Ex Vivo Pulmonary Oedema after In Vivo Blast-Induced Rat Lung Injury: Time Dependency, Blast Intensity and Beta-2 Adrenergic Receptor Role
Abstract
1. Introduction
2. Methods
2.1. In Vivo Procedure
2.1.1. Pressure Wave Monitoring
- A pressure transducer (PR-6ST-80400.XX-20, sensitivity: 39.7 mV/bar, excitation: 4 mA, natural frequency: <30 kHz; Keller AG, Winterthur, Switzerland) in the pressure reservoir determined the cracking pressure that corresponds to the rupture of the polyester film. The measured time course of the pressure wave curve indicated the velocity of the pressure increase followed by the decay.
- Two pressure transducers (2 MI PAA 110-050-020, sensitivity: 8.03/7.99 mV/bar, excitation: 1 mA, natural frequency: >400 kHz; Keller AG, Winterthur, Switzerland) on both sides of the rat measured the pressure peaks on rat thorax level.
- Another transducer (Halleffekt-IC 634-SS2, sensitivity: 7.5–10.6 mV/mT; RS Components GmbH, Mörfelden-Walldorf, Germany) recorded the breathing frequency of the animal. Therefore, a string connected with the breathing sensor was placed above the thoracic cage. Via this transducer, the valve was triggered automatically depending on the breathing situation (e.g., inspiration, expiration or resting expiratory position). In addition, the breathing frequency of the anaesthetised animals was recorded before and after the pressure wave exposure.
2.2. Ex Vivo Procedure of Preparation of the Isolated Perfused Rat Lung
2.2.1. Determination of a Haemorrhage and Oedema Score
2.2.2. Experimental Setup
2.3. Statistics
3. Results
3.1. Blast Wave Characteristics
3.2. Interrelationships between Physical and Physiological Injury
3.3. Lung Function
3.4. Time-Dependent Recovery of the Lung Function after Trauma
3.5. Pharmacological Interventions
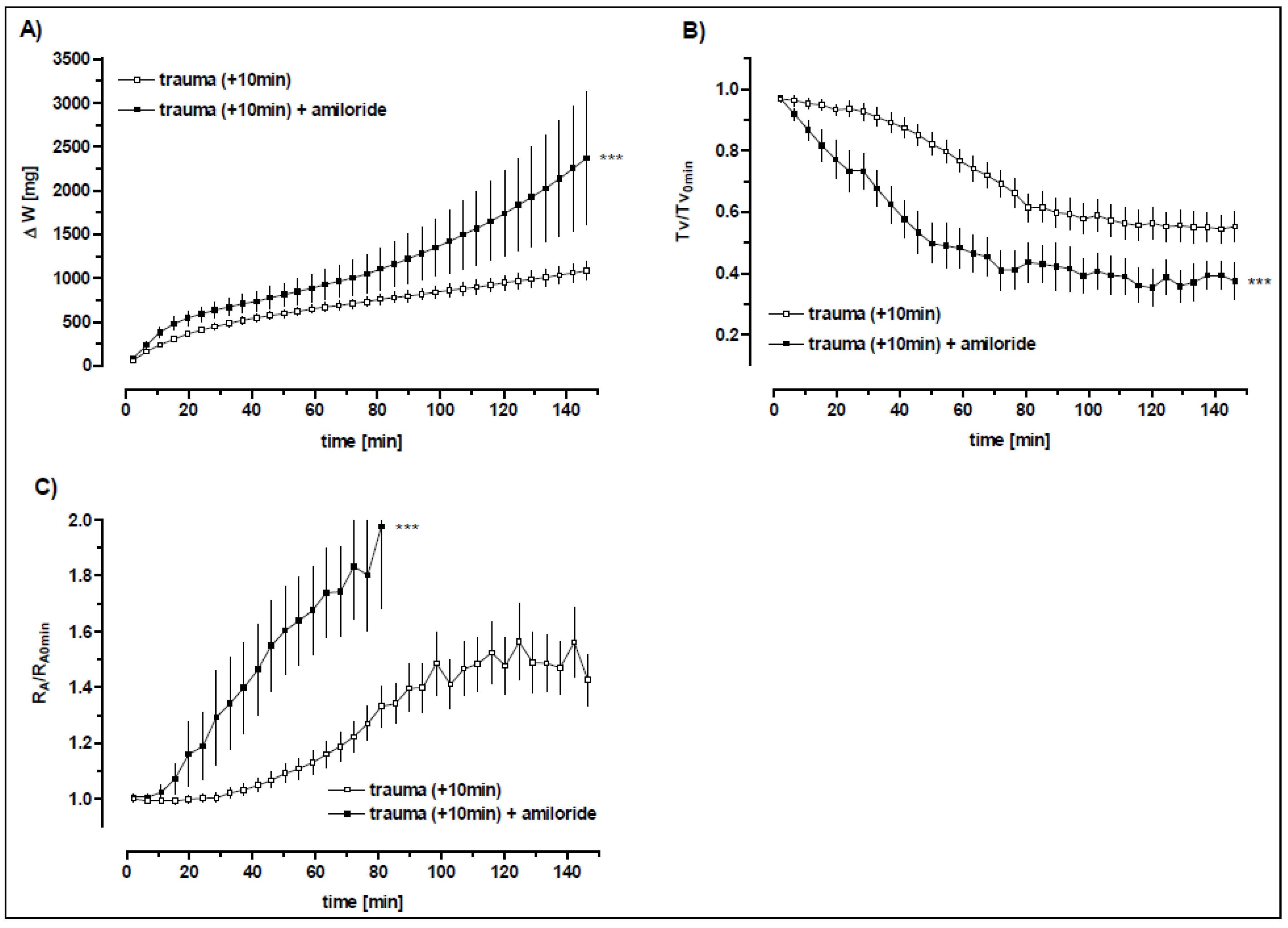
3.5.1. The Role of the Amiloride-Sensitive Sodium Channels after Trauma
3.5.2. β2-Adrenergic Receptor Stimulation
3.5.3. The Impact of the Formoterol after Trauma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, A.M.; Alderman, J.E. Thoracic Injury in Patients Injured by Explosions on the Battlefield and in Terrorist Incidents. Chest 2020, 157, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Jorolemon, M.R.; Lopez, R.A.; Krywko, D.M. Blast Injuries. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430914/ (accessed on 7 December 2020).
- Stuhmiller, J. Blast Injury: Translating Research into Operational Medicine; Office of the Surgeon General at TMM Publications: Falls Church, VA, USA, 2008; p. 41. [Google Scholar]
- Junuzovic, M. Explosion Fatalities in Sweden, 2000–2018. Med. Sci. Law 2021, 62, 258024211025228. [Google Scholar] [CrossRef] [PubMed]
- Westrol, M.S.; Donovan, C.M.; Kapitanyan, R. Blast Physics and Pathophysiology of Explosive Injuries. Ann. Emerg. Med. 2017, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Aboudara, M.; Mahoney, P.F.; Hicks, B.; Cuadrado, D. Primary blast lung injury at a NATO Role 3 hospital. J. R. Army Med Corps 2014, 160, 161. [Google Scholar] [CrossRef]
- Rendeki, S.; Tamás, F.M. Pulmonary Contusion. J. Thorac. Dis. 2019, 11, S141–S151. [Google Scholar] [CrossRef]
- Eghbalzadeh, K.; Sabashnikov, A.; Zeriouh, M.; Choi, Y.-H.; Bunck, A.C.; Mader, N.; Wahlers, T. Blunt chest trauma: A clinical chameleon. Heart 2017, 104, 719–724. [Google Scholar] [CrossRef]
- Scott, T.E.; Kirkman, E.; Haque, M.; Gibb, I.E.; Mahoney, P.; Hardman, J.G. Primary Blast Lung Injury—A Review. BJA Br. J. Anaesth. 2017, 118, 311–316. [Google Scholar] [CrossRef]
- Sziklavari, Z.; Tamas, F.M. Blast Injures to the Thorax. J. Thorac. Dis. 2019, 11, S167–S171. [Google Scholar] [CrossRef]
- Herrmann, J.; Tawhai, M.H.; Kaczka, D. Computational Modeling of Primary Blast Lung Injury: Implications for Ventilator Management. Mil. Med. 2019, 184, 273–281. [Google Scholar] [CrossRef]
- Tsokos, M.; Paulsen, F.; Petri, S.; Burkhard, M.; Püschel, K.; Türk, E.E. Histological, Immunohistochemical and Ultra-Structural Findings in Human Blast Lung Injury. Am. J. Respir. Crit. Care Med. 2003, 168, 549–555. [Google Scholar] [CrossRef]
- Peng, L.H.; Guo, G.H. Advances in the Research of Blast Lung Injury. Chin. J. Burn 2016, 32, 156–159. [Google Scholar]
- Smith, J.E.; Garner, J. Pathophysiology of primary blast injury. J. R. Army Med Corps 2018, 165, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Juffermans, N.P.; Schultz, M.; Bos, L.D.; Penuelas, O.; Laffey, J.; Lorente, J.A. Why translational research matters: Proceedings of the third international symposium on acute lung injury translational research (INSPIRES III). Intensiv. Care Med. Exp. 2019, 7 (Suppl. 1), 40. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, M.B.; Insel, P.A. Inhaled Β2 Adrenergic Agonists and Other Camp-Elevating Agents: Therapeutics for Alveolar Injury and Acute Respiratory Disease Syndrome? Pharmacol. Rev. 2021, 73, 488–526. [Google Scholar] [CrossRef] [PubMed]
- Morty, R.E.; Eickelberg, O.; Seeger, W. Alveolar Fluid Clearance in Acute Lung Injury: What Have We Learned from Animal Models and Clinical Studies? Intensive Care Med. 2007, 33, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Folkesson, H.G.; Clerici, C. Lung Epithelial Fluid Transport and the Resolution of Pulmonary Edema. Physiol. Rev. 2002, 82, 569–600. [Google Scholar] [CrossRef]
- Sigurdsson, G.H.; Christenson, J.T. Influence of terbutaline on endotoxin-induced lung injury. Circ. Shock 1988, 25, 153–163. [Google Scholar]
- Ressmeyer, A.-R.; Bai, Y.; Delmotte, P.; Uy, K.F.; Thistlethwaite, P.; Fraire, A.; Sato, O.; Ikebe, M.; Sanderson, M.J. Human Airway Contraction and Formoterol-Induced Relaxation Is Determined by Ca2+ Oscillations and Ca2+ Sensitivity. Am. J. Respir. Cell Mol. Biol. 2010, 43, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Norlin, A.; Lu, L.N.; Guggino, S.E.; Matthay, M.A.; Folkesson, H.G. Contribution of amiloride-insensitive pathways to alveolar fluid clearance in adult rats. J. Appl. Physiol. 2001, 90, 1489–1496. [Google Scholar] [CrossRef][Green Version]
- Chou, Y.-L.; Wu, C.-C.; Wang, H.-W. Effects of bambuterol and terbutaline on isolated rat’s tracheal smooth muscle. Eur. Arch. Otorhinolaryngol. 2010, 267, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Heiny, O. Measuring the weight of the isolated perfused rat lung during negative pressure ventilation. J. Pharmacol. Toxicol. Methods 1995, 33, 147–152. [Google Scholar] [CrossRef]
- Cooper, G.J.; Taylor, D.E.M. Biophysics of Impact Injury to the Chest and Abdomen. J. R. Army Med Corps 1989, 135, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Wollin, L. An improved setup for the isolated perfused rat lung. J. Pharmacol. Toxicol. Methods 1994, 31, 85–94. [Google Scholar] [CrossRef]
- Jayr, C.; Garat, C.; Meignan, M.; Pittet, J.F.; Zelter, M.; Matthay, M.A. Alveolar liquid and protein clearance in anesthetized ventilated rats. J. Appl. Physiol. 1994, 76, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Saldías, F.; Lecuona, E.; Friedman, E.; Barnard, M.L.; Ridge, K.M.; Sznajder, J.I. Modulation of lung liquid clearance by isoproterenol in rat lungs. Am. J. Physiol. Cell. Mol. Physiol. 1998, 274, L694–L701. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, J.; Stammberger, U.; Roux, J.; Kumar, S.; Yang, G.; Xiong, C.; Schmid, R.A.; Fakin, R.M.; Chakraborty, T.; Hossain, H.M.D.; et al. The lectin-like domain of tumor necrosis factor improves lung function after rat lung transplantation—Potential role for a reduction in reactive oxygen species generation. Crit. Care Med. 2010, 38, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Minnear, F.L.; DeMichele, M.A.; Leonhardt, S.; Andersen, T.T.; Teitler, M. Isoproterenol antagonizes endothelial permeability induced by thrombin and thrombin receptor peptide. J. Appl. Physiol. 1993, 75, 1171–1179. [Google Scholar] [CrossRef]
- Berthiaume, Y.; Staub, N.C.; A Matthay, M. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J. Clin. Investig. 1987, 79, 335–343. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Sznajder, J.I. Beta(2)-Agonists for Treatment of Pulmonary Edema: Ready for Clinical Studies? Crit. Care Med. 2004, 32, 1607–1608. [Google Scholar] [CrossRef]
- Goodman, B.E.; Kim, K.J.; Crandall, E.D. Evidence for active sodium transport across alveolar epithelium of isolated rat lung. J. Appl. Physiol. 1987, 62, 2460–2466. [Google Scholar] [CrossRef]
- Norlin, A.; Finley, N.; Abedinpour, P.; Folkesson, H.G. Alveolar liquid clearance in the anesthetized ventilated guinea pig. Am. J. Physiol. Content 1998, 274, L235–L243. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Shoemaker, R.L.; Matalon, S. Regulation of low-amiloride-affinity sodium channels in alveolar type II cells. Am. J. Physiol. Cell. Mol. Physiol. 1994, 267, L94–L100. [Google Scholar] [CrossRef] [PubMed]
- Robriquet, L.; Kipris, E.; Guery, B. Beta-adrenergic modulation of lung fluid balance in acute P aeruginosa pneumonia in rats. Exp. Lung Res. 2011, 37, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, J.; Hadizamani, Y.; Borgmann, M.; Mohaupt, M.; Männel, D.N.; Moehrlen, U.; Lucas, R.; Stammberger, U. Cytokine-Ion Channel Interactions in Pulmonary Inflammation. Front. Immunol. 2017, 8, 1644. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Helms, M.N.; Eaton, A.F.; Self, J.; Ramosevac, S.; Jain, L.; Bao, H.-F.; Eaton, U.C. Cholinergic regulation of epithelial sodium channels in rat alveolar type 2 epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L428–L437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Planes, C.; Blot-Chabaud, M.; Matthay, M.A.; Couette, S.; Uchida, T.; Clerici, C. Hypoxia and Beta 2-Agonists Regulate Cell Surface Expression of the Epithelial Sodium Channel in Native Alveolar Epithelial Cells. J. Biol. Chem. 2002, 277, 47318–47324. [Google Scholar] [CrossRef] [PubMed]
- Minakata, Y.; Suzuki, S.; Grygorczyk, C.; Dagenais, A.; Berthiaume, Y. Impact of Beta-Adrenergic Agonist on Na+ Channel and Na+-K+-Atpase Expression in Alveolar Type Ii Cells. Am. J. Physiol. 1998, 275, L414–L422. [Google Scholar]
- Bertorello, A.M.; Ridge, K.M.; Chibalin, A.V.; Katz, A.I.; Sznajder, J.I. Isoproterenol Increases Na+-K+-Atpase Activity by Membrane Insertion of Alpha-Subunits in Lung Alveolar Cells. Am. J. Physiol. 1999, 276, L20–L27. [Google Scholar]
- Saldias, F.J.; Lecuona, E.; Comellas, A.P.; Ridge, K.M.; Rutschman, D.H.; Sznajder, J.I. Beta-Adrenergic Stimulation Restores Rat Lung Ability to Clear Edema in Ventilator-Associated Lung Injury. Am. J. Respir. Crit. Care Med. 2000, 162, 282–287. [Google Scholar] [CrossRef]
- Dumasius, V.; Sznajder, J.I.; Azzam, Z.S.; Boja, J.; Mutlu, G.M.; Maron, M.B.; Factor, P. Beta(2)-Adrenergic Receptor Overexpression Increases Alveolar Fluid Clearance and Responsiveness to Endogenous Catecholamines in Rats. Circ. Res. 2001, 89, 907–914. [Google Scholar] [CrossRef]
- Trotta, T.; Guerra, L.; Piro, D.; D’Apolito, M.; Piccoli, C.; Porro, C.; Giardino, I.; Lepore, S.; Castellani, S.; Di Gioia, S.; et al. Stimulation of β2-adrenergic receptor increases CFTR function and decreases ATP levels in murine hematopoietic stem/progenitor cells. J. Cyst. Fibros. 2014, 14, 26–33. [Google Scholar] [CrossRef]
- Endo, M.; Kurachi, Y.; Mishina, M. Pharmacology of Ionic Channel Function: Activators and Inhibitors; Handbook of Experimental Pharmacology; Springer Science & Business Media: Berlin, Germany, 2012; Volume 147. [Google Scholar]
- Fan, Z.; Lin, W.; Lv, N.; Ye, Y.; Tan, W. R- and S-Terbutaline Activate Large Conductance and Ca2+ Dependent K+ (Bkca) Channel through Interacting with Β2 and M Receptor Respectively. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 2745–2752. [Google Scholar] [CrossRef]
- McAuley, D.F.; Matthay, M.A. Is There a Role for Beta-Adrenoceptor Agonists in the Management of Acute Lung Injury and the Acute Respiratory Distress Syndrome? Treat Respir. Med. 2005, 4, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Sartori, C.; Allemann, Y.; Duplain, H.; Lepori, M.; Egli, M.; Lipp, E.; Hutter, D.; Turini, P.; Hugli, O.; Cook, S.; et al. Salmeterol for the Prevention of High-Altitude Pulmonary Edema. N. Engl. J. Med. 2002, 346, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Festic, E.; Carr, G.E.; Cartin-Ceba, R.; Hinds, R.F.; Banner-Goodspeed, V.; Bansal, V.; Asuni, A.T.; Talmor, D.; Rajagopalan, G.; Frank, R.D.; et al. Randomized Clinical Trial of a Combination of an Inhaled Corticosteroid and Beta Agonist in Patients at Risk of Developing the Acute Respiratory Distress Syndrome*. Crit. Care Med. 2017, 45, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, Y.; Folkesson, H.G.; Matthay, M.A. Lung Edema Clearance: 20 Years of Progress: Invited Review: Alveolar Edema Fluid Clearance in the Injured Lung. J. Appl. Physiol. 2002, 93, 2207–2213. [Google Scholar] [CrossRef][Green Version]
- Briot, R.; Bayat, S.; Anglade, D.; Martiel, J.-L.; Grimbert, F. Increased cardiac index due to terbutaline treatment aggravates capillary-alveolar macromolecular leakage in oleic acid lung injury in dogs. Crit. Care 2009, 13, R166. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic Pulmonary Vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef]
- Lucas, R.; Yalda, H.; Perenlei, E.; Gabor, C.; Robert, W.C.; Harald, H.; Supriya, S.; Lever, A.A.; Hudel, M.; Ash, D.; et al. Dichotomous Role of Tumor Necrosis Factor in Pulmonary Barrier Function and Alveolar Fluid Clearance. Front. Physiol. 2022, 12, 793251. [Google Scholar] [CrossRef]
- Boutillier, J.; Deck, C.; Magnan, P.; Naz, P.; Willinger, R. A critical literature review on primary blast thorax injury and their outcomes. J. Trauma Acute Care Surg. 2016, 81, 371–379. [Google Scholar] [CrossRef]
- Agoston, D.V. Modeling the Long-Term Consequences of Repeated Blast-Induced Mild Traumatic Brain Injuries. J. Neurotrauma 2017, 34, S44–S52. [Google Scholar] [CrossRef] [PubMed]

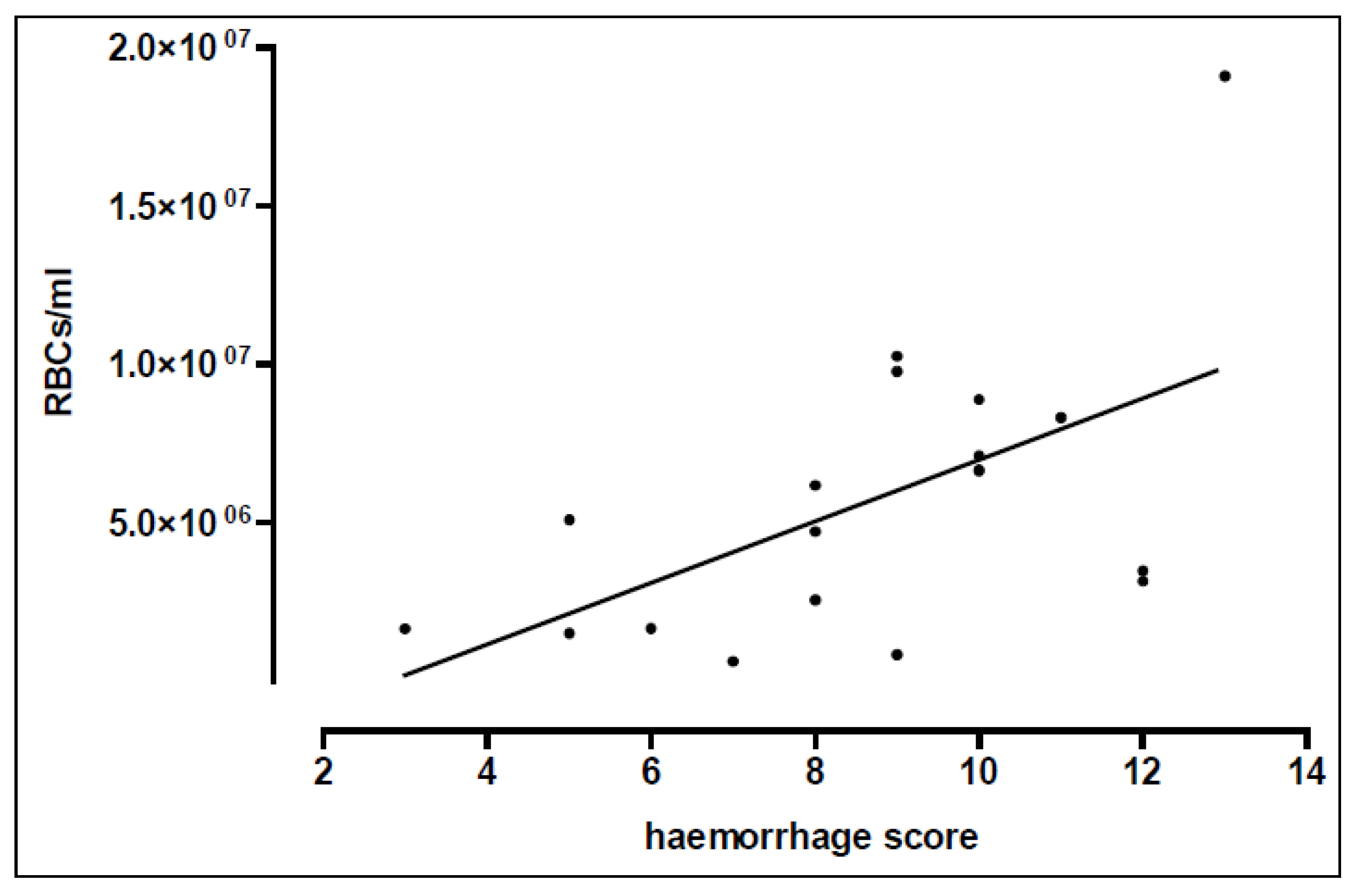

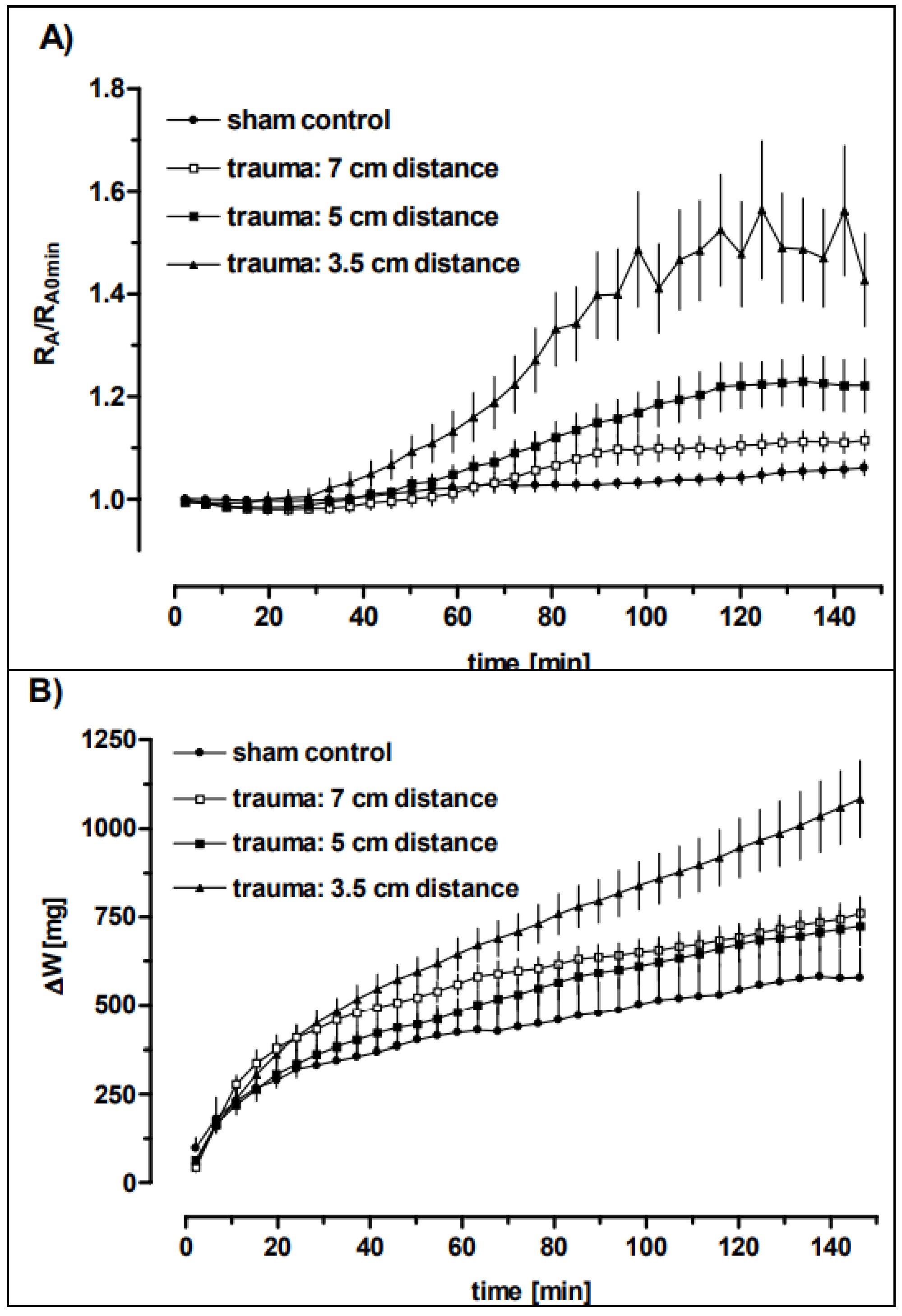
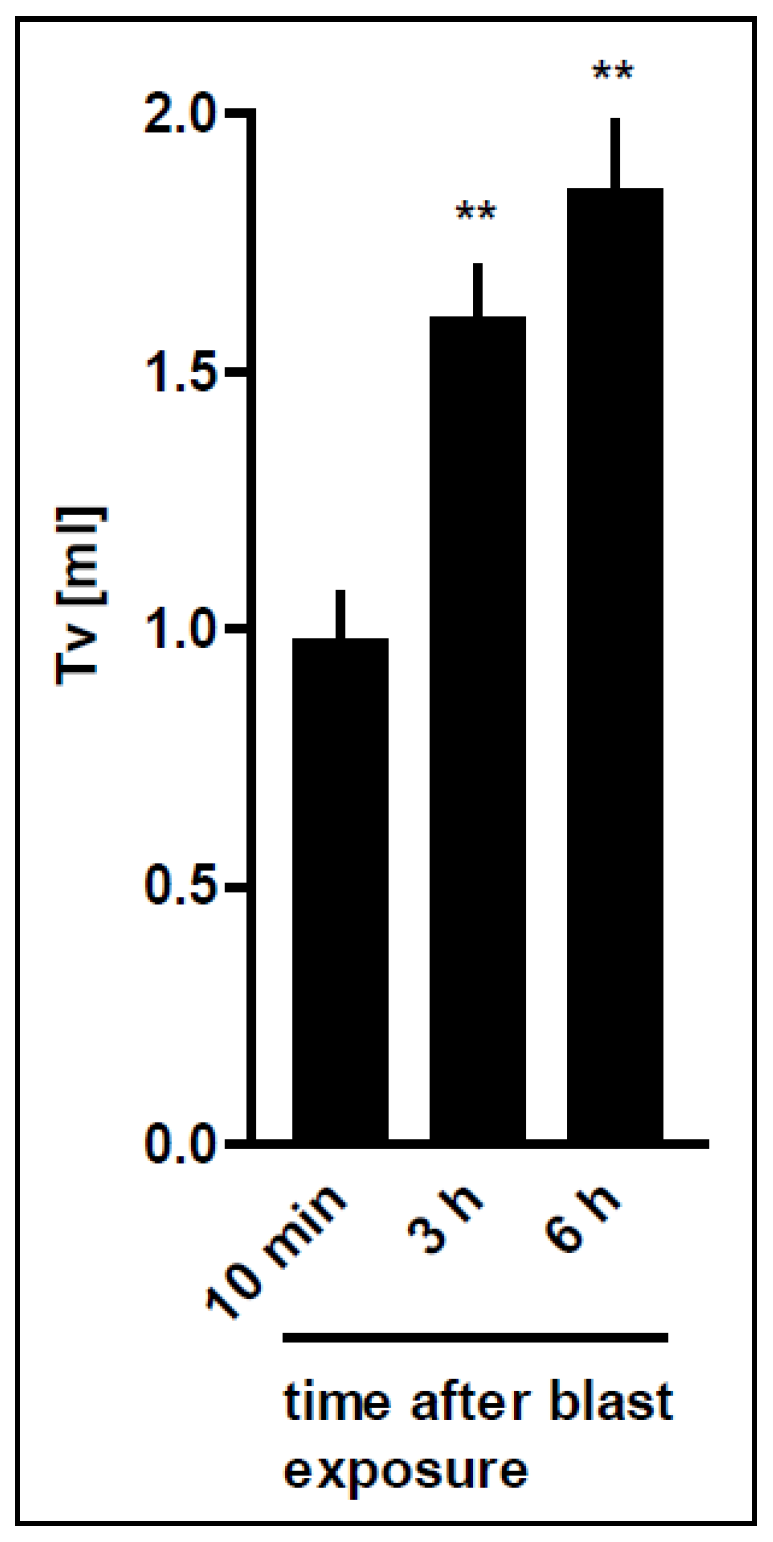
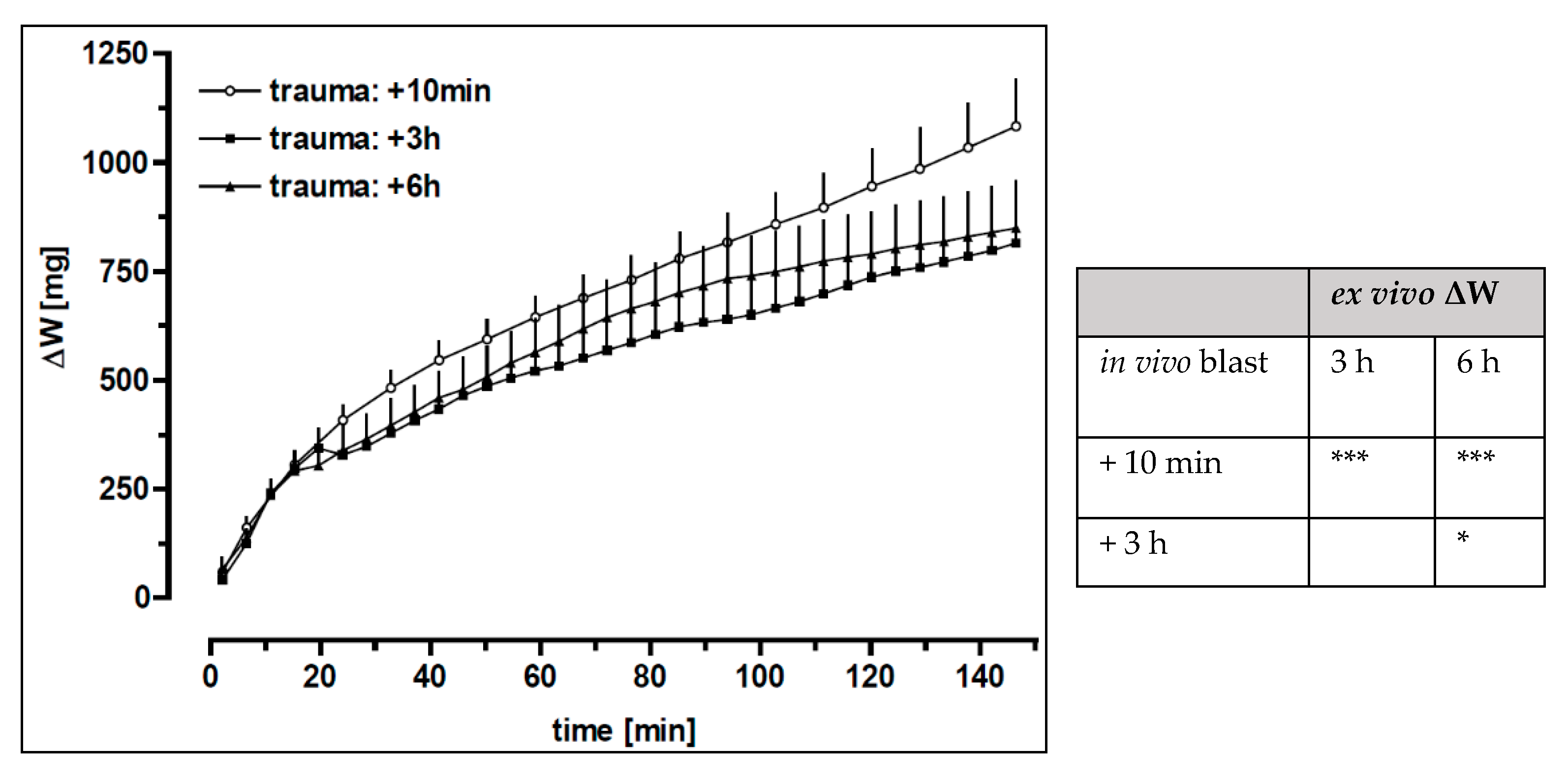

| Distance | RBCs × 106/mL | Haemorrhage Score | Oedema Score |
|---|---|---|---|
| sham control | 0.12 ± 0.15 (5) | 0 ± 0 (5) | 0 ± 0 (4) |
| 3.5 cm | 7.13 ± 4.72 (12) ** | 9.6 ± 2.2 (12) *** | 10.0 ± 3.6 (3) *** |
| 5 cm | 4.68 ± 3.11(4) | 8.3 ± 2.1 (4) *** | 7.2 ± 1.9 (5) ** |
| 7 cm | 1.33 ± 0.44 (3) | 5.7 ± 3.1 (3) ** # | 4.0 ± 1.4 (3) # |
| Distance | n | BW [g] | LWW [g] | LWR | Qi |
|---|---|---|---|---|---|
| sham control | 4 | 269 ± 30 | 1.83 ± 0.13 | 0.0069 ± 0.0013 | |
| 3.5 cm | 3 | 243 ± 6 | 3.21 ± 1.25 * | 0.0130 ± 0.0053 * | 1.94 |
| 5 cm | 5 | 241 ± 6 | 2.14 ± 0.23 | 0.0089 ± 0.0009 | 1.31 |
| 7 cm | 3 | 238 ± 12 | 1.94 ± 0.06 # | 0.0082 ± 0.0006 # | 1.20 |
| In Vivo Trauma | Ex Vivo Perfusion | |||
|---|---|---|---|---|
| ~−15 min | ~3 min | 150 min | 150 min | 150 min |
| Distance | PIP (cm H2O) | Tv (mL) | RA (cm H2Oxs/mL) | ΔW (mg) |
| Sham control | 7 ± 0 (7) | 1.60 ± 0.15 (7) | 0.33 ± 0.05 (7) | 579 ± 184 (6) |
| 3.5 cm | 8.36 ± 1.36 * (16) | 0.66 ± 0.21 *** (13) | 0.47 ± 0.15 * (15) | 1099 ± 441 ** (15) |
| 5 cm | 7.31 ± 0.64 # (11) | 0.68 ± 0.23 *** (11) | 0.34 ± 0.05 # (10) | 723 ± 158 # (9) |
| 7 cm | 7.07 ± 0.07 # (7) | 0.90 ± 0.31 *** (7) | 0.29 ± 0.02 ## (7) | 789 ± 114 (6) |
| In Vivo Trauma | Ex Vivo Perfusion | |||
|---|---|---|---|---|
| ~−15 min | 0 min | 150 min | 150 min | 150 min |
| Time after blast | PIP (cm H2O) | Tv (mL) | RA (cm H2Oxs/mL) | ΔW (mg) |
| 10 min | 8.36 ± 1.36 (16) | 0.66 ± 0.21 (13) | 0.47 ± 0.15 (15) | 1099 ± 441 (15) |
| 3 h | 7.07 ± 0.11 ** (8) | 0.83 ± 0.31 (7) | 0.36 ± 0.07 (7) | 826 ± 293 (8) |
| 6 h | 7.09 ± 0.08 * (8) | 0.76 ± 0.34 (6) | 0.38 ± 0.09 (6) | 855 ± 311 (8) |
| Tv (mL) | RA (cm H2Oxs/mL) | ΔW (mg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Trauma | +Terbutaline | +Amiloride | Trauma | +Terbutaline | +Amiloride | Trauma | +Terbutaline | +Amiloride |
| +amiloride | >0.0005 | >0.0005 | >0.0005 | ||||||
| +terbutaline | >0.0005 | >0.0005 | >0.0005 | ||||||
| +formoterol | >0.0005 | >0.0005 | >0.0005 | >0.0005 | >0.0005 | >0.0005 | |||
| +terbutaline +propranolol | ns | >0.0005 | ns | >0.0005 | >0.0005 | >0.0005 | |||
| +terbutaline +amiloride | >0.0005 | ns | >0.0005 | >0.0005 | ns | >0.0005 | >0.0005 | >0.0005 | >0.005 |
| In Vivo Trauma | Ex Vivo Perfusion | |||
|---|---|---|---|---|
| ~−15 min | ~4 min | 150 min | 150 min | 150 min |
| treatment | Tv (mL) | RA (cm H2Oxs/mL) | ΔW (mg) | |
| Sham control | 1.60 ± 0.15 (7) | 0.33 ± 0.05 (7) | 579 ± 184 (6) | |
| Trauma | 0.66 ± 0.21 (13) §§§ | 0.47 ± 0.15 (15) § | 1099 ± 441 (15) § | |
| + amiloride | 0.56 ± 0.09 (4) | 1.60 ± 0.46 (5) *** ⊗ | 2429 ± 2265 (8) * | |
| + terbutaline | 1.08 ± 0.26 (9) *** | 0.33 ± 0.04 (9) ** | 559 ± 368 (8) ** | |
| + formoterol | 1.12 ± 0.34 (12) *** | 0.32 ± 0.04 (12) ** | 827 ± 381 (12) | |
| + terbutaline + propranolol | 0.89 ± 0.51 (3) | 0.40 ± 0.12 (3) # | 3378 ± 2779 (5) ***,# | |
| + terbutaline + amiloride | 1.09 ± 0.61 (4) * | 0.35 ± 0.12 (4) | 1534 ± 799 (4) # | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huwer, H.; Hadizamani, Y.; Moehrlen, U.; Stammberger, U.; Gebhard, F.; Bally, L.; Wendel, A.; Liener, U.C.; Lucas, R.; Hamacher, J. Ex Vivo Pulmonary Oedema after In Vivo Blast-Induced Rat Lung Injury: Time Dependency, Blast Intensity and Beta-2 Adrenergic Receptor Role. Biomedicines 2022, 10, 2930. https://doi.org/10.3390/biomedicines10112930
Huwer H, Hadizamani Y, Moehrlen U, Stammberger U, Gebhard F, Bally L, Wendel A, Liener UC, Lucas R, Hamacher J. Ex Vivo Pulmonary Oedema after In Vivo Blast-Induced Rat Lung Injury: Time Dependency, Blast Intensity and Beta-2 Adrenergic Receptor Role. Biomedicines. 2022; 10(11):2930. https://doi.org/10.3390/biomedicines10112930
Chicago/Turabian StyleHuwer, Hanno, Yalda Hadizamani, Ueli Moehrlen, Uz Stammberger, Florian Gebhard, Lia Bally, Albrecht Wendel, Ulrich C. Liener, Rudolf Lucas, and Jürg Hamacher. 2022. "Ex Vivo Pulmonary Oedema after In Vivo Blast-Induced Rat Lung Injury: Time Dependency, Blast Intensity and Beta-2 Adrenergic Receptor Role" Biomedicines 10, no. 11: 2930. https://doi.org/10.3390/biomedicines10112930
APA StyleHuwer, H., Hadizamani, Y., Moehrlen, U., Stammberger, U., Gebhard, F., Bally, L., Wendel, A., Liener, U. C., Lucas, R., & Hamacher, J. (2022). Ex Vivo Pulmonary Oedema after In Vivo Blast-Induced Rat Lung Injury: Time Dependency, Blast Intensity and Beta-2 Adrenergic Receptor Role. Biomedicines, 10(11), 2930. https://doi.org/10.3390/biomedicines10112930








