Behringer, E.J.; Segal, S.S. Calcium and electrical signalling along endothelium of the resistance vasculature. Basic Clin. Pharmacol. Toxicol. 2012, 110, 80–86. [Google Scholar] [CrossRef]
- Grgic, I.; Kaistha, B.P.; Hoyer, J.; Kohler, R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery. Br. J. Pharmacol. 2009, 157, 509–526. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Clements, R.T.; Sodha, N.R.; Khabbaz, K.R.; Senthilnathan, V.; Nishimura, K.K.; Alper, S.L.; Sellke, F.W. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest. Circulation 2008, 118, S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, J.H.; Man, Y.B.; Yao, X.Q.; He, G.W. Use of intermediate/small conductance calcium-activated potassium-channel activator for endothelial protection. J. Thorac. Cardiovasc. Surg. 2011, 141, 501–510.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Li, A.Y.; Guo, Q.H.; Guo, Y.J.; Weiss, J.W.; Ji, E.S. Endothelin-1 and ET receptors impair left ventricular function by mediated coronary arteries dysfunction in chronic intermittent hypoxia rats. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Dagassan, P.H.; Breu, V.; Clozel, M.; Kunzli, A.; Vogt, P.; Turina, M.; Kiowski, W.; Clozel, J.P. Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. J. Cardiovasc. Pharmacol. 1996, 27, 147–153. [Google Scholar] [CrossRef]
- Wackenfors, A.; Emilson, M.; Ingemansson, R.; Hortobagyi, T.; Szok, D.; Tajti, J.; Vecsei, L.; Edvinsson, L.; Malmsjo, M. Ischemic heart disease induces upregulation of endothelin receptor mRNA in human coronary arteries. Eur. J. Pharmacol. 2004, 484, 103–109. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr.; Liu, Y.; Pergola, P.E. Selected contribution: Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J. Appl Physiol. (1985) 2001, 91, 2407–2411; discussion 2389–2490. [Google Scholar] [CrossRef]
- Katakam, P.V.; Snipes, J.A.; Tulbert, C.D.; Mayanagi, K.; Miller, A.W.; Busija, D.W. Impaired endothelin-induced vasoconstriction in coronary arteries of Zucker obese rats is associated with uncoupling of [Ca2+]i signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol 2006, 290, R145–R153. [Google Scholar] [CrossRef]
- Giulumian, A.D.; Molero, M.M.; Reddy, V.B.; Pollock, J.S.; Pollock, D.M.; Fuchs, L.C. Role of ET-1 receptor binding and [Ca(2+)](i) in contraction of coronary arteries from DOCA-salt hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1944–H1949. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hula, N.; Liu, R.; Spaans, F.; Pasha, M.; Quon, A.; Kirschenman, R.; Cooke, C.-L.M.; Davidge, S.T. The Long-Term Effects of Prenatal Hypoxia on Coronary Artery Function of the Male and Female Offspring. Biomedicines 2022, 10, 3019. https://doi.org/10.3390/biomedicines10123019
Hula N, Liu R, Spaans F, Pasha M, Quon A, Kirschenman R, Cooke C-LM, Davidge ST. The Long-Term Effects of Prenatal Hypoxia on Coronary Artery Function of the Male and Female Offspring. Biomedicines. 2022; 10(12):3019. https://doi.org/10.3390/biomedicines10123019
Chicago/Turabian StyleHula, Nataliia, Ricky Liu, Floor Spaans, Mazhar Pasha, Anita Quon, Raven Kirschenman, Christy-Lynn M. Cooke, and Sandra T. Davidge. 2022. "The Long-Term Effects of Prenatal Hypoxia on Coronary Artery Function of the Male and Female Offspring" Biomedicines 10, no. 12: 3019. https://doi.org/10.3390/biomedicines10123019
APA StyleHula, N., Liu, R., Spaans, F., Pasha, M., Quon, A., Kirschenman, R., Cooke, C.-L. M., & Davidge, S. T. (2022). The Long-Term Effects of Prenatal Hypoxia on Coronary Artery Function of the Male and Female Offspring. Biomedicines, 10(12), 3019. https://doi.org/10.3390/biomedicines10123019






