Eumelanin from the Black Soldier Fly as Sustainable Biomaterial: Characterisation and Functional Benefits in Tissue-Engineered Composite Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Eumelanin Extraction from Black Soldier Fly (BSF) Cuticles
2.2. Physico-Chemical and Biological Characterization of BSF-Eumel
2.2.1. Physico-Chemical Characterization
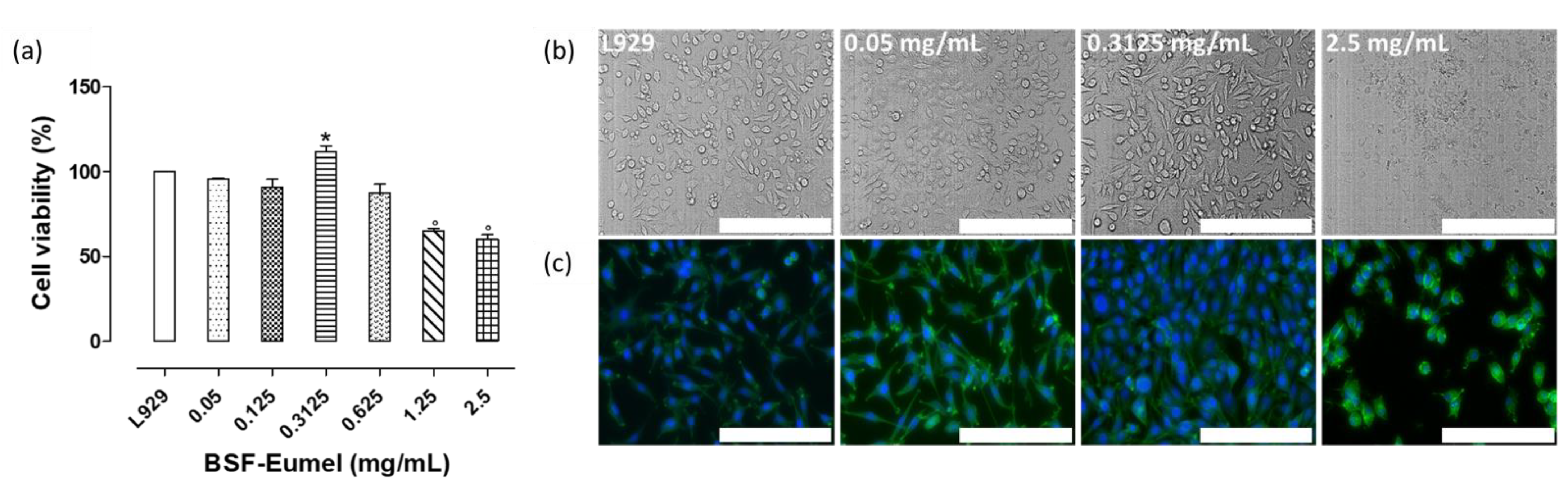
2.2.2. Cell Viability and Morphology
2.2.3. Early Cell Osteogenic Differentiation: Alkaline Phosphatase Expression
2.3. 3D Printed MEHA and MEHA/BSF-Eumel Scaffolds
2.3.1. Synthesis and Characterisation of Photocrosslinkable MEHA
2.3.2. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.3. Three-Dimensional Printing of MEHA and MEHA/BSF-Eumel Scaffolds
2.3.4. Dynamic Mechanical Analysis (DMA)
2.3.5. Swelling Studies and Stability Tests
2.3.6. Release Study of BSF-Eumel from MEHA Scaffolds
2.3.7. Biological Analyses
2.3.8. Cell Viability and Osteogenic Differentiation
2.4. Statistics and Data Analysis
3. Results and Discussion
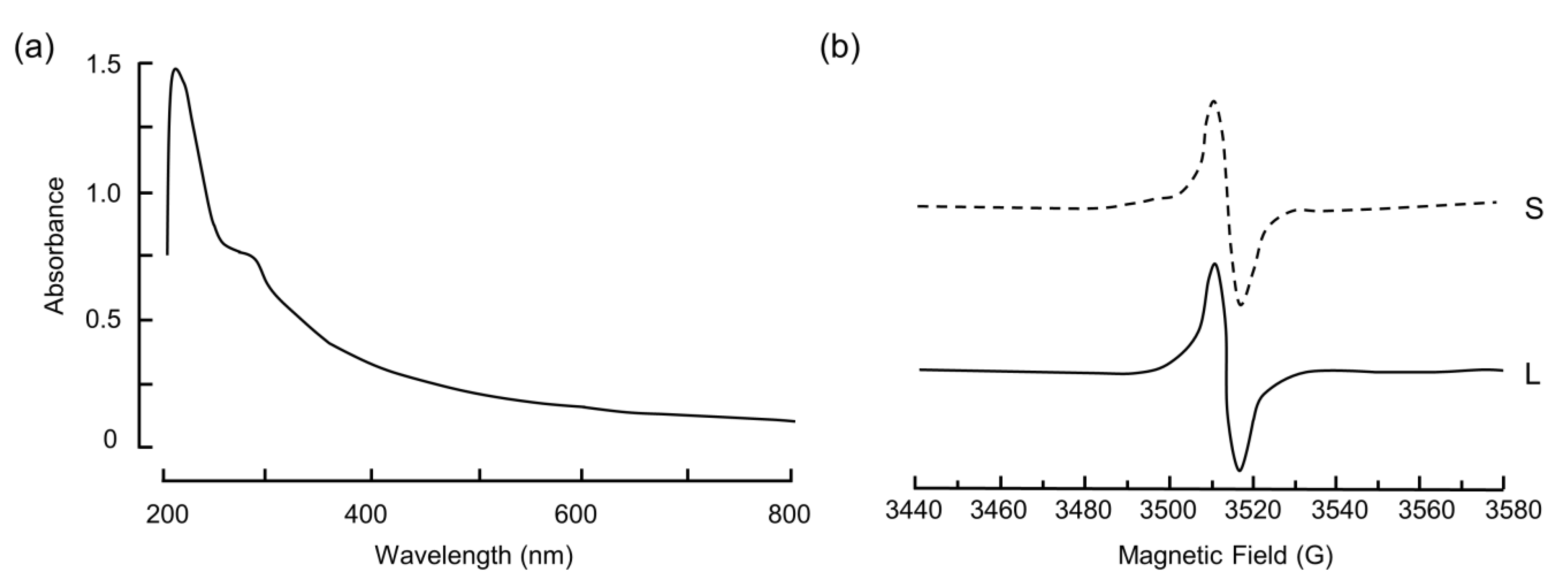
3.1. Physico-Chemical and Biological Characterization of BSF-Eumel
3.2. Three-Dimensional Printed MEHA and MEHA/BSF-Eumel Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, L.; Manini, P.; Altamura, D.; Giannini, C.; Tassini, P.; Maglione, M.G.; Minarini, C.; Pezzella, A. Evidence of Unprecedented High Electronic Conductivity in Mammalian Pigment Based Eumelanin Thin Films After Thermal Annealing in Vacuum. Front. Chem. 2019, 7, 162. [Google Scholar] [CrossRef]
- Migliaccio, L.; Gesuele, F.; Manini, P.; Maglione, M.G.; Tassini, P.; Pezzella, A. Eumelanin Precursor 2-Carboxy-5,6-Dihydroxyindole (DHICA) as Doping Factor in Ternary (PEDOT:PSS/Eumelanin) Thin Films for Conductivity Enhancement. Materials 2020, 13, 2108. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and melanin-like hybrid materials in regenerative medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Barek, H. Critical Analysis of the Melanogenic Pathway in Insects and Higher Animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef]
- Pezzella, A.; Barra, M.; Musto, A.; Navarra, A.; Alfè, M.; Manini, P.; Parisi, S.; Cassinese, A.; Criscuolo, V.; d’Ischia, M. Stem cellcompatible eumelanin biointerface fabricated by chemically controlled solid state polymerization. Mater. Horiz. 2015, 2, 212–220. [Google Scholar] [CrossRef]
- Pira, A.; Amatucci, A.; Melis, C.; Pezzella, A.; Manini, P.; d’Ischia, M.; Mula, G. The interplay of chemical structure, physical properties, and structural design as a tool to modulate the properties of melanins within mesopores. Sci. Rep. 2022, 12, 11436. [Google Scholar] [CrossRef]
- Migliaccio, L.; Aprano, S.; Iannuzzi, L.; Maglione, M.G.; Tassini, P.; Minarini, C.; Manini, P.; Pezzella, A. Eumelanin–PEDOT:PSS Complementing En Route to Mammalian-Pigment-Based Electrodes: Design and Fabrication of an ITO-Free Organic Light-Emitting Device. Adv. Electron. Mater. 2017, 3, 1600342. [Google Scholar] [CrossRef]
- Kumar, P.; Di Mauro, E.; Zhang, S.; Pezzella, A.; Soavi, F.; Santato, C.; Cicoira, F. Melanin-based flexible supercapacitors. J. Mater. Chem. C 2016, 4, 9516–9525. [Google Scholar] [CrossRef]
- Sarna, T.; Swartz, H.M.; Zadlo, A. Interaction of Melanin with Metal Ions Modulates Their Cytotoxic Potential. Appl. Magn. Reson. 2022, 53, 105–121. [Google Scholar] [CrossRef]
- Yoo, H.-S.; Chung, K.-H.; Lee, K.-J.; Kim, D.-H.; An, J.H. Melanin extract from Gallus gallus domesticus promotes proliferation and differentiation of osteoblastic MG-63 cells via bone morphogenetic protein-2 signaling. Nutr. Res. Pract. 2017, 11, 190–197. [Google Scholar] [CrossRef]
- Reali, M.; Gouda, A.; Bellemare, J.; Ménard, D.; Nunzi, J.-M.; Soavi, F.; Santato, C. Electronic transport in the biopigment sepia melanin. ACS Appl. Bio Mater. 2020, 3, 5244–5252. [Google Scholar] [CrossRef]
- Insectta Pte. Ltd. UEN: 201809941M International Publication No.: WO/2021/183058 A1. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021183058 (accessed on 19 October 2022).
- Çetingül, İ.S.; Shah, S.R.A. Black soldier fly (Hermetia illucens) larvae as an ecological, immune booster and economical feedstuff for aquaculture. Mar. Sci. Technol. Bull. 2022, 11, 52–62. [Google Scholar]
- Ushakova, N.; Dontsov, A.; Sakina, N.; Bastrakov, A.; Ostrovsky, M. Antioxidative properties of melanins and ommochromes from black soldier fly Hermetia illucens. Biomolecules 2019, 9, 408. [Google Scholar] [CrossRef]
- Burraco, P.; Orizaola, G. Ionizing radiation and melanism in Chornobyl tree frogs. Evol. Appl. 2022, 15, 1469–1479. [Google Scholar] [CrossRef]
- da Silva, L.P.; Oliveira, S.; Pirraco, R.P.; Santos, T.C.; Reis, R.L.; Marques, A.P.; Correlo, V.M. Eumelanin-releasing spongy-like hydrogels for skin re-epithelialization purposes. Biomed. Mater. 2017, 12, 025010. [Google Scholar] [CrossRef]
- Fasolino, I.; Bonadies, I.; Ambrosio, L.; Raucci, M.G.; Carfagna, C.; Caso, F.M.; Cimino, F.; Pezzella, A. Eumelanin coated PLA electrospun micro fibers as bioinspired cradle for SH-SY5Y neuroblastoma cells growth and maturation. ACS Appl. Mater. Interfaces 2017, 9, 40070–40076. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 32001. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; D’Amora, U.; D’Arrigo, D.; Tomasini, M.; Candrian, C.; Ambrosio, L.; Moretti, M. Musculoskeletal tissues-on-a-chip: Role of natural polymers in reproducing tissue-specific microenvironments. Biofabrication 2022, 14, 042001. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calzà, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of mesenchymal stem cells delivered from methacrylated hyaluronic acid patch improve the regenerative properties of endothelial and dermal cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; D’Amora, U.; Ronca, A.; Li, Y.; Mo, X.; Zhou, F.; Yuan, M.; Ambrosio, L.; Wu, J.; Raucci, M.G. In vitro and in vivo biocompatibility and inflammation response of methacrylated and maleated hyaluronic acid for wound healing. RSC Adv. 2020, 10, 32183–32192. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Lin, H.; Soriente, A.; Fan, Y.; Zhang, X.; Ambrosio, L. Bioactive composites based on double network approach with tailored mechanical, physico-chemical, and biological features. J. Biomed. Mater. Res.—Part A 2018, 106, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Dozio, S.M.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. In situ sol-gel synthesis of hyaluronan derivatives bio-nanocomposite hydrogels. Regen. Biomater. 2019, 6, 249–258. [Google Scholar] [CrossRef]
- Kunwar, A.; Adhikary, B.; Jayakumar, S.; Barik, A.; Chattopadhyay, S.; Raghukumar, S.; Priyadarsini, K.I. Melanin, a promising radioprotector: Mechanisms of actions in a mice model. Toxicol. Appl. Pharmacol. 2012, 264, 202–211. [Google Scholar] [CrossRef]
- Chirila, T.V. Melanized Poly(HEMA) Hydrogels: Basic Research and Potential Use. J. Biomater. Appl. 1993, 8, 106–144. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, Q.; Yang, M.; Cheng, K.; Lu, X.; Zhang, L.; Huang, W.; Cheng, Z. Engineering Melanin Nanoparticles as an Efficient Drug-Delivery System for Imaging-Guided Chemotherapy. Adv. Mater. 2015, 27, 5063–5069. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; De Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. J. Funct. Biomater. 2022, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Simon, J. Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J. Phys. Chem. B 2007, 111, 7938–7947. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; d’Ischia, M. Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew. Chem. Int. Ed. 2013, 52, 12684–12687. [Google Scholar] [CrossRef] [PubMed]
- Adel, I.M.; ElMeligy, M.F.; Elkasabgy, N.A. Conventional and recent trends of scaffolds fabrication: A superior mode for tissue engineering. Pharmaceutics 2022, 14, 306. [Google Scholar] [CrossRef]





| Samples | BSF-Eumel | 5,6-Dihydroxyindole |
|---|---|---|
| Carbon (C, % w) | 52.6 | 64.4 |
| Hydrogen (H, % w) | 5.8 | 4.7 |
| Nitrogen (N, % w) | 10.1 | 9.4 |
| Others (O, % w) | 31.5 | 21.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amora, U.; Soriente, A.; Ronca, A.; Scialla, S.; Perrella, M.; Manini, P.; Phua, J.W.; Ottenheim, C.; Di Girolamo, R.; Pezzella, A.; et al. Eumelanin from the Black Soldier Fly as Sustainable Biomaterial: Characterisation and Functional Benefits in Tissue-Engineered Composite Scaffolds. Biomedicines 2022, 10, 2945. https://doi.org/10.3390/biomedicines10112945
D’Amora U, Soriente A, Ronca A, Scialla S, Perrella M, Manini P, Phua JW, Ottenheim C, Di Girolamo R, Pezzella A, et al. Eumelanin from the Black Soldier Fly as Sustainable Biomaterial: Characterisation and Functional Benefits in Tissue-Engineered Composite Scaffolds. Biomedicines. 2022; 10(11):2945. https://doi.org/10.3390/biomedicines10112945
Chicago/Turabian StyleD’Amora, Ugo, Alessandra Soriente, Alfredo Ronca, Stefania Scialla, Martina Perrella, Paola Manini, Jun Wei Phua, Christoph Ottenheim, Rocco Di Girolamo, Alessandro Pezzella, and et al. 2022. "Eumelanin from the Black Soldier Fly as Sustainable Biomaterial: Characterisation and Functional Benefits in Tissue-Engineered Composite Scaffolds" Biomedicines 10, no. 11: 2945. https://doi.org/10.3390/biomedicines10112945
APA StyleD’Amora, U., Soriente, A., Ronca, A., Scialla, S., Perrella, M., Manini, P., Phua, J. W., Ottenheim, C., Di Girolamo, R., Pezzella, A., Raucci, M. G., & Ambrosio, L. (2022). Eumelanin from the Black Soldier Fly as Sustainable Biomaterial: Characterisation and Functional Benefits in Tissue-Engineered Composite Scaffolds. Biomedicines, 10(11), 2945. https://doi.org/10.3390/biomedicines10112945