Acetylcholine Reduces L-Type Calcium Current without Major Changes in Repolarization of Canine and Human Purkinje and Ventricular Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Conventional Microelectrode Recordings
2.1.1. Dog Heart Preparations
2.1.2. Human Heart Preparations
Human Atrial Preparation (Left Atrial Appendage)
Human Left Ventricular Myocardial Slices
2.2. Patch Clamp Recordings from hiPSC-CMs
2.2.1. hiPSC Culture and Cardiomyocytes Differentiation
2.2.2. Preparation of hiPSC-CMs for Patch Clamp Recordings
2.2.3. Data Acquisition
2.2.4. L-Type Calcium Current Measurements
2.2.5. Action Potential Measurements
2.3. Computer Simulations
2.4. Statistics
3. Results
3.1. Standard Microelectrode Measurements
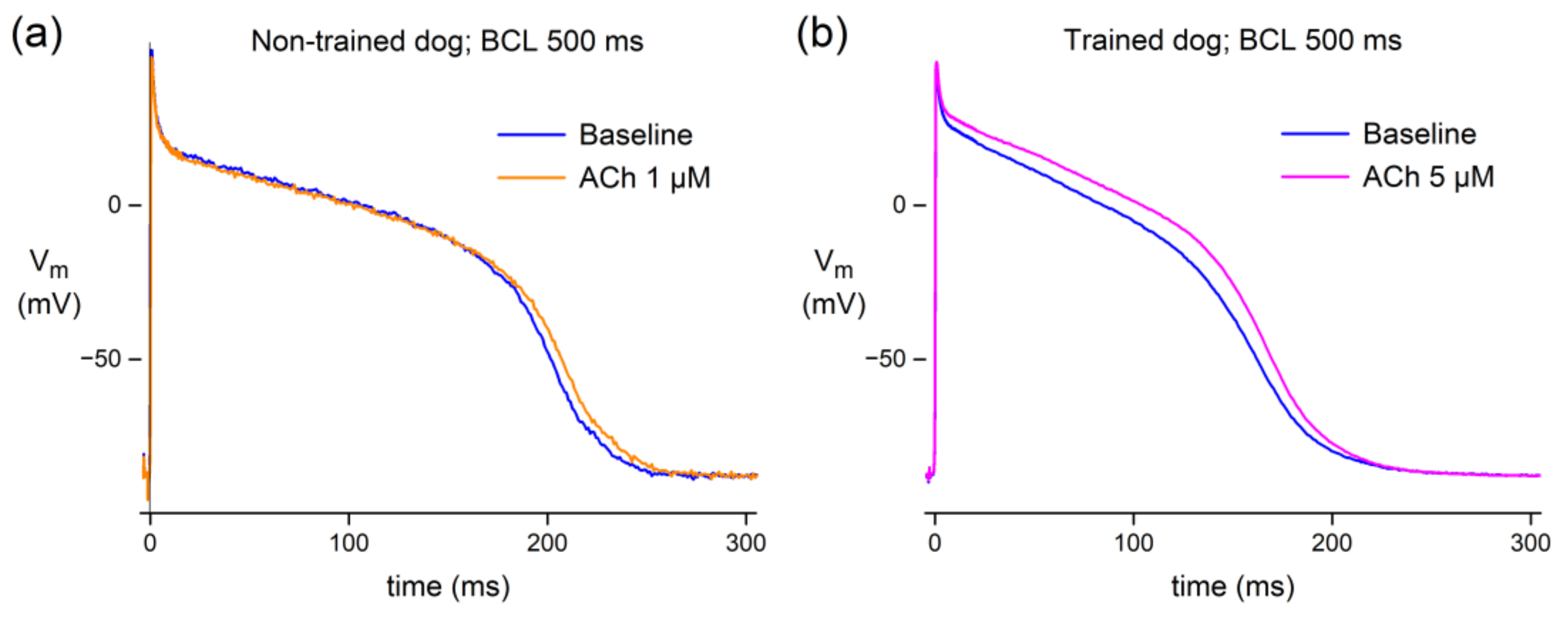
3.1.1. Effect of ACh in Canine Purkinje Fibers
3.1.2. Effect of ACh in Human Atrial and Ventricular Preparations
3.2. Patch Clamp Measurements
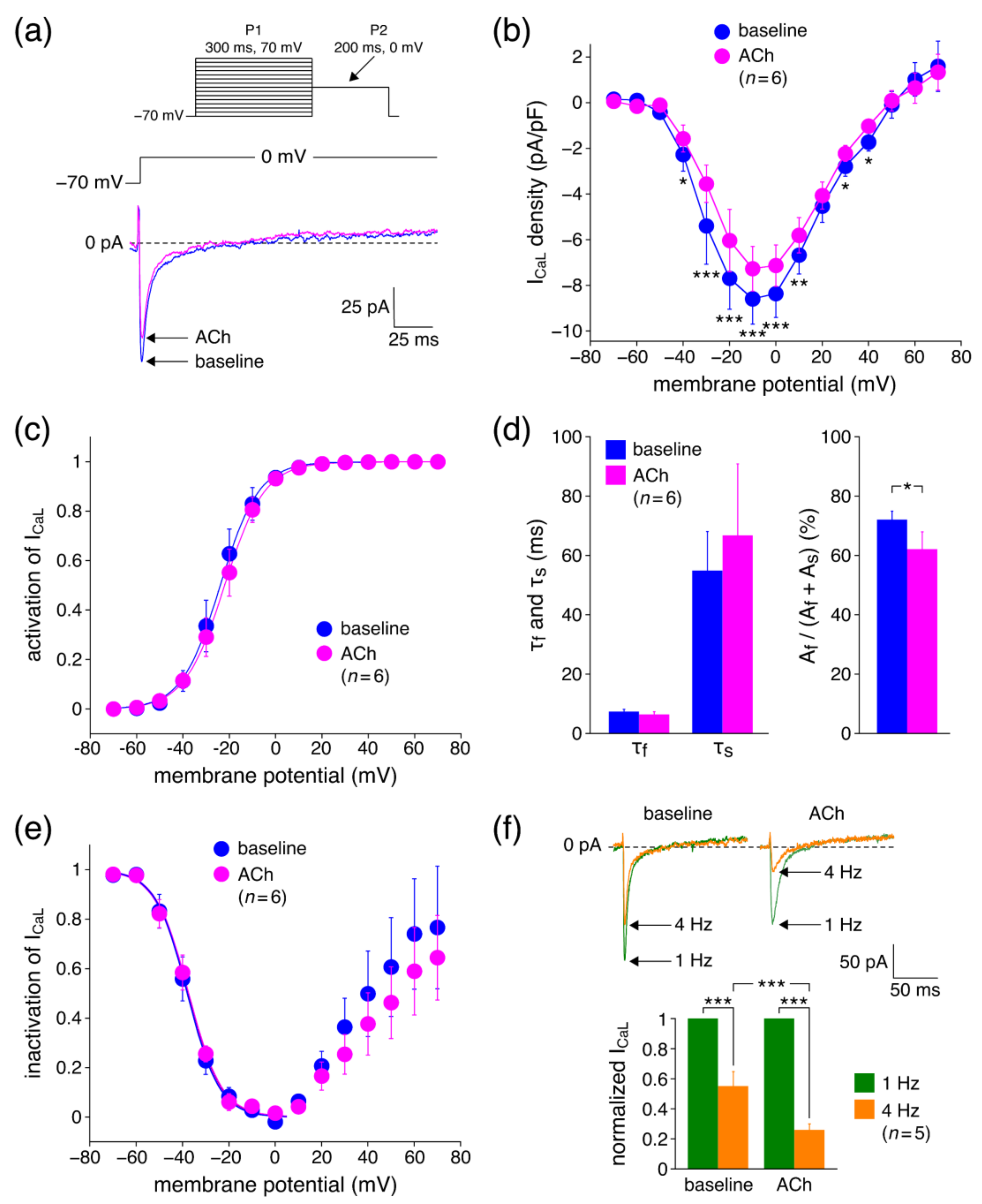
3.2.1. Effects of ACh on ICaL Density
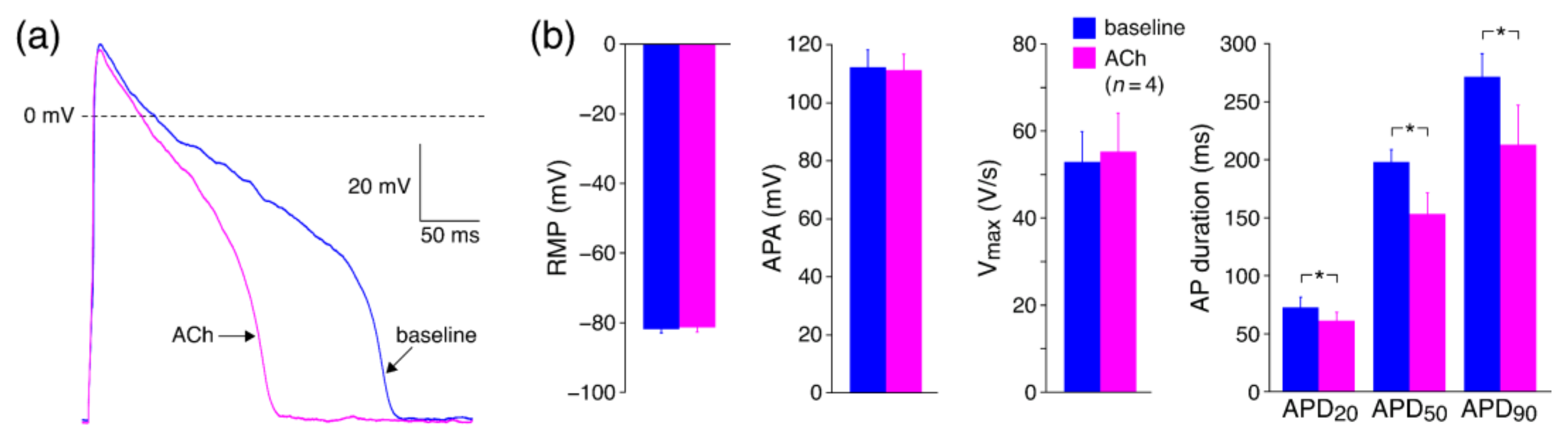
3.2.2. Effects of ACh on APs with Limited Interference of K+ Currents
3.3. In Silico Experiments
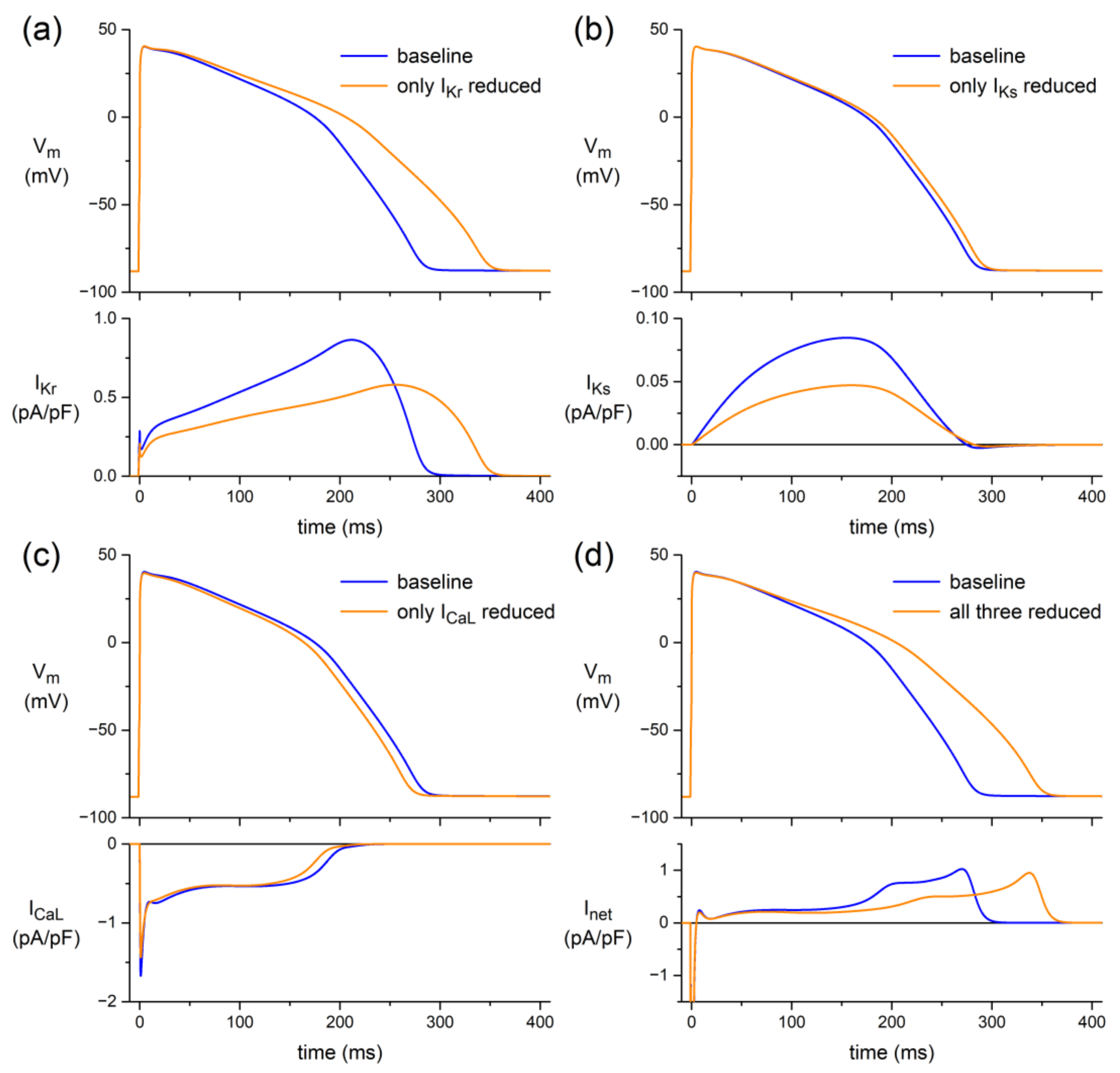
3.3.1. Human Ventricular Cell Model
3.3.2. Human Atrial Cell Model
4. Discussion
4.1. Overview
4.2. Standard Microelectrode Measurements
4.2.1. Canine Purkinje Fibers
4.2.2. Human Tissue
4.3. Patch Clamp Measurements
4.3.1. Effects of ACh on ICaL Density
4.3.2. Effects of ACh on ICaL Gating Properties
4.3.3. Effects of ACh on APs with Limited Interference of K+ Currents
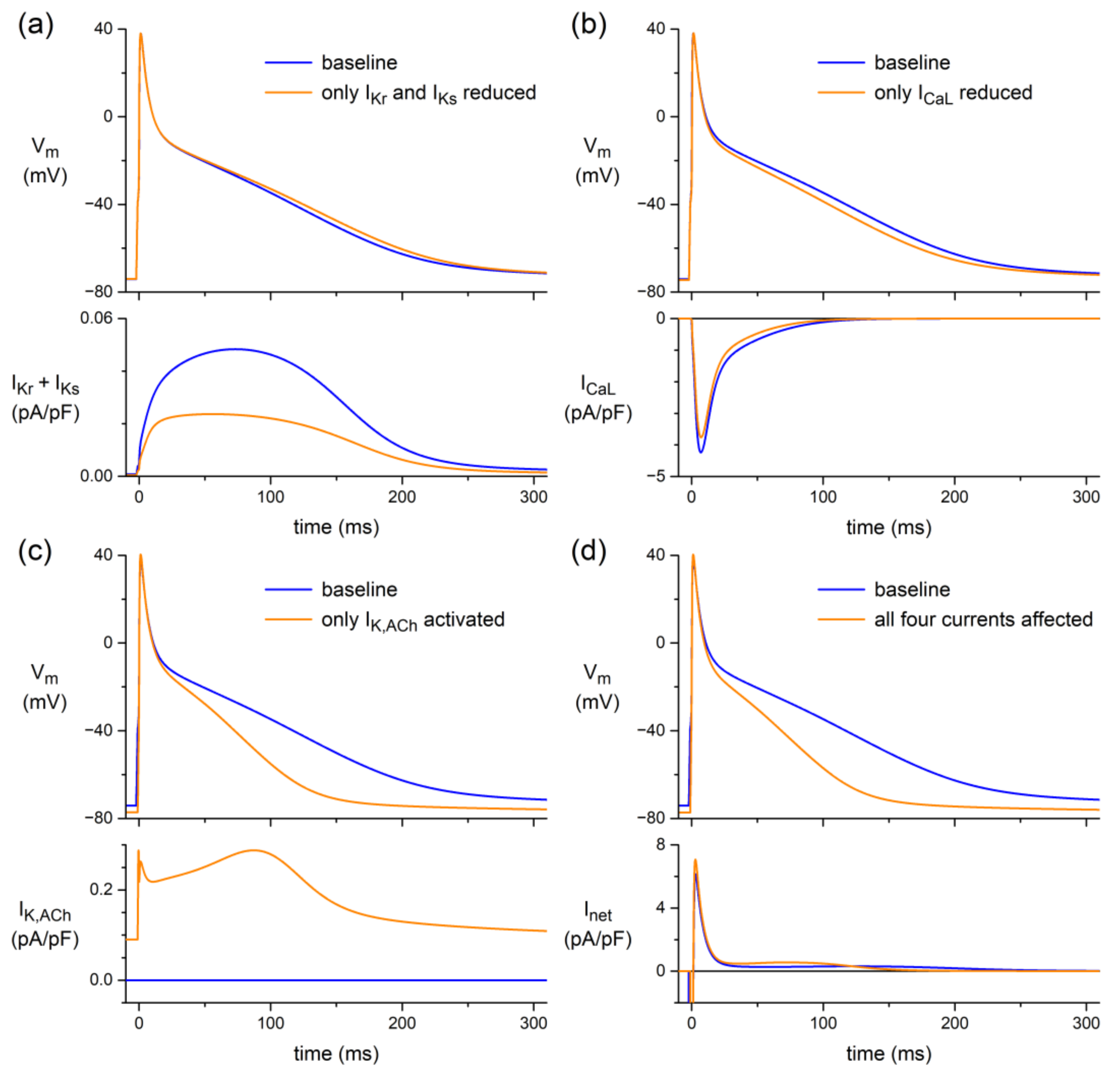
4.4. In Silico Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kishi, T. Heart failure as an autonomic nervous system dysfunction. J. Cardiol. 2012, 59, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; De Ferrari, G.M. Vagal stimulation in heart failure. Herz 2021, 46, 541–549. [Google Scholar] [CrossRef]
- Premchand, R.K.; Sharma, K.; Mittal, S.; Monteiro, R.; Dixit, S.; Libbus, I.; DiCarlo, L.A.; Ardell, J.L.; Rector, T.S.; Amurthur, B.; et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: Results of the ANTHEM-HF trial. J. Card. Fail. 2014, 20, 808–816. [Google Scholar] [CrossRef]
- Zannad, F.; De Ferrari, G.M.; Tuinenburg, A.E.; Wright, D.; Brugada, J.; Butter, C.; Klein, H.; Stolen, C.; Meyer, S.; Stein, K.M.; et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: Results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur. Heart J. 2015, 36, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.R.; Van Veldhuisen, D.J.; Hauptman, P.J.; Borggrefe, M.; Kubo, S.H.; Lieberman, R.A.; Milasinovic, G.; Berman, B.J.; Djordjevic, S.; Neelagaru, S.; et al. Vagus nerve stimulation for the treatment of heart failure: The INOVATE-HF trial. J. Am. Coll. Cardiol. 2016, 68, 149–158. [Google Scholar] [CrossRef]
- Konstam, M.A.; Udelson, J.E.; Butler, J.; Klein, H.U.; Parker, J.D.; Teerlink, J.R.; Wedge, P.M.; Saville, B.R.; Ardell, J.L.; Libbus, I.; et al. Impact of autonomic regulation therapy in patients with heart failure: ANTHEM-HFrEF pivotal study design. Circ. Heart Fail. 2019, 12, e005879. [Google Scholar] [CrossRef] [PubMed]
- Chinushi, M.; Nakagawa, I.; Hori, T.; Yamashita, F.; Washizuka, T.; Aizawa, Y. QT interval prolongation and torsades de pointes unmasked by intracoronary acetylcholine administration. Pacing Clin. Electrophysiol. 2001, 24, 1561–1562. [Google Scholar] [CrossRef]
- Furushima, H.; Niwano, S.; Chinushi, M.; Shiba, M.; Fujita, S.; Abe, A.; Ohhira, K.; Taneda, K.; Aizawa, Y. Intracoronary acetylcholine-induced prolongation of monophasic action potential in long QT syndrome. Jpn. Heart J. 1998, 39, 225–233. [Google Scholar] [CrossRef][Green Version]
- Antzelevitch, C.; Sicouri, S. Clinical relevance of cardiac arrhythmias generated by afterdepolarizations: Role of M cells in the generation of U waves, triggered activity and torsade de pointes. J. Am. Coll. Cardiol. 1994, 23, 259–277. [Google Scholar] [CrossRef]
- Roden, D.M.; Lazzara, R.; Rosen, M.; Schwartz, P.J.; Towbin, J.; Vincent, G.M. Multiple mechanisms in the long-QT syndrome: Current knowledge, gaps, and future directions. Circulation 1996, 94, 1996–2012. [Google Scholar] [CrossRef]
- El-Sherif, N.; Turitto, G.; Boutjdir, M. Acquired long QT syndrome and electrophysiology of torsade de pointes. Arrhythm. Electrophysiol. Rev. 2019, 8, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Burashnikov, A.; Antzelevitch, C. Late-phase 3 EAD: A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin. Electrophysiol. 2006, 29, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Locati, E.T.; Bagliani, G.; Cecchi, F.; Johny, H.; Lunati, M.; Pappone, C. Arrhythmias due to inherited and acquired abnormalities of ventricular repolarization. Card. Electrophysiol. Clin. 2019, 11, 345–362. [Google Scholar] [CrossRef]
- Calloe, K.; Goodrow, R.; Olesen, S.-P.; Antzelevitch, C.; Cordeiro, J.M. Tissue-specific effects of acetylcholine in the canine heart. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H66–H75. [Google Scholar] [CrossRef]
- Litovsky, S.H.; Antzelevitch, C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol: A direct effect of acetylcholine in ventricular myocardium. Circ. Res. 1990, 67, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-K.; Boyett, M.R.; Janvier, N.C.; McMorn, S.O.; Shui, Z.; Karim, F. Regional differences in the negative inotropic effect of acetylcholine within the canine ventricle. J. Physiol. 1996, 492, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, E.; Ramon, J. Electrophysiological effects of acetylcholine in sheep cardiac Purkinje fibers. Pflüg. Arch. 1980, 387, 197–205. [Google Scholar] [CrossRef]
- Mubagwa, K.; Carmeliet, E. Effects of acetylcholine on electrophysiological properties of rabbit cardiac Purkinje fibers. Circ. Res. 1983, 53, 740–751. [Google Scholar] [CrossRef]
- Boyett, M.R.; Kirby, M.S.; Orchard, C.H.; Roberts, A. The negative inotropic effect of acetylcholine on ferret ventricular myocardium. J. Physiol. 1988, 404, 613–635. [Google Scholar] [CrossRef]
- Lipsius, S.L.; Gibbons, W.R. Acetylcholine lengthens action potentials of sheep cardiac Purkinje fibers. Am. J. Physiol. 1980, 238, H237–H243. [Google Scholar] [CrossRef]
- Gadsby, D.C.; Wit, A.L.; Cranefield, P.F. The effects of acetylcholine on the electrical activity of canine cardiac Purkinje fibers. Circ. Res. 1978, 43, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.C.; Watanabe, A.M.; Besch, H.R., Jr.; Lathrop, D.A. Acetylcholine antagonism of the electrophysiological effects of isoproterenol on canine cardiac Purkinje fibers. Circ. Res. 1979, 44, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, R.F., Jr.; Zipes, D.P. Positive inotropic effect of acetylcholine in canine cardiac Purkinje fibers. Am. J. Physiol. 1985, 249, H735–H740. [Google Scholar] [CrossRef]
- Horváth, A.; Lemoine, M.D.; Löser, A.; Mannhardt, I.; Flenner, F.; Uzun, A.U.; Neuber, C.; Breckwoldt, K.; Hansen, A.; Girdauskas, E.; et al. Low resting membrane potential and low inward rectifier potassium currents are not inherent features of hiPSC-derived cardiomyocytes. Stem Cell Rep. 2018, 10, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.C.; Mengarelli, I.; Koopman, C.D.; Wilders, R.; Van Amersfoorth, S.C.; Bakker, D.; Wolswinkel, R.; Hababa, M.; De Boer, T.P.; Guan, K.; et al. Genetic variation in GNB5 causes bradycardia by augmenting the cholinergic response via increased acetylcholine-activated potassium current (IK,ACh). Dis. Model. Mech. 2019, 12, dmm037994. [Google Scholar] [CrossRef] [PubMed]
- Koncz, I.; Verkerk, A.O.; Nicastro, M.; Wilders, R.; Árpádffy-Lovas, T.; Magyar, T.; Tóth, N.; Nagy, N.; Madrid, M.; Lin, Z.; et al. Acetylcholine reduces IKr and prolongs action potentials in human ventricular cardiomyocytes. Biomedicines 2022, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.C.; Kass, R.S. Cholinergic inhibition of slow delayed-rectifier K+ current in guinea pig sino-atrial node is not mediated by muscarinic receptors. Mol. Pharmacol. 1995, 47, 1248–1254. [Google Scholar]
- Treinys, R.; Bogdelis, A.; Rimkutė, L.; Jurevičius, J.; Skeberdis, V.A. Differences in the control of basal L-type Ca2+ current by the cyclic AMP signaling cascade in frog, rat, and human cardiac myocytes. J. Physiol. Sci. 2016, 66, 327–336. [Google Scholar] [CrossRef]
- Zanesco, A.; Antunes, E. Effects of exercise training on the cardiovascular system: Pharmacological approaches. Pharmacol. Ther. 2007, 114, 307–317. [Google Scholar] [CrossRef]
- Harvard Personal Genome Project. Available online: https://pgp.med.harvard.edu/ (accessed on 24 September 2022).
- Maas, R.G.C.; Lee, S.; Harakalova, M.; Snijders Blok, C.J.B.; Goodyer, W.R.; Hjortnaes, J.; Doevendans, P.A.F.M.; Van Laake, L.W.; Van der Velden, J.; Asselbergs, F.W.; et al. Massive expansion and cryopreservation of functional human induced pluripotent stem cell-derived cardiomyocytes. STAR Protoc. 2021, 2, 100334. [Google Scholar] [CrossRef]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef]
- Markwardt, F.; Nilius, B. Changes of calcium channel inactivation during run-down. Gen. Physiol. Biophys. 1990, 9, 209–218. [Google Scholar]
- Zhou, Z.; January, C.T. Both T- and L-type Ca2+ channels can contribute to excitation-contraction coupling in cardiac Purkinje cells. Biophys. J. 1998, 74, 1830–1839. [Google Scholar] [CrossRef]
- Tajima, Y.; Ono, K.; Akaike, N. Perforated patch-clamp recording in cardiac myocytes using cation-selective ionophore gramicidin. Am. J. Physiol. 1996, 271, C524–C532. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, Y.; Asano, Y.; Takikawa, R.; Sugimoto, T. Cardiac Ca current does not run down and is very sensitive to isoprenaline in the nystatin-method of whole cell recording. Naunyn Schmiedebergs Arch. Pharmacol. 1989, 340, 219–222. [Google Scholar] [CrossRef]
- Barry, P.H.; Lynch, J.W. Liquid junction potentials and small cell effects in patch-clamp analysis. J. Membr. Biol. 1991, 121, 101–117. [Google Scholar] [CrossRef]
- Wilders, R. Dynamic clamp: A powerful tool in cardiac electrophysiology. J. Physiol. 2006, 576, 349–359. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Wilders, R. Dynamic clamp in electrophysiological studies on stem cell-derived cardiomyocytes—Why and how? J. Cardiovasc. Pharmacol. 2021, 77, 267–279. [Google Scholar] [CrossRef]
- Meijer van Putten, R.M.E.; Mengarelli, I.; Guan, K.; Zegers, J.G.; Van Ginneken, A.C.G.; Verkerk, A.O.; Wilders, R. Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: A dynamic clamp study with virtual IK1. Front. Physiol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Veerman, C.C.; Zegers, J.G.; Mengarelli, I.; Bezzina, C.R.; Wilders, R. Patch-clamp recording from human induced pluripotent stem cell-derived cardiomyocytes: Improving action potential characteristics through dynamic clamp. Int. J. Mol. Sci. 2017, 18, 1873. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Marchal, G.A.; Zegers, J.G.; Kawasaki, M.; Driessen, A.H.G.; Remme, C.A.; De Groot, J.R.; Wilders, R. Patch-clamp recordings of action potentials from human atrial myocytes: Optimization through dynamic clamp. Front. Pharmacol. 2021, 12, 649414. [Google Scholar] [CrossRef] [PubMed]
- Bett, G.C.L.; Kaplan, A.D.; Lis, A.; Cimato, T.R.; Tzanakakis, E.S.; Zhou, Q.; Morales, M.J.; Rasmusson, R.L. Electronic “expression” of the inward rectifier in cardiocytes derived from human-induced pluripotent stem cells. Heart Rhythm 2013, 10, 1903–1910. [Google Scholar] [CrossRef]
- O’Hara, T.; Virág, L.; Varró, A.; Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput. Biol. 2011, 7, e1002061. [Google Scholar] [CrossRef] [PubMed]
- Maleckar, M.M.; Greenstein, J.L.; Giles, W.R.; Trayanova, N.A. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1398–H1410. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Lawson, J.R.; Hunter, P.J.; Nielsen, P.F. The CellML Model Repository. Bioinformatics 2008, 24, 2122–2123. [Google Scholar] [CrossRef]
- Garny, A.; Kohl, P.; Noble, D. Cellular Open Resource (COR): A public CellML based environment for modelling biological function. Int. J. Bifurcat. Chaos 2003, 13, 3579–3590. [Google Scholar] [CrossRef]
- Kemi, O.J.; Ellingsen, O.; Smith, G.L.; Wisloff, U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Front. Biosci. 2008, 13, 356–368. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Fitts, R.H. Cardiomyocyte slowly activating delayed rectifier potassium channel: Regulation by exercise and β-adrenergic signaling. J. Appl. Physiol. 2020, 128, 1177–1185. [Google Scholar] [CrossRef]
- Kui, P.; Polyák, A.; Morvay, N.; Tiszlavicz, L.; Nagy, N.; Ördög, B.; Takács, H.; Leprán, I.; Farkas, A.; Papp, J.G.; et al. Long-term endurance exercise training alters repolarization in a new rabbit athlete’s heart model. Front. Physiol. 2022, 12, 741317. [Google Scholar] [CrossRef]
- Varró, A.; Tomek, J.; Nagy, N.; Virág, L.; Passini, E.; Rodriguez, B.; Baczkó, I. Cardiac transmembrane ion channels and action potentials: Cellular physiology and arrhythmogenic behavior. Physiol. Rev. 2021, 101, 1083–1176. [Google Scholar] [CrossRef]
- Benitah, J.-P.; Alvarez, J.L.; Gómez, A.M. L-type Ca2+ current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 48, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Maleckar, M.M.; Greenstein, J.L.; Trayanova, N.A.; Giles, W.R. Mathematical simulations of ligand-gated and cell-type specific effects on the action potential of human atrium. Prog. Biophys. Mol. Biol. 2008, 98, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Mantravadi, R.; Gabris, B.; Liu, T.; Choi, B.-R.; De Groat, W.C.; Ng, G.A.; Salama, G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ. Res. 2007, 100, e72–e80. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.G.; Louch, W.E. Species-dependent mechanisms of cardiac arrhythmia: A cellular focus. Clin. Med. Insights Cardiol. 2017, 11, 1179546816686061. [Google Scholar] [CrossRef]
- Dhein, S.; Van Koppen, C.J.; Brodde, O.-E. Muscarinic receptors in the mammalian heart. Pharmacol. Res. 2001, 44, 161–182. [Google Scholar] [CrossRef]
- Bayer, J.D.; Boukens, B.J.; Krul, S.P.J.; Roney, C.H.; Driessen, A.H.G.; Berger, W.R.; Van den Berg, N.W.E.; Verkerk, A.O.; Vigmond, E.J.; Coronel, R.; et al. Acetylcholine delays atrial activation to facilitate atrial fibrillation. Front. Physiol. 2019, 10, 1105. [Google Scholar] [CrossRef]
- Petersen, J.; Castro, L.; Bengaard, A.K.P.; Pecha, S.; Ismaili, D.; Schulz, C.; Sahni, J.; Steenpass, A.; Meier, C.; Reichenspurner, H.; et al. Muscarinic receptor activation reduces force and arrhythmias in human atria independent of IK,ACh. J. Cardiovasc. Pharmacol. 2022, 79, 678–686. [Google Scholar] [CrossRef]
- Koumi, S.-I.; Arentzen, C.E.; Backer, C.L.; Wasserstrom, J.A. Alterations in muscarinic K+ channel response to acetylcholine and to G protein-mediated activation in atrial myocytes isolated from failing human hearts. Circulation 1994, 90, 2213–2224. [Google Scholar] [CrossRef]
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation 2005, 112, 3697–3706. [Google Scholar] [CrossRef]
- Jakob, H.; Oelert, H.; Rupp, J.; Nawrath, H. Functional role of cholinoceptors and purinoceptors in human isolated atrial and ventricular heart muscle. Br. J. Pharmacol. 1989, 97, 1199–1208. [Google Scholar] [CrossRef]
- Fu, G.-S.; Meissner, A.; Simon, R. Repolarization dispersion and sudden cardiac death in patients with impaired left ventricular function. Eur. Heart J. 1997, 18, 281–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, G.-X.; Joshi, A.; Guo, D.; Hlaing, T.; Martin, J.; Xu, X.; Kowey, P.R. Phase 2 reentry as a trigger to initiate ventricular fibrillation during early acute myocardial ischemia. Circulation 2004, 110, 1036–1041. [Google Scholar] [CrossRef]
- Antzelevitch, C. Brugada syndrome. Pacing Clin. Electrophysiol. 2006, 29, 1130–1159. [Google Scholar] [CrossRef] [PubMed]
- Janse, M.J.; Wit, A.L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 1989, 69, 1049–1169. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, T.E.; Mohr, G.H.; Blom, M.T.; Verkerk, A.O.; Souverein, P.C.; Torp-Pedersen, C.; Folke, F.; Wissenberg, M.; Van den Brink, L.; Davis, R.P.; et al. Differential effects on out-of-hospital cardiac arrest of dihydropyridines: Real-world data from population-based cohorts across two European countries. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 347–355. [Google Scholar] [CrossRef]
- Petit-Jacques, J.; Bois, P.; Bescond, J.; Lenfant, J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflüg. Arch. 1993, 423, 21–27. [Google Scholar] [CrossRef]
- Wang, Y.G.; Lipsius, S.L. Acetylcholine elicits a rebound stimulation of Ca2+ current mediated by pertussis toxin-sensitive G protein and cAMP-dependent protein kinase A in atrial myocytes. Circ. Res. 1995, 76, 634–644. [Google Scholar] [CrossRef]
- Iijima, T.; Irisawa, H.; Kameyama, M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J. Physiol. 1985, 359, 485–501. [Google Scholar] [CrossRef]
- Carmeliet, E.; Ramon, J. Effects of acetylcholine on time-dependent currents in sheep cardiac Purkinje fibers. Pflüg. Arch. 1980, 387, 217–223. [Google Scholar] [CrossRef]
- Hino, N.; Ochi, R. Effect of acetylcholine on membrane currents in guinea-pig papillary muscle. J. Physiol. 1980, 307, 183–197. [Google Scholar] [CrossRef]
- Shimoni, Y.; Spindler, A.J.; Noble, D. The control of calcium current reactivation by catecholamines and acetylcholine in single guinea-pig ventricular myocytes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1987, 230, 267–278. [Google Scholar] [CrossRef]
- Fischmeister, R.; Hartzell, H.C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J. Physiol. 1986, 376, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Fischmeister, R.; Shrier, A. Interactive effects of isoprenaline, forskolin and acetylcholine on Ca2+ current in frog ventricular myocytes. J. Physiol. 1989, 417, 213–239. [Google Scholar] [CrossRef] [PubMed]
- Giles, W.; Noble, S.J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J. Physiol. 1976, 261, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, Y.; Goto, M. Effects of ACh on slow inward current and tension components of the bullfrog atrium. J. Mol. Cell. Cardiol. 1977, 9, 313–326. [Google Scholar] [CrossRef]
- Veerman, C.C.; Kosmidis, G.; Mummery, C.L.; Casini, S.; Verkerk, A.O.; Bellin, M. Immaturity of human stem-cell-derived cardiomyocytes in culture: Fatal flaw or soluble problem? Stem Cells Dev. 2015, 24, 1035–1052. [Google Scholar] [CrossRef]
- Kane, C.; Couch, L.; Terracciano, C.M.N. Excitation-contraction coupling of human induced pluripotent stem cell-derived cardiomyocytes. Front. Cell Dev. Biol. 2015, 3, 59. [Google Scholar] [CrossRef]
- Kroll, K.; Chabria, M.; Wang, K.; Häusermann, F.; Schuler, F.; Polonchuk, L. Electro-mechanical conditioning of human iPSC-derived cardiomyocytes for translational research. Prog. Biophys. Mol. Biol. 2017, 130, 212–222. [Google Scholar] [CrossRef]
- Bett, G.C.L.; Dai, S.; Campbell, D.L. Cholinergic modulation of the basal L-type calcium current in ferret right ventricular myocytes. J. Physiol. 2002, 542, 107–117. [Google Scholar] [CrossRef]
- Liang, H.-M.; Tang, M.; Liu, C.-J.; Luo, H.-Y.; Song, Y.-L.; Hu, X.-W.; Xi, J.-Y.; Gao, L.-L.; Nie, B.; Li, S.-Y.; et al. Muscarinic cholinergic regulation of L-type calcium channel in heart of embryonic mice at different developmental stages. Acta Pharmacol. Sin. 2004, 25, 1450–1457. [Google Scholar]
- Courtemanche, M.; Ramirez, R.J.; Nattel, S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. Am. J. Physiol. 1998, 275, H301–H321. [Google Scholar] [CrossRef] [PubMed]

| Species | APD | Reference |
|---|---|---|
| Rabbit | ↓ | Carmeliet and Ramon [17] |
| ↓ | Mubagwa and Carmeliet [18] | |
| Ferret | ↓ | Boyett et al. [19] |
| Cow | ↔ | Carmeliet and Ramon [17] |
| Sheep | ↑ | Lipsius and Gibbons [20] |
| ↑ | Carmeliet and Ramon [17] | |
| Cat | ↑ | Carmeliet and Ramon [17] |
| Dog | ↓ | Gadsby et al. [21] |
| ↔ | Bailey et al. [22] | |
| ↔ | Gilmour and Zipes [23] | |
| ↔ | Calloe et al. [14] |
| Non-Trained Dogs (n = 6) | Trained Dogs (n = 4) | |||
|---|---|---|---|---|
| Baseline | 1 µM ACh | Baseline | 5 µM ACh | |
| RMP (mV) | −85.3 ± 0.7 | −85.7 ± 1.7 | −84.6 ± 1.9 | −84.6 ± 1.7 |
| APA (mV) | 118.5 ± 5.5 | 117.9 ± 4.8 | 135.2 ± 3.0 # | 138.1 ± 2.4 * |
| Vmax (V/s) | 272.2 ± 54.8 | 268.6 ± 51.5 | 486.6 ± 51.6 # | 514.1 ± 65.4 |
| APD25 (ms) | 70.1 ± 26.4 | 72.0 ± 26.1 | 35.0 ± 5.4 | 45.4 ± 7.3 * |
| APD50 (ms) | 197.3 ± 10.9 | 202.3 ± 11.2 *** | 147.3 ± 9.3 # | 158.5 ± 10.1 * |
| APD90 (ms) | 248.9 ± 11.7 | 254.1 ± 11.7 *** | 203.5 ± 12.9 # | 209.0 ± 13.4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verkerk, A.O.; Doszpod, I.J.; Mengarelli, I.; Magyar, T.; Polyák, A.; Pászti, B.; Efimov, I.R.; Wilders, R.; Koncz, I. Acetylcholine Reduces L-Type Calcium Current without Major Changes in Repolarization of Canine and Human Purkinje and Ventricular Tissue. Biomedicines 2022, 10, 2987. https://doi.org/10.3390/biomedicines10112987
Verkerk AO, Doszpod IJ, Mengarelli I, Magyar T, Polyák A, Pászti B, Efimov IR, Wilders R, Koncz I. Acetylcholine Reduces L-Type Calcium Current without Major Changes in Repolarization of Canine and Human Purkinje and Ventricular Tissue. Biomedicines. 2022; 10(11):2987. https://doi.org/10.3390/biomedicines10112987
Chicago/Turabian StyleVerkerk, Arie O., Illés J. Doszpod, Isabella Mengarelli, Tibor Magyar, Alexandra Polyák, Bence Pászti, Igor R. Efimov, Ronald Wilders, and István Koncz. 2022. "Acetylcholine Reduces L-Type Calcium Current without Major Changes in Repolarization of Canine and Human Purkinje and Ventricular Tissue" Biomedicines 10, no. 11: 2987. https://doi.org/10.3390/biomedicines10112987
APA StyleVerkerk, A. O., Doszpod, I. J., Mengarelli, I., Magyar, T., Polyák, A., Pászti, B., Efimov, I. R., Wilders, R., & Koncz, I. (2022). Acetylcholine Reduces L-Type Calcium Current without Major Changes in Repolarization of Canine and Human Purkinje and Ventricular Tissue. Biomedicines, 10(11), 2987. https://doi.org/10.3390/biomedicines10112987









